Abstract
The E3 ligase TRIM7 has emerged as a critical player in viral infection and pathogenesis. However, the mechanism governing the TRIM7–substrate association remains to be defined. Here we report the crystal structures of TRIM7 in complex with 2C peptides of human enterovirus. Structure-guided studies reveal the C-terminal glutamine residue of 2C as the primary determinant for TRIM7 binding. Leveraged by this finding, we identify norovirus and SARS-CoV-2 proteins, and physiological proteins, as new TRIM7 substrates. Crystal structures of TRIM7 in complex with multiple peptides derived from SARS-CoV-2 proteins display the same glutamine-end recognition mode. Furthermore, TRIM7 could trigger the ubiquitination and degradation of these substrates, possibly representing a new Gln/C-degron pathway. Together, these findings unveil a common recognition mode by TRIM7, providing the foundation for further mechanistic characterization of antiviral and cellular functions of TRIM7.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The structure factors and atomic coordinates for TRIM7 in complex with substrate peptides have been deposited to the Protein Data Bank with accession numbers of 7W0Q (TRIM7–2C, 1.1 Å), 7W0S (TRIM7–2C, 1.4 Å), 7W0T (TRIM7–2C, 1.57 Å), 7X6Y (TRIM7–NSP5, 1.39 Å), 7X70 (TRIM7–NSP8, 1.25 Å) and 7X6Z (TRIM7–NSP12, 1.43 Å). Source data are provided with this paper.
References
Hatakeyama, S. TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem. Sci. 42, 297–311 (2017).
Reymond, A. et al. The tripartite motif family identifies cell compartments. EMBO J. 20, 2140–2151 (2001).
Shen, Z. et al. The roles of TRIMs in antiviral innate immune signaling. Front. Cell. Infect. Microbiol. 11, 628275 (2021).
Giraldo, M. I., Hage, A., van Tol, S. & Rajsbaum, R. TRIM proteins in host defense and viral pathogenesis. Curr. Clin. Microbiol. Rep. 7, 101–114 (2020).
Koepke, L., Gack, M. U. & Sparrer, K. M. The antiviral activities of TRIM proteins. Curr. Opin. Microbiol. 59, 50–57 (2021).
Hage, A. & Rajsbaum, R. To TRIM or not to TRIM: the balance of host–virus interactions mediated by the ubiquitin system. J. Gen. Virol. 100, 1641 (2019).
van Gent, M., Sparrer, K. M. & Gack, M. U. TRIM proteins and their roles in antiviral host defenses. Annu. Rev. Virol. 5, 385–405 (2018).
D’Cruz, A. A., Babon, J. J., Norton, R. S., Nicola, N. A. & Nicholson, S. E. Structure and function of the SPRY/B30. 2 domain proteins involved in innate immunity. Protein Sci. 22, 1–10 (2013).
Montori-Grau, M. et al. GNIP1 E3 ubiquitin ligase is a novel player in regulating glycogen metabolism in skeletal muscle. Metabolism 83, 177–187 (2018).
Chakraborty, A., Diefenbacher, M. E., Mylona, A., Kassel, O. & Behrens, A. The E3 ubiquitin ligase Trim7 mediates c-Jun/AP-1 activation by Ras signalling. Nat. Commun. 6, 1–12 (2015).
Hu, X. et al. Tripartite motif-containing protein 7 regulates hepatocellular carcinoma cell proliferation via the DUSP6/p38 pathway. Biochem. Biophys. Res. Commun. 511, 889–895 (2019).
Fan, W. et al. TRIM7 inhibits enterovirus replication and promotes emergence of a viral variant with increased pathogenicity. Cell 184, 3410–3425 (2021).
Giraldo, M. I. et al. Envelope protein ubiquitination drives entry and pathogenesis of Zika virus. Nature 585, 414–419 (2020).
Orchard, R. C. et al. Identification of antinorovirus genes in human cells using genome-wide CRISPR activation screening. J. Virol. 93, e01324–01318 (2019).
Yang, B. et al. RNF90 negatively regulates cellular antiviral responses by targeting MITA for degradation. PLoS Pathog. 16, e1008387 (2020).
Yang, B. et al. Negative regulation of RNF90 on RNA virus-triggered antiviral immune responses targeting MAVS. Front. Immunol. 12, 730483 (2021).
