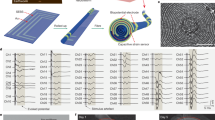
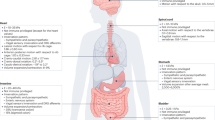
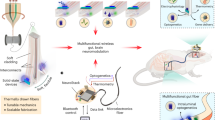
Abstract
There is an increasing demand for multimodal sensing and stimulation bioelectronic fibres for both research and clinical applications1,2. However, existing fibres suffer from high rigidity, low component layout precision, limited functionality and low density of active components. These limitations arise from the challenge of integrating many components into one-dimensional fibre devices, especially owing to the incompatibility of conventional microfabrication methods (for example, photolithography) with curved, thin and long fibre structures2. As a result, limited applications have been demonstrated so far. Here we use ‘spiral transformation’ to convert two-dimensional thin films containing microfabricated devices into one-dimensional soft fibres. This approach allows for the fabrication of high-density multimodal soft bioelectronic fibres, termed Spiral-NeuroString (S-NeuroString), while enabling precise control on the longitudinal, angular and radial positioning and distribution of the functional components. Taking advantage of the biocompatibility of our soft fibres with the dynamic and soft gastrointestinal system, we proceed to show the feasibility of our S-NeuroString for post-operative multimodal continuous motility mapping and tissue stimulation in awake pigs. We further demonstrate multi-channel single-unit electrical recording in mouse brain for up to 4 months, and a fabrication capability to produce 1,280 channels within a 230-μm-diameter soft fibre. Our soft bioelectronic fibres offer a powerful platform for minimally invasive implantable electronics, where diverse sensing and stimulation functionalities can be effectively integrated.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the main figures are available at https://purl.stanford.edu/cy798jt0703. Additional supplementary data and instructions for data analysis and interpretation can be obtained from the corresponding authors upon request. Source data are provided with this paper.
Code availability
All codes used in this study are either routine analytical codes or have been previously developed and reported elsewhere.
References
Canales, A., Park, S., Kilias, A. & Anikeeva, P. Multifunctional fibers as tools for neuroscience and neuroengineering. Acc. Chem. Res. 51, 829–838 (2018).
Zhang, Y. et al. Multifunctional fibers to shape future biomedical devices. Adv. Funct. Mater. 29, 1902834 (2019).
Lozano, A. M. et al. Deep brain stimulation: current challenges and future directions. Nat. Rev. Neurol. 15, 148–160 (2019).
Kahrilas, P. J. & Sifrim, D. High-resolution manometry and impedance-pH/manometry: valuable tools in clinical and investigational esophagology. Gastroenterology 135, 756–769 (2008).
Zeng, K., Shi, X., Tang, C., Liu, T. & Peng, H. Design, fabrication and assembly considerations for electronic systems made of fibre devices. Nat. Rev. Mater. 8, 552–561 (2023).
Richard, I., Schyrr, B., Aiassa, S., Carrara, S. & Sorin, F. All-in-fiber electrochemical sensing. ACS Appl. Mater. Interfaces 13, 43356–43363 (2021).
Kim, I. H. et al. Human-muscle-inspired single fibre actuator with reversible percolation. Nat. Nanotechnol. 17, 1198–1205 (2022).
Hwang, S. et al. Integration of multiple electronic components on a microfibre towards an emerging electronic textile platform. Nat. Commun. 13, 3173 (2022).
Ding, T. et al. Scalable thermoelectric fibers for multifunctional textile-electronics. Nat. Commun. 11, 6006 (2020).
Rein, M. et al. Diode fibres for fabric-based optical communications. Nature 560, 214–218 (2018).
Kanik, M. et al. Strain-programmable fiber-based artificial muscle. Science 365, 145–150 (2019).
Sahasrabudhe, A. et al. Multifunctional microelectronic fibers enable wireless modulation of gut and brain neural circuits. Nat. Biotechnol. 42, 892–904 (2023).
Xu, Z. & Gao, C. Graphene chiral liquid crystals and macroscopic assembled fibres. Nat. Commun. 2, 571 (2011).
Zhang, S. et al. Biomimetic spinning of soft functional fibres via spontaneous phase separation. Nat. Electron. 6, 338–348 (2023).
