Abstract
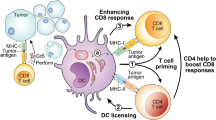
The dendritic cell (DC)-initiated and sustained cancer immunity cycle is indispensable for effective endogenous and therapeutically mobilized antitumour T cell responses1,2,3,4,5,6,7,8. This necessitates the continuous migration of antigen-carrying DCs from the tumour microenvironment (TME) to the tumour draining lymph nodes (tdLNs)7,8,9,10,11,12,13. Here, through longitudinal analysis of human and mouse tumours, we observed a progressive decrease in migratory conventional DCs (mig-cDCs) in the tdLNs during tumour progression. This decline compromised tumour-specific T cell priming and subsequent T cell supply to the TME. Using a genome-wide in vivo CRISPR screen, we identified phosphodiesterase 5 (PDE5) and its substrate cyclic guanosine monophosphate (cGMP) as key modulators of DC migration. Advanced tumours disrupted cGMP synthesis in DCs to decrease their motility, while PDE5 perturbation preserved the cGMP pool to restore DC migration. Mechanistically, cGMP enhanced myosin-II activity through Rho-associated factors, extending the paradigm of cGMP-regulated amoeboid migration from Dictyostelium to mammalian immune cells. Pharmacological inhibition of PDE5 using sildenafil restored mig-cDC homing to late-stage tdLNs and sustained antitumour immunity in a DC-dependent manner. Our findings bridge fundamental DC interstitial motility to antitumour immunity, revealing that its disruption in chaotic TME promotes immune evasion, and its enhancement offers a promising direction for DC-centric immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available in the Article and its Supplementary Information. RNA-seq data files are available at the Gene Expression Omnibus (GEO) under accession number GSE246849. All other data are available in the Article and its Supplementary Information. Source data are provided with this paper.
References
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Anderson, D. A. 3rd, Dutertre, C. A., Ginhoux, F. & Murphy, K. M. Genetic models of human and mouse dendritic cell development and function. Nat. Rev. Immunol. 21, 101–115 (2021).
Palucka, K. & Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 12, 265–277 (2012).
Merad, M., Sathe, P., Helft, J., Miller, J. & Mortha, A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 31, 563–604 (2013).
Cabeza-Cabrerizo, M., Cardoso, A., Minutti, C. M., Pereira da Costa, M. & Reis e Sousa, C. Dendritic cells revisited. Annu. Rev. Immunol. 39, 131–166 (2021).
Wculek, S. K. et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 (2020).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Pittet, M. J., Di Pilato, M., Garris, C. & Mempel, T. R. Dendritic cells as shepherds of T cell immunity in cancer. Immunity 56, 2218–2230 (2023).
Heuze, M. L. et al. Migration of dendritic cells: physical principles, molecular mechanisms, and functional implications. Immunol Rev. 256, 240–254 (2013).
Worbs, T., Hammerschmidt, S. I. & Forster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 17, 30–48 (2017).
Alvarez, D., Vollmann, E. H. & von Andrian, U. H. Mechanisms and consequences of dendritic cell migration. Immunity 29, 325–342 (2008).
de Winde, C. M., Munday, C. & Acton, S. E. Molecular mechanisms of dendritic cell migration in immunity and cancer. Med. Microbiol. Immunol. 209, 515–529 (2020).
Liu, J., Zhang, X., Cheng, Y. & Cao, X. Dendritic cell migration in inflammation and immunity. Cell. Mol. Immunol. 18, 2461–2471 (2021).
Forster, R., Davalos-Misslitz, A. C. & Rot, A. CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 8, 362–371 (2008).
Weber, M. et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 339, 328–332 (2013).
Bursch, L. S. et al. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 204, 3147–3156 (2007).
Broz, M. L. et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 938 (2014).
Roberts, E. W. et al. Critical role for CD103+/CD141+ dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 30, 324–336 (2016).
Salmon, H. et al. Expansion and activation of CD103+ dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44, 924–938 (2016).