Sosa, C. J. M., Issoglio, F. M. & Carrizo, M. E. Crystal structure and mutational analysis of the human TRIM7 B30. 2 domain provide insights into the molecular basis of its binding to glycogenin-1. J. Biol. Chem. 296, 100772 (2021).
Ashkenazy, H. et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44, W344–W350 (2016).
Zheng, Z. et al. Human norovirus NTPase antagonizes interferon-β production by interacting with IkB kinase ε. Front. Microbiol. 12, 687933 (2021).
Xia, H. et al. Evasion of type I interferon by SARS-CoV-2. Cell Rep. 33, 108234 (2020).
Liu, Y. et al. SARS-CoV-2 Nsp5 demonstrates two distinct mechanisms targeting RIG-I and MAVS to evade the innate immune response. MBio 12, e02335–02321 (2021).
Zheng, Y. et al. SARS-CoV-2 NSP5 and N protein counteract the RIG-I signaling pathway by suppressing the formation of stress granules. Signal Transduct. Target. Ther. 7, 1–12 (2022).
Shemesh, M. et al. SARS-CoV-2 suppresses IFNβ production mediated by NSP1, 5, 6, 15, ORF6 and ORF7b but does not suppress the effects of added interferon. PLoS Pathog. 17, e1009800 (2021).
Yang, Z. et al. Suppression of MDA5-mediated antiviral immune responses by NSP8 of SARS-CoV-2. Preprint at https://doi.org/10.1101/2020.08.12.247767 (2020).
Banerjee, A. K. et al. SARS-CoV-2 disrupts splicing, translation, and protein trafficking to suppress host defenses. Cell 183, 1325–1339 (2020).
Zhou, C. et al. N6-Methyladenosine modification of the TRIM7 positively regulates tumorigenesis and chemoresistance in osteosarcoma through ubiquitination of BRMS1. EBioMedicine 59, 102955 (2020).
Zhai, L., Dietrich, A., Skurat, A. V. & Roach, P. J. Structure–function analysis of GNIP, the glycogenin-interacting protein. Arch. Biochem. Biophys. 421, 236–242 (2004).
Wang, Z. et al. Tripartite motif containing 11 interacts with DUSP6 to promote the growth of human osteosarcoma cells through regulating ERK1/2 pathway. BioMed Res. Int. 25, 9612125 (2019).
Luck, K. et al. A reference map of the human binary protein interactome. Nature 580, 402–408 (2020).
Campillay-Véliz, C. P. et al. Human norovirus proteins: implications in the replicative cycle, pathogenesis, and the host immune response. Front. Immunol. 11, 961 (2020).
Mariano, G., Farthing, R. J., Lale-Farjat, S. L. & Bergeron, J. R. Structural characterization of SARS-CoV-2: where we are, and where we need to be. Front. Mol. Biosci. 7, 344 (2020).
Chen, J. et al. Structural basis for helicase-polymerase coupling in the SARS-CoV-2 replication-transcription complex. Cell 182, 1560–1573. e1513 (2020).
Kneller, D. W. et al. Structural plasticity of SARS-CoV-2 3CL Mpro active site cavity revealed by room temperature X-ray crystallography. Nat. Commun. 11, 1–6 (2020).
Tan, J. et al. 3C protease of enterovirus 68: structure-based design of Michael acceptor inhibitors and their broad-spectrum antiviral effects against picornaviruses. J. Virol. 87, 4339–4351 (2013).
Sosnovtsev, S. V. et al. Cleavage map and proteolytic processing of the murine norovirus nonstructural polyprotein in infected cells. J. Virol. 80, 7816–7831 (2006).
Ziebuhr, J., Heusipp, G. & Siddell, S. G. Biosynthesis, purification, and characterization of the human coronavirus 229E 3C-like proteinase. J. Virol. 71, 3992–3997 (1997).
Parker, E. et al. Multi‐tissue epigenetic and gene expression analysis combined with epigenome modulation identifies RWDD2B as a target of osteoarthritis susceptibility. Arthritis Rheumatol. 73, 100–109 (2021).
Varshavsky, A. N-degron and C-degron pathways of protein degradation. Proc. Natl Acad. Sci. 116, 358–366 (2019).