Pi, Q. et al. Digitally tunable microfluidic bioprinting of multilayered cannular tissues. Adv. Mater. 30, 1706913 (2018).
Nan, K. et al. Low-cost gastrointestinal manometry via silicone–liquid-metal pressure transducers resembling a quipu. Nat. Biomed. Eng. 6, 1092–1104 (2022).
Lee, J. et al. Stretchable and suturable fibre sensors for wireless monitoring of connective tissue strain. Nat. Electron. 4, 291–301 (2021).
Kalidasan, V. et al. Wirelessly operated bioelectronic sutures for the monitoring of deep surgical wounds. Nat. Biomed. Eng. 5, 1217–1227 (2021).
Qu, Y. et al. Superelastic multimaterial electronic and photonic fibers and devices via thermal drawing. Adv. Mater. 30, 1707251 (2018).
Driscoll, N. et al. Multifunctional neural probes enable bidirectional electrical, optical, and chemical recording and stimulation in vivo. Adv. Mater. https://doi.org/10.1002/adma.202408154 (2024).
Lee, Y. et al. Selectively micro-patternable fibers via in-fiber photolithography. ACS Cent. Sci. 6, 2319–2325 (2020).
Steinmetz, N. A. et al. Neuropixels 2.0: a miniaturized high-density probe for stable, long-term brain recordings. Science 372, eabf4588 (2021).
Tian, Y. et al. An ultraflexible electrode array for large‐scale chronic recording in the nonhuman primate brain. Adv. Sci. 10, 2302333 (2023).
Le Floch, P. et al. 3D spatiotemporally scalable in vivo neural probes based on fluorinated elastomers. Nat. Nanotechnol. 19, 319–329 (2024).
Rivkin, B. et al. Electronically integrated microcatheters based on self-assembling polymer films. Sci. Adv. 7, eabl5408 (2021).
Wang, S. et al. A self-assembled implantable microtubular pacemaker for wireless cardiac electrotherapy. Sci. Adv. 9, eadj0540 (2023).
Huang, W. et al. Monolithic mtesla-level magnetic induction by self-rolled-up membrane technology. Sci. Adv. 6, eaay4508 (2020).
Gabler, F., Karnaushenko, D. D., Karnaushenko, D. & Schmidt, O. G. Magnetic origami creates high performance micro devices. Nat. Commun. 10, 3013 (2019).
Lipomi, D. J., Chiechi, R. C., Reus, W. F. & Whitesides, G. M. Laterally ordered bulk heterojunction of conjugated polymers: nanoskiving a jelly roll. Adv. Funct. Mater. 18, 3469–3477 (2008).
Ruijie, X. et al. Rolling 2D bioelectronic film into 1D: a suturable long-term implantable soft microfiber. Preprint at bioRxiv https://doi.org/10.1002/adma.202408154 (2023).
Liu, Y. et al. A high-density 1,024-channel probe for brain-wide recordings in non-human primates. Nat. Neurosci. 27, 1620–1631 (2024).
Khatib, M. et al. Spiral NeuroString: high-density soft bioelectronic fibers for multimodal sensing and stimulation. Preprint at bioRxiv https://doi.org/10.1101/2023.10.02.560482 (2023).
Jiang, Y. et al. A universal interface for plug-and-play assembly of stretchable devices. Nature 614, 456–462 (2023).
Li, J. et al. A tissue-like neurotransmitter sensor for the brain and gut. Nature 606, 94–101 (2022).
Nan, K. et al. Mucosa-interfacing electronics. Nat. Rev. Mater. 7, 908–925 (2022).
Steiger, C. et al. Ingestible electronics for diagnostics and therapy. Nat. Rev. Mater. 4, 83–98 (2019).
Abramson, A. et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 363, 611–615 (2019).
Pannala, R. et al. Devices for esophageal function testing. VideoGIE 7, 1–20 (2022).
Scholz, S., Sood, V. & Sharbaugh, E. in The SAGES Manual of Physiologic Evaluation of Foregut Diseases (eds Patel, A. D. et al.) 591–624 (Springer, 2023).
Jiang, Y. et al. Topological supramolecular network enabled high-conductivity, stretchable organic bioelectronics. Science 375, 1411–1417 (2022).
Salimi-Jazi, F. et al. Perioperative gastrointestinal myoelectric activity measurement using wireless external patches. J. Surg. Res. 302, 186–199 (2024).