Allan, R. S. et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25, 153–162 (2006).
Lammermann, T. & Sixt, M. Mechanical modes of ‘amoeboid’ cell migration. Curr. Opin. Cell Biol. 21, 636–644 (2009).
Paluch, E. K., Aspalter, I. M. & Sixt, M. Focal adhesion-independent cell migration. Annu. Rev. Cell Dev. Biol. 32, 469–490 (2016).
Moreau, H. D., Piel, M., Voituriez, R. & Lennon-Dumenil, A. M. Integrating physical and molecular insights on immune cell migration. Trends Immunol 39, 632–643 (2018).
Lammermann, T. et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55 (2008).
Vicente-Manzanares, M., Ma, X., Adelstein, R. S. & Horwitz, A. R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790 (2009).
Renkawitz, J. et al. Adaptive force transmission in amoeboid cell migration. Nat. Cell Biol. 11, 1438–1443 (2009).
Bergert, M. et al. Force transmission during adhesion-independent migration. Nat. Cell Biol. 17, 524–529 (2015).
Faure-Andre, G. et al. Regulation of dendritic cell migration by CD74, the MHC class II-associated invariant chain. Science 322, 1705–1710 (2008).
Vargas, P. et al. Innate control of actin nucleation determines two distinct migration behaviours in dendritic cells. Nat. Cell Biol. 18, 43–53 (2016).
de Saint-Vis, B. et al. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity 9, 325–336 (1998).
Bender, A. T. & Beavo, J. A. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol. Rev. 58, 488–520 (2006).
Lucas, K. A. et al. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol. Rev. 52, 375–414 (2000).
Francis, S. H., Busch, J. L., Corbin, J. D. & Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 62, 525–563 (2010).
van Haastert, P. J. M. et al. Forty-five years of cGMP research in Dictyostelium: understanding the regulation and function of the cGMP pathway for cell movement and chemotaxis. Mol. Biol. Cell 32, ar8 (2021).
Bosgraaf, L. & van Haastert, P. J. The regulation of myosin II in Dictyostelium. Eur. J. Cell Biol. 85, 969–979 (2006).
Chabaud, M. et al. Cell migration and antigen capture are antagonistic processes coupled by myosin II in dendritic cells. Nat. Commun. 6, 7526 (2015).
Goldberg, J. M., Bosgraaf, L., Van Haastert, P. J. & Smith, J. L. Identification of four candidate cGMP targets in Dictyostelium. Proc. Natl Acad. Sci. USA 99, 6749–6754 (2002).
Beavo, J. A. & Brunton, L. L. Cyclic nucleotide research–still expanding after half a century. Nat. Rev. Mol. Cell Biol. 3, 710–718 (2002).
Cruz-Burgos, M. et al. New approaches in oncology for repositioning drugs: the case of PDE5 inhibitor sildenafil. Front. Oncol. 11, 627229 (2021).
Huang, W., Sundquist, J., Sundquist, K. & Ji, J. Use of phosphodiesterase 5 inhibitors is associated with lower risk of colorectal cancer in men with benign colorectal neoplasms. Gastroenterology 157, 672–681 (2019).
Huang, W., Sundquist, J., Sundquist, K. & Ji, J. Phosphodiesterase-5 inhibitors use and risk for mortality and metastases among male patients with colorectal cancer. Nat. Commun. 11, 3191 (2020).
Sutton, S. S., Magagnoli, J., Cummings, T. H. & Hardin, J. W. The association between phosphodiesterase-5 inhibitors and colorectal cancer in a national cohort of patients. Clin. Transl. Gastroenterol. 11, e00173 (2020).
Weed, D. T. et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 21, 39–48 (2015).
Califano, J. A. et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 21, 30–38 (2015).
Luginbuhl, A. J. et al. Tadalafil enhances immune signatures in response to neoadjuvant nivolumab in resectable head and neck squamous cell carcinoma. Clin. Cancer Res. 28, 915–927 (2022).
