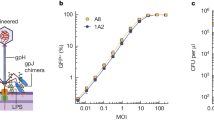
Abstract
Oral administration of biologic drugs is challenging because of the degradative activity of the upper gastrointestinal tract. Strategies that use engineered microbes to produce biologics in the lower gastrointestinal tract are limited by competition with resident commensal bacteria. Here we demonstrate the engineering of bacteriophage (phage) that infect resident commensals to express heterologous proteins released during cell lysis. Working with the virulent T4 phage, which targets resident, nonpathogenic Escherichia coli, we first identify T4-specific promoters with maximal protein expression and minimal impact on T4 phage titers. We engineer T4 phage to express a serine protease inhibitor of a pro-inflammatory enzyme with increased activity in ulcerative colitis and observe reduced enzyme activity in a mouse model of colitis. We also apply the approach to reduce weight gain and inflammation in mouse models of diet-induced obesity. This work highlights an application of virulent phages in the mammalian gut as engineerable vectors to release therapeutics from resident gut bacteria.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
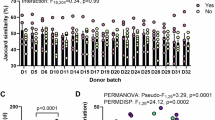
Source data for figures in the main text and Supplementary Information are provided with this paper. 16S amplicon reads are available on Mendeley Data85. Source data are provided with this paper.
Code availability
No code was generated in this study.
References
Hua, S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract—influence of physiological, pathophysiological and pharmaceutical factors. Front. Pharmacol. 11, 524 (2020).
Tripathi, J., Thapa, P., Maharjan, R. & Jeong, S. H. Currentstate and future perspectives on gastroretentive drug delivery systems. Pharmaceutics 11, 193 (2019).
New, R. Oral delivery of biologics via the intestine. Pharmaceutics 13, 18 (2020).
Dahlgren, D. & Lennernas, H. Intestinal permeability and drug absorption: predictive experimental, computational and in vivo approaches. Pharmaceutics 11, 411 (2019).
Brown, M. T. & Bussell, J. K. Medication adherence: WHO cares? Mayo Clin. Proc. 86, 304–314 (2011).
Merril, C. R., Scholl, D. & Adhya, S. L. The prospect for bacteriophage therapy in Western medicine. Nat. Rev. Drug Discov. 2, 489–497 (2003).
Barr, J. J. et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc. Natl Acad. Sci. USA 110, 10771–10776 (2013).
Van Belleghem, J. D., Dabrowska, K., Vaneechoutte, M., Barr, J. J. & Bollyky, P. L. Interactions between bacteriophage, bacteria, and the mammalian immune system. Viruses 11, 10 (2018).
Shkoporov, A. N. et al. The human gut virome is highly diverse, stable, and individual specific. Cell Host Microbe 26, 527–541 e525 (2019).
Minot, S. et al. Rapid evolution of the human gut virome. Proc. Natl Acad. Sci. USA 110, 12450–12455 (2013).
Shkoporov, A. N. et al. PhiCrAss001 represents the most abundant bacteriophage family in the human gut and infects Bacteroides intestinalis. Nat. Commun. 9, 4781 (2018).
Weiss, M. et al. In vivo replication of T4 and T7 bacteriophages in germ-free mice colonized with Escherichia coli. Virology 393, 16–23 (2009).
Hsu, B. B. et al. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe 25, 803–814 (2019).
Maura, D. et al. Intestinal colonization by enteroaggregative Escherichia coli supports long-term bacteriophage replication in mice. Environ. Microbiol. 14, 1844–1854 (2012).
Javaudin, F., Latour, C., Debarbieux, L. & Lamy-Besnier, Q. Intestinal bacteriophage therapy: looking for optimal efficacy. Clin. Microbiol. Rev. 34, e0013621 (2021).
Tennoune, N. et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide α-MSH, at the origin of eating disorders. Transl. Psychiatry 4, e458 (2014).
Legrand, R. et al. Commensal Hafnia alvei strain reduces food intake and fat mass in obese mice—a new potential probiotic for appetite and body weight management. Int. J. Obes. 44, 1041–1051 (2020).