Dubrovsky, G. et al. Intestinal electrical stimulation to increase the rate of peristalsis. J. Surg. Res. 236, 153–158 (2019).
Southwell, B. R. Electro-neuromodulation for colonic disorders—review of meta-analyses, systematic reviews, and RCTs. Neuromodulation 23, 1061–1081 (2020).
Liu, J. et al. Intrinsically stretchable electrode array enabled in vivo electrophysiological mapping of atrial fibrillation at cellular resolution. Proc. Natl Acad. Sci. USA 117, 14769–14778 (2020).
Minev, I. R. et al. Electronic dura mater for long-term multimodal neural interfaces. Science 347, 159–163 (2015).
Zhou, T. et al. 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces. Nat. Mater. 22, 895–902 (2023).
Tchoe, Y. et al. Human brain mapping with multithousand-channel PtNRGrids resolves spatiotemporal dynamics. Sci. Transl. Med. 14, eabj1441 (2022).
Viswam, V., Obien, M. E. J., Franke, F., Frey, U. & Hierlemann, A. Optimal electrode size for multi-scale extracellular-potential recording from neuronal assemblies. Front. Neurosci. 13, 385 (2019).
Boehler, C., Carli, S., Fadiga, L., Stieglitz, T. & Asplund, M. Tutorial: guidelines for standardized performance tests for electrodes intended for neural interfaces and bioelectronics. Nat. Protoc. 15, 3557–3578 (2020).
Zhao, E. T. et al. A CMOS-based highly scalable flexible neural electrode interface. Sci. Adv. 9, eadf9524 (2023).
Won, C. et al. Mechanically tissue‐like and highly conductive au nanoparticles embedded elastomeric fiber electrodes of brain–machine interfaces for chronic in vivo brain neural recording. Adv. Funct. Mater. 32, 2205145 (2022).
Le Floch, P. et al. 3D spatiotemporally scalable in vivo neural probes based on fluorinated elastomers. Nat. Nanotechnol. 19, 319–329 (2023).
Wang, W. et al. Neuromorphic sensorimotor loop embodied by monolithically integrated, low-voltage, soft e-skin. Science 380, 735–742 (2023).
Du, Z. J. et al. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 53, 46–58 (2017).
Joo, H. R. & Frank, L. M. The hippocampal sharp wave-ripple in memory retrieval for immediate use and consolidation. Nat. Rev. Neurosci. 19, 744–757 (2018).
Kim, H. D., Huh, J. H., Kim, E. Y. & Park, C. C. Comparison of properties of thermoplastic polyurethane elastomers with two different soft segments. J. Appl. Polym. Sci. 69, 1349–1355 (1998).
Lin, J. et al. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 5, 5714 (2014).
Hashemi, P., Dankoski, E. C., Petrovic, J., Keithley, R. B. & Wightman, R. Voltammetric detection of 5-hydroxytryptamine release in the rat brain. Anal. Chem. 81, 9462–9471 (2009).
Lee, H. et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 11, 566–572 (2016).
Khatib, M., Zohar, O., Saliba, W. & Haick, H. A multifunctional electronic skin empowered with damage mapping and autonomic acceleration of self‐healing in designated locations. Adv. Mater. 32, 2000246 (2020).
Guinovart, T., Crespo, G. A., Rius, F. X. & Andrade, F. J. A reference electrode based on polyvinyl butyral (PVB) polymer for decentralized chemical measurements. Anal. Chim. Acta 821, 72–80 (2014).
Oh, S. Y. et al. Skin-attachable, stretchable electrochemical sweat sensor for glucose and pH detection. ACS Appl. Mater. Interfaces 10, 13729–13740 (2018).
Swaminathan, M. et al. Video imaging and spatiotemporal maps to analyze gastrointestinal motility in mice. J. Vis. Exp. 108, e53828 (2016).
Patel, B. Electroanalytical approaches to study signaling mechanisms in the gastrointestinal tract. Neurogastroenterol. Motility 23, 595–605 (2011).
Marcelli, G. & Patel, B. A. Understanding changes in uptake and release of serotonin from gastrointestinal tissue using a novel electroanalytical approach. Analyst 135, 2340–2347 (2010).