Hassel, J. C. et al. Tadalafil has biologic activity in human melanoma. Results of a pilot trial with tadalafil in patients with metastatic melanoma (TaMe). Oncoimmunology 6, e1326440 (2017).
Das, A., Durrant, D., Salloum, F. N., Xi, L. & Kukreja, R. C. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol. Ther. 147, 12–21 (2015).
Ohno, M., Gibbons, G. H., Dzau, V. J. & Cooke, J. P. Shear stress elevates endothelial cGMP. Role of a potassium channel and G protein coupling. Circulation 88, 193–197 (1993).
Hofmann, F., Ammendola, A. & Schlossmann, J. Rising behind NO: cGMP-dependent protein kinases. J. Cell Sci. 113, 1671–1676 (2000).
Weed, D. T. et al. The reversal of immune exclusion mediated by tadalafil and an anti-tumor vaccine also induces PDL1 upregulation in recurrent head and neck squamous cell carcinoma: interim analysis of a phase i clinical trial. Front. Immunol. 10, 1206 (2019).
Arber, N. et al. Sporadic adenomatous polyp regression with exisulind is effective but toxic: a randomised, double blind, placebo controlled, dose-response study. Gut 55, 367–373 (2006).
Philip, M. et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 545, 452–456 (2017).
Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 15, 554 (2014).
Hänzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 14, 7 (2013).
Aran, D., Hu, Z. & Butte, A. J. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 18, 220 (2017).
Li, T. et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 48, W509–W514 (2020).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics 16, 284–287 (2012).
Zilionis, R. et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity 50, 1317–1334 (2019).
Cheng, S. et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 184, 792–809.e23 (2021).
Pelka, K. et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell 184, 4734–4752 (2021).
Lun, A. T., McCarthy, D. J. & Marioni, J. C. A step-by-step workflow for low-level analysis of single-cell RNA-seq data with Bioconductor. F1000Res. 5, 2122 (2016).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Pertz, O., Hodgson, L., Klemke, R. L. & Hahn, K. M. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 440, 1069–1072 (2006).
Surks, H. K. et al. Regulation of myosin phosphatase by a specific interaction with cGMP-dependent protein kinase Iα. Science 286, 1583–1587 (1999).
Chung, K. K. H. et al. Fluorogenic DNA-PAINT for faster, low-background super-resolution imaging. Nat. Methods 19, 554–559 (2022).
Gunn, M. D. et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189, 451–460 (1999).
Acknowledgements
We thank all of the members of the Ting Zhou and Yongdeng Zhang laboratories for discussions and support; the staff at the Laboratory Animal Resources Center, High-Performance Computing Center, Flow Cytometry Core, Genomic Core and Microscopy Core at Westlake University for support with techniques; and K. Guan, Q. Ma, D. Li, J. Wang, X. Zhou, D. He, H. Xu and C. Rosen for discussions and suggestions. This study was supported by National Natural Science Foundation of China grant 32270968, the Noncommunicable Chronic Diseases-National Science and Technology Major Program (2023ZD0500402 to T.Z.), the ‘High Risk High Impact’ (HRHI) program of Westlake Laboratory of Life Sciences and Biomedicine W101110566022301, ‘Pioneer’ and ‘Leading Goose’ R&D Program of the Department of Science and Technology of Zhejiang Province 2023SDXHDX0001, Westlake Multidisciplinary Research Initiative Center (MRIC) Seed Fund MRIC20210202 (T.Z.) and the Education Foundation of Westlake University.