Miller, E. S. et al. Bacteriophage T4 genome. Microbiol. Mol. Biol. Rev. 67, 86–156 (2003).
Luke, K. et al. Microarray analysis of gene expression during bacteriophage T4 infection. Virology 299, 182–191 (2002).
Grigonyte, A. Engineering Bacteriophages to Enhance their Potential Use in Therapy (University of Warwick, 2018).
Duong, M. M., Carmody, C. M. & Nugen, S. R. Phage-based biosensors: in vivo analysis of native T4 phage promoters to enhance reporter enzyme expression. Analyst 145, 6291–6297 (2020).
Tiemann, B. et al. ModA and ModB, two ADP-ribosyltransferases encoded by bacteriophage T4: catalytic properties and mutation analysis. J. Bacteriol. 186, 7262–7272 (2004).
Fokine, A. et al. Molecular architecture of the prolate head of bacteriophage T4. Proc. Natl Acad. Sci. USA 101, 6003–6008 (2004).
Yap, M. L. & Rossmann, M. G. Structure and function of bacteriophage T4. Future Microbiol. 9, 1319–1327 (2014).
Leatham, M. P. et al. Mouse intestine selects nonmotile flhDC mutants of Escherichia coli MG1655 with increased colonizing ability and better utilization of carbon sources. Infect. Immun. 73, 8039–8049 (2005).
Sweeney, N. J. et al. The Escherichia coli K-12 gntP gene allows E. coli F-18 to occupy a distinct nutritional niche in the streptomycin-treated mouse large intestine. Infect. Immun. 64, 3497–3503 (1996).
Curciarello, R. et al. Human neutrophil elastase proteolytic activity in ulcerative colitis favors the loss of function of therapeutic monoclonal antibodies. J. Inflamm. Res. 13, 233–243 (2020).
Barry, R. et al. Faecal neutrophil elastase-antiprotease balance reflects colitis severity. Mucosal Immunol. 13, 322–333 (2020).
Herrero-Cervera, A., Soehnlein, O. & Kenne, E. Neutrophils in chronic inflammatory diseases. Cell Mol. Immunol. 19, 177–191 (2022).
Morohoshi, Y. et al. Inhibition of neutrophil elastase prevents the development of murine dextran sulfate sodium-induced colitis. J. Gastroenterol. 41, 318–324 (2006).
Leborgne, N. G. F., Taddeo, A., Freigang, S. & Benarafa, C. Serpinb1a is dispensable for the development and cytokine response of invariant natural killer T cell subsets. Front. Immunol. 11, 562587 (2020).
Lin, R. et al. Resolving neutrophils due to TRAM deletion renders protection against experimental sepsis. Inflamm. Res. 72, 1733–1744 (2023).
Zhang, Y. et al. Immune-enhancing neutrophils reprogrammed by subclinical low-dose endotoxin in cancer treatment. EMBO Mol. Med. 16, 1886–1900 (2024).
Chen, G. Y., Tang, J., Zheng, P. & Liu, Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 323, 1722–1725 (2009).
Boyapati, R. K., Rossi, A. G., Satsangi, J. & Ho, G. T. Gut mucosal DAMPs in IBD: from mechanisms to therapeutic implications. Mucosal Immunol. 9, 567–582 (2016).
Geng, S. et al. Monocytes reprogrammed by 4-PBA potently contribute to the resolution of inflammation and atherosclerosis. Circ. Res. 135, 856–872 (2024).
Wang, X. et al. CD24-Siglec axis is an innate immune checkpoint against metaflammation and metabolic disorder. Cell Metab. 34, 1088–1103 (2022).
Ahmed, M. A. et al. CD24 is upregulated in inflammatory bowel disease and stimulates cell motility and colony formation. Inflamm. Bowel Dis. 16, 795–803 (2010).