Pachitariu, M., Steinmetz, N. A., Kadir, S. N., Carandini, M. & Harris, K. D. Fast and accurate spike sorting of high-channel count probes with KiloSort. Adv. Neural Inf. Process. Syst. 29, 2199 (2016).
Buccino, A. P. et al. SpikeInterface, a unified framework for spike sorting. eLife 9, e61834 (2020).
Hill, D. N., Mehta, S. B. & Kleinfeld, D. Quality metrics to accompany spike sorting of extracellular signals. J. Neurosci. 31, 8699–8705 (2011).
Gonzalez, A. & Giocomo, L. M. Parahippocampal neurons encode task-relevant information for goal-directed navigation. eLife 12, RP85646 (2024).
Jones, E. A., Gillespie, A. K., Yoon, S. Y., Frank, L. M. & Huang, Y. Early hippocampal sharp-wave ripple deficits predict later learning and memory impairments in an Alzheimer’s disease mouse model. Cell Rep. 29, 2123–2133.e2124 (2019).
Canales, A. et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 33, 277–284 (2015).
Wang, L. et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 4, 159–171 (2020).
Kessing, B. F., Weijenborg, P. W., Smout, A. J., Hillenius, S. & Bredenoord, A. J. Water-perfused esophageal high-resolution manometry: normal values and validation. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G491–G495 (2014).
Wang, K., Duan, L.-p., Ge, Y., Xia, Z.-w. & Xu, Z.-j. A comparative study of 22-channel water-perfusion system and solid-state system with 36-sensors in esophageal manometry. BMC Gastroenterol. 12, 157 (2012).
Liem, O. et al. Solid‐state vs water‐perfused catheters to measure colonic high‐amplitude propagating contractions. Neurogastroenterol. Motility 24, 345–e167 (2012).
Rasijeff, A. M., Withers, M., Burke, J. M., Jackson, W. & Scott, S. M. High‐resolution anorectal manometry: a comparison of solid‐state and water‐perfused catheters. Neurogastroenterol. Motility 29, e13124 (2017).
Koppen, I. et al. Motility of the left colon in children and adolescents with functional constpation; a retrospective comparison between solid‐state and water‐perfused colonic manometry. Neurogastroenterol. Motility 30, e13401 (2018).
de Leon, A., Thörn, S.-E. & Wattwil, M. High-resolution solid-state manometry of the upper and lower esophageal sphincters during anesthesia induction: a comparison between obese and non-obese patients. Anesth. Analg. 111, 149–153 (2010).
Corsetti, M. et al. Pan-colonic pressurizations associated with relaxation of the anal sphincter in health and disease: a new colonic motor pattern identified using high-resolution manometry. Am. J. Gastroenterol. 112, 479–489 (2017).
Lee, T. H. & Bharucha, A. E. How to perform and interpret a high-resolution anorectal manometry test. J. Neurogastroenterol. Motility 22, 46 (2016).
Arkwright, J. et al. In-vivo demonstration of a high resolution optical fiber manometry catheter for diagnosis of gastrointestinal motility disorders. Opt. Express 17, 4500–4508 (2009).
Arkwright, J. W. et al. Design of a high-sensor count fibre optic manometry catheter for in-vivo colonic diagnostics. Opt. Express 17, 22423–22431 (2009).
Dinning, P. et al. Low‐resolution colonic manometry leads to a gross misinterpretation of the frequency and polarity of propagating sequences: initial results from fiber‐optic high‐resolution manometry studies. Neurogastroenterol. Motility 25, e640–e649 (2013).
Dinning, P. et al. Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high‐resolution fiber‐optic manometry. Neurogastroenterol. Motility 26, 1443–1457 (2014).
Racz, R. R. et al. jULIEs: nanostructured polytrodes for low traumatic extracellular recordings and stimulation in the mammalian brain. J. Neural Eng. 19, 016041 (2022).
Lee, S. H. et al. Scalable thousand channel penetrating microneedle arrays on flex for multimodal and large area coverage brainmachine interfaces. Adv. Funct. Mater. 32, 2112045 (2022).
Raducanu, B. C. et al. Time multiplexed active neural probe with 1356 parallel recording sites. Sensors 17, 2388 (2017).