Author information
Authors and Affiliations
Contributions
H.T. and Z.W. performed all of the experiments. Z.W. and H.T. performed imaging experiments. L.W. and H.T. analysed RNA-seq data. Z.W., B.Z., Y. Chen, Z.Y., Y.Q. and Yongdeng Zhang performed super-resolution imaging. H.W., Y. Cai, S.S., B.X., R.Z., J.L. and J.G. collected and prepared human patient samples. H.T. and X.M. performed western blot experiments. P.W., X.L. and H.T. performed biotin–cGMP pull-down assays and MS analysis. J.X. and Y. Zhao helped to maintain the mice. Z.L. and F.G. provided DC-related genetic mice. Ying Zhang, J.M., Z.X. and Q.X. helped to provide agents and assays. T.Z., H.T. and Z.W. wrote the paper. T.Z., H.T., Z.W. and L.Z. edited and discussed the paper. T.Z. supervised the research. T.Z. conceived the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Ana-Maria Lennon-Duménil, Caetano Reis e Sousa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Decreased migratory cDCs in both human and mouse tdLNs.
a,b, Immunofluorescent staining of cDCs (CD11C and LAMP3), T cells (CD3), or B cells (CD19) in early- or late-stage SLNs from breast cancer (n = 5 vs 6 patients) (a) and colon cancer (n = 5 vs 5 patients) (b) patients. Scale bars, overview, 1 mm; zone a and b, 100 μm. Quantifications on the right panel were CD11C/LAMP3 double positive area out of the total SLN area (%) (upper), or area value of 2 randomly selected areas (500 × 500 μm2) within each sample (lower) (n = 10 vs 12 areas in a; n = 10 areas each in b from corresponding patients). Lower quantification is displayed as area per 10 μm2. c,d, Quantifications of tumour volume and tdLN mig-cDCs (CD11c+MHC-IIhi) at day 7, 10, and 14 (c, B16F10, n = 4, 4, 6 mice; d, MC38, n = 4 mice per group). e, Quantification of IFNγ and TOX frequencies in CD44+PD1+CD8+ T cells in B16F10 tumour (n = 3 vs 6 mice). f, Quantifications of mig-cDCs, mig-CD103+ cDCs, or mig-CD11b+ cDCs in tdLNs of MC38 tumour-bearing mice (n = 5 vs 6 mice). g, Flow cytometric gating strategy for DC populations in tdLNs (sample derived from B16F10-OVA-ZsGreen tumour bearing mice). ZsGreen negative control was indicated as grey. Migratory cDC1s or cDC2s were pre-gated on migratory cDCs (CD11c+MHC-IIhi). h, Quantifications of migratory XCR1+ and SIRPα+ cDCs in tdLNs of B16F10-OVA-ZsGreen -bearing mice (n = 6 mice per group). i, Quantifications of migratory CD103+ and CD11b+ cDCs in non-tdLNs of B16F10-OVA-ZsGreen-bearing mice (n = 6 mice per group). j, Quantifications of total CD8+ T cells, CD44+CD8+ T cells and PD1+CD8+ T cells in tdLNs from mice bearing early- or late-stage tumours (n = 6 mice per group). Data are merged (a, b) or representative (c-j) of at least two to three independent experiments and present as mean ± s.e.m. Statistical analysis was performed using two-tailed Student’s unpaired t-test.
Extended Data Fig. 2 DC frequency in TME and in vivo CRISPR screening QC.
a, Flow cytometric gating strategy for DC populations in TME. b,c, Quantifications of total cDCs, CD103+ cDCs, and CD11b+ cDCs in TME from early or late stage (b, B16F10, n = 5 mice; c, MC38, n = 6 mice). d, LAMP3+ DC signatures in early- or late-stage cancer patients based on TCGA database. e,i, All the mapped sgRNA hits for the pre-round (e) or major-round (i). Data are merged from 5 (e) or 3 (i) independent experiments. The x-axis indicates sgRNA numbers and y-axis indicates the calculated log2 fold change. f,j, Coverage calculation in each step from pre-round (f) or major-round (j) screening. g,k, Gini-Index analysis for pre-round (g) or major-round (k) screening. h, Minipool gRNAs selection strategy after the pre-round screening. l, Distribution of the enriched sgRNAs for top-hit genes from the major-round screening. m, Western blot analysis of Pde5 expression in BMDCs derived from Pde5+/+ or Pde5−/− mice. β-actin was used as control. n, Frequencies of CTV-labelled Pde5+/+ and CFSE-labelled Pde5−/− DCs in the TME two days post peritumour transfer (n = 6 mice). Quantifications on right panel were normalized to the mixture ratio before cell transfer. o, Whole-mount tissue 3D reconstitution (left) and cross-section image (middle) of tdLNs from mice co-transferred with Pde5+/+ and Pde5−/− DCs. Cells were surface rendered and DC signals were processed as dots. Scale bars, 500 μm. Data quantified from full tissue sections from each mouse (n = 2 mice). p, Representative staining and quantification of CTV-labelled Pde5+/+ and CFSE-labelled Pde5−/− mature BMDCs in mouse ear explants (n = 7 lymphatic vessels). Scale bar, 100 μm. Data are representative (b, c, m, n) or merged (e, g, i, k, l, o, p) of at least two to three independent experiments and present as mean ± s.e.m. Statistical analysis was performed using two-tailed Student’s unpaired (b, c) or paired (n, p) t-test or two tailed Mann–Whitney U-tests (d).