Dechelotte, P. et al. The probiotic strain H. alvei HA4597((R)) improves weight loss in overweight subjects under moderate hypocaloric diet: a proof-of-concept, multicenter randomized, double-blind placebo-controlled study. Nutrients 13, 1902 (2021).
Dominique, M. et al. Effects of bacterial CLPB protein fragments on food intake and PYY secretion. Nutrients 13, 2223 (2021).
Panaro, B. L. et al. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metab. 20, 1018–1029 (2014).
Yeo, G. S. H. et al. The melanocortin pathway and energy homeostasis: from discovery to obesity therapy. Mol. Metab. 48, 101206 (2021).
Lourenco, M. et al. The spatial heterogeneity of the gut limits predation and fosters coexistence of bacteria and bacteriophages. Cell Host Microbe 28, 390–401 (2020).
Mangalea, M. R. & Duerkop, B. A. Fitness trade-offs resulting from bacteriophage resistance potentiate synergistic antibacterial strategies. Infect. Immun. 88, e00926 (2020).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Wellen, K. E. & Hotamisligil, G. S. Inflammation, stress, and diabetes. J. Clin. Invest. 115, 1111–1119 (2005).
Tang, C., Chen, S., Qian, H. & Huang, W. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology 135, 112–124 (2012).
Martins, L. M. S. et al. Interleukin-23 promotes intestinal T helper type17 immunity and ameliorates obesity-associated metabolic syndrome in a murine high-fat diet model. Immunology 154, 624–636 (2018).
Sumarac-Dumanovic, M. et al. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int. J. Obes. 33, 151–156 (2009).
Iwakura, Y. & Ishigame, H. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 116, 1218–1222 (2006).
Kanda, H. et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505 (2006).
Panee, J. Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine 60, 1–12 (2012).
Sartipy, P. & Loskutoff, D. J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl Acad. Sci. USA 100, 7265–7270 (2003).
Cani, P. D., Osto, M., Geurts, L. & Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 3, 279–288 (2012).
Tetart, F., Repoila, F., Monod, C. & Krisch, H. M. Bacteriophage T4 host range is expanded by duplications of a small domain of the tail fiber adhesin. J. Mol. Biol. 258, 726–731 (1996).
Zhang, H. et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl Acad. Sci. USA 106, 2365–2370 (2009).
Yun, Y. et al. Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol. 17, 151 (2017).
Edwards, R. A. et al. Global phylogeography and ancient evolution of the widespread human gut virus crAssphage. Nat. Microbiol. 4, 1727–1736 (2019).
Charbonneau, M. R., Isabella, V. M., Li, N. & Kurtz, C. B. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat. Commun. 11, 1738 (2020).
Isabella, V. M. et al. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat. Biotechnol. 36, 857–864 (2018).
Kurtz, C. B. et al. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci. Transl. Med. 11, eaau7975 (2019).
Maura, D., Galtier, M., Le Bouguenec, C. & Debarbieux, L. Virulent bacteriophages can target O104:H4 enteroaggregative Escherichia coli in the mouse intestine. Antimicrob. Agents Chemother. 56, 6235–6242 (2012).
Geiduschek, E. P. & Kassavetis, G. A. Transcription of the T4 late genes. Virol J. 7, 288 (2010).
Stirling, F. et al. Rational design of evolutionarily stable microbial kill switches. Mol. Cell 68, 686–697 (2017).
Rottinghaus, A. G., Ferreiro, A., Fishbein, S. R. S., Dantas, G. & Moon, T. S. Genetically stable CRISPR-based kill switches for engineered microbes. Nat. Commun. 13, 672 (2022).
Smati, M. et al. Quantitative analysis of commensal Escherichia coli populations reveals host-specific enterotypes at the intra-species level. Microbiologyopen 4, 604–615 (2015).
Tenaillon, O., Skurnik, D., Picard, B. & Denamur, E. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 8, 207–217 (2010).
Strathdee, S. A., Hatfull, G. F., Mutalik, V. K. & Schooley, R. T. Phage therapy: from biological mechanisms to future directions. Cell 186, 17–31 (2023).