Woeppel, K. et al. Explant analysis of Utah electrode arrays implanted in human cortex for brain–computer-interfaces. Front. Bioeng. Biotechnol. 9, 1137 (2021).
Obaid, A. et al. Massively parallel microwire arrays integrated with CMOS chips for neural recording. Sci. Adv. 6, eaay2789 (2020).
Sahasrabuddhe, K. et al. The Argo: a high channel count recording system for neural recording in vivo. J. Neural Eng. 18, 015002 (2021).
Patel, P. R. et al. Chronic in vivo stability assessment of carbon fiber microelectrode arrays. J. Neural Eng. 13, 066002 (2016).
Patel, P. R. et al. High density carbon fiber arrays for chronic electrophysiology, fast scan cyclic voltammetry, and correlative anatomy. J. Neural Eng. 17, 056029 (2020).
Wei, X. et al. Nanofabricated ultraflexible electrode arrays for high‐density intracortical recording. Adv. Sci. 5, 1700625 (2018).
Zhao, S. et al. Tracking neural activity from the same cells during the entire adult life of mice. Nat. Neurosci. 26, 696–710 (2023).
Yang, X. et al. Bioinspired neuron-like electronics. Nat. Mater. 18, 510–517 (2019).
Zhao, Z. et al. Ultraflexible electrode arrays for months-long high-density electrophysiological mapping of thousands of neurons in rodents. Nat. Biomed. Eng. 7, 520–532 (2023).
Middya, S. et al. Multishank thin‐film neural probes and implantation system for high‐resolution neural recording applications. Adv. Electron. Mater. 9, 2200883 (2022).
Park, S. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat. Commun. 12, 3435 (2021).
Lee, K. et al. Flexible, scalable, high channel count stereo-electrode for recording in the human brain. Nat. Commun. 15, 218 (2024).
Guan, S. et al. Elastocapillary self-assembled neurotassels for stable neural activity recordings. Sci. Adv. 5, eaav2842 (2019).
Chung, J. E. et al. High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays. Neuron 101, 21–31.e25 (2019).
Musk, E. An integrated brain-machine interface platform with thousands of channels. J. Med. Internet Res. 21, e16194 (2019).
Yuk, H. et al. 3D printing of conducting polymers. Nat. Commun. 11, 1604 (2020).
Acknowledgements
This work was partly supported by the Wu Tsai Neurosciences (Big Ideas in Neuroscience), Maternal and Child Health Research Institute at Stanford University, CZ Biohub San Francisco Investigator programme, the Arc Institute Innovation Investigator programme and the Stanford Wearable Electronics Initiative (eWEAR) seed funding. Part of this work was performed at the Stanford Nanofabrication (SNF) and Stanford Nano Shared Facilities (SNSF) RRID:SCR_023230, supported by the National Science Foundation under award ECCS-2026822. Part of this work was performed at Stanford Cell Sciences Imaging Facility (CSIF), which is funded by NIH ORIP funding: 1 S10 OD032300-01. We thank M. Sosa and E. A. Jones for discussions on hippocampus electrophysiology; and W. Reineking for help with veterinary histopathology analysis of the animal samples. M.K. acknowledges postdoctoral fellowship support from the Fulbright Foundation and Israeli Council for Higher Education (VATAT). E.T.Z. acknowledges support from the Bio-X Stanford Interdisciplinary Fellowship and Croucher Fellowship for Postdoctoral Research. M.J.W. acknowledges support by Schmidt Science Fellows, in partnership with Schmidt Sciences and Rhodes Trust. K.P. acknowledges the Swiss national science foundation for fellowship number P500PN_222266. C.Z. acknowledges the funding from an F32 fellowship from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (F32EB034156). A.A. acknowledges funding from an NIH F32 fellowship (grant 1F32EB029787). Z.B. is a Chan Zuckerberg Biohub San Francisco investigator and an Arc Institute innovation investigator. We thank K. Li and L. Gao, Xiaoji Wu Family Foundation for their generous support of the Bao Group’s research at Stanford University. X.C., X.Q. and Z.B. acknowledge support from the Tianqiao and Chrissy Chen Institute (TCCI) and the Tianqiao and Chrissy Chen Ideation and Prototyping Lab at Stanford University.