Extended Data Fig. 3 Late-stage tumours dampen DC interstitial motility.
a, Gene set enrichment analysis of down-regulated genes associated with cytoskeletal protein binding in late-stage tumoural DCs. NES, normalized enrichment score. b, Gene ontology enrichment analysis of downregulated pathways in late-stage tumoural DCs. c, Differentially expressed genes associated with GTPase in early- or late-stage tumoural DCs. d, Representative images and quantifications of tumoural DCs within early- or late-stage tumour margin lymphatic vessels (white arrowheads; n = 57 lymphatic vessels). Scale bars, 50 μm. e, Transcriptional analysis of Ccr7 (left, n = 2 biological samples) and flow cytometric quantification of CCR7 on tumoural DCs (right, n = 6 vs 5 mice). f,g, Representative trajectories of BMDC migration (corresponding to experiments and velocity statistics in Fig. 3f,g). h, ELISA examining intracellular cGMP levels in Pde5+/+ and Pde5−/− BMDCs (n = 3 biological replicates). i, Mean velocities of BMDCs treated with vehicle (n = 169 cells), 10 μM (n = 157 cells), 100 μM (n = 182 cells), or 500 μM 8-Br-cGMP (n = 142 cells) in 3D migration assay. j,k, Trajectories and mean velocities of mature BMDCs in 3D collagen gel assay migration assay treated with vehicle (n = 181 cells) or 500 μM 8-Br-cGMP (n = 175 cells) (j), or using Pde5−/− BMDCs (Pde5+/+, n = 193 cells; Pde5−/−, n = 116 cells) (k). l, Schematic of cGMP pathway components in regulating DC interstitial motility. The diagrams were created in BioRender. Zhou, T. (2025) https://BioRender.com/lyhhvye. m, t-SNE plot visualization of flow cytometric quantified cell populations (left) and NOS2 fluorescence intensity (right) in B16F10 TME. n, Representative trajectories of BMDC migration (corresponding to Fig. 3l). o, Trajectories and mean velocities of mature BMDCs either treated with vehicle (n = 188 cells) or 100 μM DETA NONOate (n = 154 cells). Data are representative (e, h, m) or merged (d, f, g, i-k, n, o) of at least two to three independent experiments and present as mean ± s.e.m. Statistical analysis was performed using two-tailed Mann–Whitney U-tests (d, j, k, o), two-tailed Student’s unpaired t-test (e, h) or Kruskal–Wallis test with multiple comparisons (i). Trajectories were randomly selected from cells after quantifying their velocities under the same conditions.