Gogokhia, L. et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe 25, 285–299 (2019).
Nguyen, S. et al. Bacteriophage transcytosis provides a mechanism to cross epithelial cell layers. mBio 8, e01874 (2017).
Clooney, A. G. et al. Whole-virome analysis sheds light on viral dark matter in inflammatory bowel disease. Cell Host Microbe 26, 764–778 (2019).
Engler, C., Kandzia, R. & Marillonnet, S. A one pot, one step, precision cloning method with high throughput capability. PLoS ONE 3, e3647 (2008).
Yan, Q. & Fong, S. S. Study of in vitro transcriptional binding effects and noise using constitutive promoters combined with UP element sequences in Escherichia coli. J. Biol. Eng. 11, 33 (2017).
Qi, L. S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013).
Silver, S. Acriflavine resistance: a bacteriophage mutation affecting the uptake of dye by the infected bacterial cells. Proc. Natl Acad. Sci. USA 53, 24–30 (1965).
Wang, F. J. & Ripley, L. S. The spectrum of acridine resistant mutants of bacteriophage T4 reveals cryptic effects of the tsL141 DNA polymerase allele on spontaneous mutagenesis. Genetics 148, 1655–1665 (1998).
Park, S., Reyer, M. A., McLean, E. L., Liu, W. & Fei, J. An improved method for bacterial immunofluorescence staining to eliminate antibody exclusion from the fixed nucleoid. Biochemistry 58, 4457–4465 (2019).
De Paepe, M. & Taddei, F. Viruses’ life history: towards a mechanistic basis of a trade-off between survival and reproduction among phages. PLoS Biol. 4, e193 (2006).
Weichhart, T. mTor: Methods and Protocols (Humana Press, 2012).
Charles River Research Model. C57BL/6 Mouse Clinical Pathology Data, C57BL/6 mouse hematology: North American colonies. (2012).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Hasegawa, Y., Mark Welch, J. L., Rossetti, B. J. & Borisy, G. G. Preservation of three-dimensional spatial structure in the gut microbiome. PLoS ONE 12, e0188257 (2017).
Baker, Z. 16S Illumina sequencing reads. Mendeley Data data.mendeley.com/datasets/pt9m6b9s4z/1 (2024).
Acknowledgements
We thank T. Thorn, S. DiLorenzo and J. Cartwright at Virginia Tech for technical assistance, as well as E. Ng at the FLSI Light Microscopy Facility at Virginia Tech for fluorescence microscopy support. Research reported in this publication was supported in part by the National Institutes of Health (R35 GM147484 (to B.B.H.) and R01 AI172133 (to L.L.)) and Virginia Tech Dean’s Discovery Fund (fund 446728 to B.B.H. and. L.L.).
Author information
Authors and Affiliations
Contributions
B.B.H. and L.L. conceptualized the project. Z.B. and B.B.H. developed the methodology. Z.B., Y.Z., H.Z., H.F., P.B.S.S. and T.S. conducted the investigation. Z.B. and B.B.H. wrote the original draft of the paper. L.L. and B.B.H. reviewed and edited the paper, provided supervision and secured funding.
Corresponding authors
Ethics declarations
Competing interests
Z.B., L.L. and B.B.H. are inventors on a pending patent application related to the phage-based heterologous gene expression system described in this paper. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks Jeremy Barr, Laurent Debarbieux and Tae Seok Moon for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7 and Supplementary Tables 1 and 2.
Supplementary Data 1
Supporting data for Supplementary Figs. 1, 3–5 and 7.
Supplementary Data 2
Oligonucleotide sequences used in this study.
Source data
Source Data Figs. 1–5
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baker, Z.R., Zhang, Y., Zhang, H. et al. Sustained in situ protein production and release in the mammalian gut by an engineered bacteriophage. Nat Biotechnol (2025). https://doi.org/10.1038/s41587-025-02570-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41587-025-02570-7