Author information
Authors and Affiliations
Contributions
M.K. and Z.B. conceived of the project and designed the experiments. M.K. designed and fabricated the devices, and performed the mechanical, electrical and electrochemical measurements. D.C. helped in the characterization of the chemical and pressure sensors. W.Y. performed the scanning electron microscopy. C.X. performed the Raman characterization and the surface roughness analysis of the laser-induced graphene, and in the design of the photographic illustrations. J.P. helped in the preparation of connections and wiring parts used for the measurements and in the design of the schematic illustrations. Y.L., D.Z., K.K.K., Y.N., Y.J., K.P., C.W. and W.W. helped with the development of the materials and the fabrication processes. S.E.R. captured the digital microscopy images of the fibres. T.Z. performed finite element analysis modelling of fibres under force. M.J.W. did the crosstalk evaluation experiments. J.L. advised the preparation and testing of the serotonin electrochemical sensors. M.K., R.H. and E.S.B. carried out all the ex vivo experiments. E.S.B. analysed the ex vivo data. A.A. and M.K. designed, performed and analysed the colonic biocompatibility tests and in vivo colonic recording in anaesthetised mice. M.K., C.-H.C., A.-L.T., P.E., F.S.-J. and T.A.R. performed the anaesthetised pig experiments for the gut-related applications. M.K., E.T.Z. and S.W. designed the neural recording experiments with help from L.Y. and N.M.H. M.K., E.T.Z. and S.W. performed all the neural recording experiments. E.T.Z. analysed the neural recording data. S.W. and M.K. performed the histology experiments. S.W. analysed the brain histology data. A.Z. helped with the histology study. A.A., E.T.Z., S.W. and C.Z. prepared the different mice protocols used in this study. A.-L.T. and J.C.Y.D. prepared the pig protocol. X.C. contributed to the neural recording study. J.A.K. contributed to the ex vivo gastrointestinal studies. M.K., E.T.Z., S.W., J.C.Y.D. and Z.B. prepared the paper. J.B.-H.T. and X.Q. help revise this work. All authors commented on the paper and gave approval to the final version. Z.B. and J.C.Y.D. directed the project.
Corresponding authors
Ethics declarations
Competing interests
Stanford University has filed a patent application related to this technology that lists M.K. and Z.B. as inventors. The patent application number is 63/528846. A.A. is a co-inventor on a patent application describing ingestible devices for electrical stimulation. A full list of A.A.’s competing interests can be found at https://www.abramsonlab.com/aa-conflicts-of-interest.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Ex-vivo mouse colonic motility sensing.
a, A photograph showing the system used for ex-vivo monitoring of colonic motility. b, Motility signals recorded by one of the S-NeuroString sensors (S7) (300 µm in diameter, 5 MPa Young’s modulus) inside the colon. c, Sensor layout inside the S-NeuroString used for motility detection in the colon. d, A photograph showing the colon from Supplementary Video 2 and the locations for colon diameter measurements (X1-X8). The circles indicate the locations of pressure sensors (S1-S8). The distance between each two sensors is 0.5 cm. e, Motility signals recorded by sensors in the colon. The propagation of pressure signals from sensor 7 to sensor 4 was observed in the sensors’ output (marked with an arrow). f, The measured colon diameter from the camera video recording (Supplementary Video 2) also shows the propagation of diameter change from location X7 to X4. g, Comparison between the sensor outputs and video analysis outputs, showing high similarity in the signals, which confirms good the motility sensing provided by our fibers. All traces were normalized and shifted for clarity (see methods). h, Two examples illustrating the sensor output and camera output (diameter change with time) at diameter locations: X5/X7 and sensor locations S5/S7. The cross-correlation analysis yielded coefficients of 0.7 for X7/S7 and 0.5 for X5/S5, indicating a good level of correlation for the entire recording. Specific regions exhibit even higher similarity. For instance, the marked boxed regions in h show cross correlation coefficients (ccc) of 0.9 and 0.8 for X7/S7 and X5/S5, respectively.