Extended Data Fig. 4 Pde5 perturbation restores DC migration through cGMP-regulated myosin-II activity.
a, pMLC signal visualization using DNA-PAINT imaging at nanoscale resolution in whole cell (overlay) or y-z slice view (x = 200 nm) of BMDCs treated with vehicle or 8-Br-cGMP. pMLC signal quantifications are presented on the right (n = 75 steps per group, 1 step = 50 nm). White arrows indicate enhanced pMLC signals in peri-nuclear regions. Colour bar denotes depth of z axis. Scale bars, 1 μm. b, pMLC signal visualization in mature BMDCs treated with vehicle or 500 μM 8-Br-cGMP. Colour bar denotes depth. Scale bar, 1 μm. c, Quantifications of the pMLC signal ratio between the back and front of DCs treated with vehicle or 500 μM 8-Br-cGMP (n = 20 cells per group) (see Methods). d, Western blot analysis of pMLC expression in mature BMDCs treated with 500 μM 8-Br-cGMP for varying durations (0, 5, and 15 min). GAPDH was used as control. e-f, Western blot analysis of pMLC and pROCK levels in mature BMDCs treated with 500 μM 8-Br-cGMP plus 10 μM Y27632 (e) or 50 μM Rhosin hydrochloride (f) for 5 min. g, Quantifications of RhoA activity in BMDCs treated with vehicle (n = 26 cells) or 500 μM 8-Br-cGMP (n = 50 cells) using FRET sensor, with FRET ratios plotted. h, Mean velocities of BMDCs treated with vehicle (n = 125 cells), 500 μM 8-Br-cGMP (n = 158 cells), 8-Br-cGMP plus 50 μM Rhosin (n = 100 cells) or 10 μM Y27632 (n = 132 cells). i,j, Transcript analysis of PRKG1 and PRKG2 in different cell populations of Pan-cancer (i) or colorectal cancer (CRC) (j) patients (see Methods). k, Mean velocities of mature BMDCs treated with vehicle (n = 132 cells), 500 μM 8-Br-cGMP (n = 115 cells), 30 μM sildenafil (n = 137 cells), or each plus 86 μM PKG inhibitor (n = 121, 130, 123 cells correspondingly) in 3D collagen gel assay. Data are representative (a-f) or merged (g, h, k) of at least two to three independent experiments and present as mean ± s.e.m. Statistical analysis was performed using two-tailed Mann–Whitney U-tests (g) or Kruskal–Wallis test with multiple comparisons (h, k).
Extended Data Fig. 5 Sildenafil enhances antitumour immunity.
a,b, Trajectories and mean velocities of immature BMDCs (n = 147 vs 136 cells) (a) or mature BMDCs (n = 150 vs 174 cells) (b) treated with 30 μM sildenafil in 3D collagen gel assay. c, Mean velocities of BMDCs treated with vehicle (n = 151 cells), 10 μM (n = 137 cells), 30 μM (n = 173 cells), or 50 μM sildenafil (n = 137 cells) in 3D migration assay. d-i, Comparative characteristics between B16F10/B16F10Pde5−/− (d-f) and MC38/MC38Pde5−/− tumour cells (g-i) (n = 3 biological replicates for d, g; n = 6 mice per group for e, f, h, i). j,k, Tumour growth and Kaplan–Meier survival curves of MC38Pde5−/− bearing mice treated with 7 doses of vehicle or sildenafil every other day via intraperitoneal (i.p.) injection (n = 10 vs 13 mice) (j), or oral gavage (gava.) (n = 11 vs 10 mice) (k). l, Tumour growth curves of MC38Pde5−/− bearing mice treated with vehicle or sildenafil at specified doses (vehicle, n = 7; 200 μg sildenafil, n = 6; 400 μg sildenafil, n = 7). m, Tumour growth curves of Batf3+/− and Batf3−/− mice bearing MC38Pde5−/− tumour and treated with vehicle or sildenafil (Batf3+/− + vehicle, n = 5; Batf3+/− + sildenafil, n = 6; Batf3−/− + vehicle, n = 5; Batf3−/− + sildenafil, n = 6). n, Tumour growth curves of MC38Pde5−/− bearing mice treated with vehicle plus isotype, sildenafil plus isotype, vehicle plus anti-CD8, or sildenafil plus anti-CD8 antibody (n = 3, 3, 6, 4 mice). o, Quantifications of mig-CD103+ or CD11b+ cDCs in tdLNs from each group (n = 5, 3, 4 mice). p, Tumour growth curve of MC38Pde5−/− bearing mice treated with vehicle, sildenafil, sildenafil plus 50 μM blebbistatin, or 50 μM Y27632 (n = 3, 3, 6, 6 mice). q, IF staining of DCs in tumoural lymphatic vessels (n = 22, 31, 30 vessels). Scale bar, 50 μm. r, Quantifications of H-2Kb/SIINFEKL, CD80 and CD86 levels on mig-cDCs in tdLNs from each group (n = 5, 5, 3, 4 mice). Control, resident cDCs. Data are merged (a-c, j-l, q) or representative (d-i, m-p, r) of at least two to three independent experiments and present as mean ± s.e.m. Statistical analysis was performed using two-tailed Mann–Whitney U-tests (a, b), two-tailed Student’s unpaired t-test (d, f, g, i, o) or Kruskal–Wallis test with multiple comparisons (c, q), two-way ANOVA with multiple comparisons corrected using Šidák’s multiple comparison test (d, e, g, h and j–n) or one-way ANOVA with Tukey’s multiple-comparison test (r).
Extended Data Fig. 6 Sildenafil specifically acts on DCs.
a, Quantifications of TCF1+TIM3− CD8+ T cells in the TME in each group (n = 6 vs 8 mice). b, Quantifications of CD44+PD-1+TIM3−CD8+ T cells, IFNγ+CD44+PD-1+CD8+ T cells, and CD44+PD-1+IFNγ+CD8+ T cells in the TME in each group (n = 6 vs 8 mice). c, Tumour growth and survival curves of B16F10Pde5−/− bearing mice treated with 7 doses of vehicle or sildenafil every other day via subcutaneous (s.c.) injection (n = 10 vs 12 mice). d, Kaplan–Meier survival curve of previously cured mice rechallenged with a double dose of MC38Pde5−/− cells (WT, n = 4; cured, n = 5). Age- and sex-matched mice served as controls. e, Quantifications of CD44+CD8+ and Ki67+CD8+ T cells in tdLNs from each group (n = 6). f, Representative flow cytometric histograms of IFNγ or GZMB in CD44+PD-1+CD8+ and quantifications of CD44+PD-1+CD8+ T cells and GZMB+CD44+PD-1+CD8+ T cells in the TME from each treatment group (n = 6 per group). GZMB, Granzyme B. g,h, TCGA pan-cancer analysis using DC gene signature combined with PDE5 expression (n = 1066 patients). Kaplan–Meier survival curve of patients stratified by median PDE5 expression into high or low (n = 537 vs 529 patients) DC gene signature groups (g). Correlation between PDE5 and CD8 T cell infiltration levels (h). i, Quantifications of mig-CD103+ and CD11b+ cDCs in Zbtb46crePde5fl/fl mice bearing MC38Pde5−/− tumour and treated with vehicle or sildenafil (vehicle, n = 5; sildenafil, n = 4). j, Quantifications of tdLNs CD44+CD8+ or TME IFNγ+CD44+PD-1+CD8+ T cells in Zbtb46crePde5fl/fl mice bearing tumour and treated with vehicle or sildenafil (n = 5 vs 4 mice). k, Schematic of the design strategy for LSL-Pde5 mice. l, Survival curves corresponding to results in Fig. 5o. m, Quantifications of tdLN mig-CD103+ and CD11b+ cDCs in Zbtb46crePde5LSL mice bearing MC38Pde5−/− tumour and treated with vehicle or sildenafil (n = 6 per group). n, Quantifications of tdLN CD44+CD8+ or IFNγ+CD44+PD-1+CD8+ T cells in Zbtb46crePde5LSL mice bearing MC38Pde5−/− tumour and treated with vehicle or sildenafil (n = 6 per group). o, Tumour growth curves of Zbtb46cre or Zbtb46crePde5LSL mice inoculated with MC38Pde5−/− tumour. Data were re-analysed from Fig. 5o (Zbtb46cre, n = 8; Zbtb46crePde5LSL, n = 7). p,q, Quantifications of tdLN migratory cDCs (p), or migratory CD103+ and CD11b+ cDCs (q) in Zbtb46cre and Zbtb46crePde5LSL mice (n = 4 per group). r,s, Quantifications of CD44+CD8+ T cells in tdLNs (r) or IFNγ+CD44+PD-1+CD8+ T cells in the TME (s) in Zbtb46cre and Zbtb46crePde5LSL mice (n = 4 per group). Data are representative (a, b, d, e-j, m, n, p-s) or merged (c, l, o) of at least two to three independent experiments and present as mean ± s.e.m. Statistical analysis was performed using two-tailed Student’s unpaired t-test (a, b, i, j, m, n, p-s), two-way ANOVA with multiple comparisons corrected using Šidák’s multiple comparison test (c, o), or one-way ANOVA with Tukey’s multiple-comparison test (e, f).