Extended Data Fig. 2 Ex-vivo mouse colonic motility sensing and stimulation.
a, Schematic structure of the sensing-stimulation regions of S-NeuroString which included 8 piezoresistive sensors (labeled as S1-S8, 0.5 cm distance between sensors) and 4 stimulation electrodes (labeled as SE1-SE4, 1 cm distance between electrodes). b, A schematic illustration of the stimulation study, beginning with a 5-min baseline period, followed by five biphasic stimulation trials (2 V vs AgCl reference electrode, applied simultaneously to all stimulation channels). Each stimulation trial consisted of 50 msec stimulation pulses (1.5 mA) and lasted for 5 s and followed by recovery time (no stimulation) for 55 s. c, Output of piezoresistive sensors before and during electrical stimulation of the tissue (1.5 mA, 100 Hz). All traces are normalized and shifted for clarity (see methods). S1 and S5 were broken. d, Time course of the motility index obtained by different piezoresistive sensor (S2, S4, S6, S8) before and during tissue stimulation (n = 5 colons/mice). Error bars indicate SD. e, Summary of motility index values before and during stimulation, which is calculated from the output of the different sensors (n = 5 mice). Boxes represent the interquartile range, spanning from the 25th to the 75th percentile of the data. Squares represent the mean. Whiskers represent the outlier (coefficient of 1.5). P values: 0.053, 0.015, 0.003, 0.012, 0.049, and 0.007 for S2, S3, S4, S6, S7, and S8, respectively. ns p > 0.05; * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; paired, two-tailed Student’s t-test. f, Overlay of sensor output and measured diameter as a function of time. Dashed lines mark the beginning of stimulation. S4 and S6 are located at X4 and X6, respectively. A clear change in diameter is observed with each stimulation. g, Frequency of slow wave and CMMC events before and during stimulation. Each data point is the average of 8 different locations on the colon (n = 5 mice). Bars represent the mean. Error bars indicate SE. Although no statistical significance was observed between the no-stimulation and stimulation groups, a clear trend toward increased frequency with stimulation was evident. Additional repetitions, longer recordings, or more frequent stimulation may be required to achieve statistical significance.
Extended Data Fig. 3 S-NeuroString for sensing and stimulation in the GI tracts of anesthetized pigs.
a, Comparison of our GI S-NeuroString with existing manometry probes in terms of diameter and sensor density (Extended Data Table 2). b, Schematic and photographic illustrations showing an S-NeuroString inserted into the small intestine of an anesthetized pig to monitor and modulate GI functions. Parts of the schematic were created using BioRender.com. c, Recording of natural motility in the small intestine using a S-NeuroString with 8 pressure sensors (S1-S8). All traces are normalized and shifted for clarity (see methods). d, Schematic illustration of the 2D design used to roll-up into a bifunctional S-NeuroString used for simultaneous motility sensing (S1-S5) and stimulation (SE1-SE5). e, A photograph showing the bifunctional S-NeuroString obtained from the 2D design shown in (d). f, Pressure sensor output in response to stimulation at 2 and 4 V. g, Summary of the response obtained under stimulation at different voltages (n = 7 stimulation experiments, 2 S-NeuroStrings, 2 pigs). Bars represent the mean, with error bars indicating SD. The results in (f) and (g) were obtained from sensors and stimulation electrodes that are located at the same point. h, Schematic illustration showing the use of distributed stimulation electrodes to induce programmable contraction events over the length of the intestinal segments. i, Demonstration of the programmable stimulation and induced contractions. Stimulation of the pig intestine at different locations generates progressive contractions, as indicated by the propagation of the pressure wave using the arrows. Distributed pressure sensors provide feedback about the location and progression of contractions. j, Schematic illustration showing the use of SSRI injection (0.5 ml of 10 µM) to induce serotonin release inside the intestine. k, Drug-induced luminal serotonin concentration change using an SSRI solution and PBS as control (n = 5 experiments, 3 S-NeuroStrings, 3 pigs). Short horizontal lines represent the mean. Each point is the average of 5 electrochemical sensors as shown in Supplementary Fig. 23. Paired, two-tailed Student’s t-test. l, Electrochemical sensing of SSRI-induced serotonin using cycling voltammetry. The red dotted line boxed region indicates the electrochemical oxidation of serotonin.
Extended Data Fig. 4 Histological analysis of the porcine small intestine after S-NeuroString implantation.