Extended Data Fig. 7 Pde5-cGMP-mediated DC migration in inflammation and homeostasis.
a, Representative flow cytometric plots and quantifications of FITC+ migratory and resident cDCs in tdLNs from Pde5fl/fl and Zbtb46crePde5fl/fl mice (Pde5fl/fl, n = 6; Zbtb46crePde5fl/fl, n = 7). Mice were painted with FITC-LPS mixture and tdLNs were analysed 48 h post painting to assess FITC uptake. b, Quantifications of cDC percentages, CCR7 expression, or surface CD80/CD86 expression from Pde5+/+ and Pde5−/− BMDCs differentiated with FLT3L for 9 days (n = 3 biological replicates per group). c,d, Quantifications of migratory cDCs (c), and migratory XCR1+ and SIRPα+ cDCs (d) in mediastinal LNs from Pde5fl/fl and Zbtb46crePde5fl/fl mice at homeostasis (n = 6 per group). e, Quantification of total cDCs in lung tissue from Pde5fl/fl and Zbtb46crePde5fl/fl mice (n = 6 per group). Cells were gated on live CD45+CD3−B220−Gr1−F4/80−. Data are representative of at least two to three independent experiments and present as mean ± s.e.m. Statistical analysis was performed using two-tailed Student’s unpaired t-test.
Supplementary information
Supplementary Figs. 1 and 2
Supplementary Fig. 1: the gating strategies for flow cytometry analysis. a, The gating strategy for mig-cDCs, CD103+ cDCs, CD11b+ cDCs, XCR1+ cDCs, SIRPα+ cDCs and ZsG+ cDCs in tdLNs. b, The gating strategy for analysis of immune cells in the TME. Supplementary Fig. 2: uncropped immunoblot images with size marker indications. The loading control of β-actin or GAPDH was run on the same gels.
Supplementary Table 1
Results from the pre-round and major-round in vivo CRISPR screens. This table includes gene-level and sgRNA-level results from both the pre-round and major-round in vivo CRISPR library screens, as well as the composition of the minipool used in the major round. LFC, log2 fold change; RRA, robust rank aggregation [RRA] lo value, reflecting positive selection.
Supplementary Table 2
Identification of cGMP-binding proteins by MS. This table lists proteins enriched in the cGMP-pull-down assay, including protein identities and their corresponding MS intensities. NC, negative control group treated with vehicle.
Supplementary Table 3
Patient information for SLN staining and DC motility analysis. This table presents the clinical and pathological characteristics of the patient cohort evaluated for SLN staining and DC motility.
Supplementary Table 4
Flow cytometry antibodies used in this study and the associated product information.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, H., Wei, Z., Zheng, B. et al. Rescuing dendritic cell interstitial motility sustains antitumour immunity. Nature 645, 244–253 (2025). https://doi.org/10.1038/s41586-025-09202-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09202-9
This article is cited by
-
Single-cell omics in tumor lymph node metastasis: mechanisms and therapeutic implications
Molecular Cancer (2026)
-
PDE5 inhibitor restores dendritic cell migration
Nature Reviews Drug Discovery (2025)