Representative hematoxylin-and-eosin-stained cross-sections of porcine small intestine, showing different section from each condition: control porcine jejunum and porcine jejunum implanted with S-NeuroString in the lumen for 7 days. No differences in cellularity and cellular composition were present in the lamina propria mucosae between the control and fiber-implanted segment from the different animal samples. There are no signs of lesions or inflammatory response, highlighting the importance of our soft technology for long term interfacing with the GI tissue. There was a focally accentuated goblet cell (enterocytes that secrete mucus in the lumen) hyperplasia and increased amounts of mucus within the intestinal crypts and the intestinal lumen of the fiber section as compared to the control sample (observed only in Pig 3 out of a total of 4 pigs). Depending on the sampling location and experimental surgical procedures, mural myodegeneration is most likely unrelated to the intraluminal fiber. Observed mesothelial hyperplasia can occur with intra-abdominal irritations, such as surgical manipulation – very common in abdominal surgeries. This observation was made on all samples, both controls and fiber-implanted segments. Desquamation of epithelial cells is present in all samples and interpreted as postmortem finding, a common degradation and separation of the epithelial cells from the mucosa of intestine that happens after death.
Extended Data Fig. 5 Representative examples of activation of intestinal activity by electrical stimulation.
The electrical stimulation lasted for ~10 sec and is indicated by the shaded area. The graphs show the normalized outputs of pressure sensors ~30 sec before, during, and ~30 sec after stimulation. Each graph represents a single stimulation trial from the three tested pigs. The appearance of peaks in the sensor output indicates an increase in intestinal motility.
Extended Data Fig. 6 Representative examples of de-activation of intestinal activity by electrical stimulation.
The electrical stimulation lasted for ~10 sec and is indicated by the shaded area. The graphs show the normalized outputs of pressure sensors ~30 sec before, during, and ~30 sec after stimulation. Each graph represents a single stimulation trial from the three tested pigs. The disappearance of peaks in the sensor output indicates a decrease in intestinal motility.
Extended Data Fig. 7 1280-channel high-density electrical-recording fiber.
a, A photograph of the 2D film with the 1280 channels before rolling. i, A zoom-on image showing the 10-µm interconnects. ii, A zoom-on image showing the exposed recording area. b, A microscope image of the exposed site showing 10 recording electrodes. c-d, Microscope images showing the 230-µm fiber obtained after rolling. The length of the active recording area was 19.2 mm and the density (per unit length) is 66.7 mm−1 (compared to 9.6 mm and 133.33 mm−1 in NeuroPixles 2.0)22. e, Fiber cross-section showing the different rolled layers and the Au/PEDOT interconnects. Red lines in the inset mark some of the Au/PEDOT traces. f-g, SEM images of the 320-channel fiber. h, Impedance analysis of 32 channels showing a good yield after the transformation process.
Extended Data Fig. 8 S-NeuroString for LFP recording in the mouse brain.
a, Waterfall plot of the raw LFP data recorded one week after a S-NeuroString was implanted in the hippocampus, band-passed Butterworth filtered between 0.1 – 300 Hz. Colors correspond to the depth of the electrodes where blue is more ventral and purple more dorsal. SWR electrophysiological signatures are apparent and consistent with literature, most notably with the co-occurrence of CA1 and CA3 ripple events as well as an inverse of polarity with depth. b, Spectrogram showing an increase of power centered around 160 Hz. c, Spatial representation of the SWR spectral power at 160 Hz across multiple electrodes revealing stronger engagement in CA1. d, Discrete Fourier transform of channel 7, with a clear peak at 160 Hz indicative of the SWR. e, Firing patterns of individual neurons around SWRs, averaged across 14 events. Multiple neurons show elevated firing rates around a SWR event.
Supplementary information
Supplementary Information
Supplementary Methods, Notes 1–4, Figs. 1–55, Table 1 and References.
Supplementary Video 1
The transformation process of a 3-cm-wide and 12-cm-long film into a 300-μm fibre.
Supplementary Video 2
Ex vivo detection of progressive contractions using S-NeuroString.
Supplementary Video 3
The video shows the bioelectronic fibre inserted in the small intestine of pig and used to record the observed natural motility patterns.
Supplementary Video 4
The video shows the programmable stimulation system, which allows the stimulation of specific tissue parts.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khatib, M., Zhao, E.T., Wei, S. et al. High-density soft bioelectronic fibres for multimodal sensing and stimulation. Nature 645, 656–664 (2025). https://doi.org/10.1038/s41586-025-09481-2
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09481-2