Koren, I. et al. The eukaryotic proteome is shaped by E3 ubiquitin ligases targeting C-terminal degrons. Cell 173, 1622–1635 (2018).
Lin, H.-C. et al. C-terminal end-directed protein elimination by CRL2 ubiquitin ligases. Mol. cell 70, 602–613. e603 (2018).
Chen, X. et al. Molecular basis for arginine C-terminal degron recognition by Cul2FEM1 E3 ligase. Nat. Chem. Biol. 17, 254–262 (2021).
Yan, X. et al. Molecular basis for ubiquitin ligase CRL2FEM1C-mediated recognition of C-degron. Nat. Chem. Biol. 17, 263–271 (2021).
Rusnac, D.-V. et al. Recognition of the diglycine C-end degron by CRL2KLHDC2 ubiquitin ligase. Mol. cell 72, 813–822. e814 (2018).
Li, Y. et al. Structural basis of the phosphorylation-independent recognition of cyclin D1 by the SCFFBXO31 ubiquitin ligase. Proc. Natl Acad. Sci. 115, 319–324 (2018).
Zhao, S. et al. Structural insights into SMCR8 C-degron recognition by FEM1B. Biochem. Biophys. Res. Commun. 557, 236–239 (2021).
Tsu, B. V. et al. Diverse viral proteases activate the NLRP1 inflammasome. eLife 10, e60609 (2021).
Saita, S. et al. PARL mediates Smac proteolytic maturation in mitochondria to promote apoptosis. Nat. Cell Biol. 19, 318–328 (2017).
Robinson, K. S. et al. Enteroviral 3C protease activates the human NLRP1 inflammasome in airway epithelia. Science 370, eaay2002 (2020).
Qiao, J. et al. SARS-CoV-2 Mpro inhibitors with antiviral activity in a transgenic mouse model. Science 371, 1374–1378 (2021).
Zhang, W.-Z. et al. The protein complex crystallography beamline (BL19U1) at the Shanghai Synchrotron Radiation Facility. Nucl. Sci. Tech. 30, 1–11 (2019).
Potterton, L. et al. CCP4i2: the new graphical user interface to the CCP4 program suite. Acta Crystallogr. Sect. D Struct. Biol. 74, 68–84 (2018).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr., Sect. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. Sect. D Biol. Crystallogr. 58, 1948–1954 (2002).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix. refine. Acta Crystallogr. Sect. D Biol. Crystallogr. 68, 352–367 (2012).
Maier, J. A. et al. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 (2015).
Ryckaert, J.-P., Ciccotti, G. & Berendsen, H. J. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 (1977).
Roe, D. R. & Cheatham, T. E. III PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 9, 3084–3095 (2013).
Acknowledgements
We thank J. Lei for supporting plasmids encoding SARS-CoV-2 proteins. We thank the staffs from BL17B/BL18U1/BL19U1/BL19U2/BL01B beamlines of the National Facility for Protein Science in Shanghai at Shanghai Synchrotron Radiation Facility, for assistance during data collection. This work is supported by the National Natural Science Foundation of China Grants (32071218 to H.Z.) and the National Key Research and Development Program of China (2018YFC1313002 to L.L.).
Author information
Authors and Affiliations
Contributions
X.L., Y.W., Y.M. and Y.Z. performed the sample preparation, biochemistry and crystallization experiments. Xu.L. collected the diffraction data and solved the structure with the help from X.L. and Y. M. X.C. performed the molecular dynamics analysis. J.X., Y. Li., Y. Lu, X.L., Z.L., X.Z., D.J. and P.W. carried out the cellular experiments. C.X., L.L., G.Y. and H.Z. analyzed the data. H.Z., G.Y. and L.L. designed the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
X.C. is the founder of YDS Pharmatech. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Chao Xu, Matthew Ravalin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 TRIM7 interacts with enteroviruses 2C.
a, Sequence alignment of 2C proteins from different enteroviruses. The last eight residues at the C-terminus are displayed. Invariant and conserved residues are shaded green and yellow, respectively. b, ITC measurements of peptides derived from the C-terminal fragments of 2C proteins from different enteroviruses, including enterovirus 71, poliovirus and enterovirus D68, to TRIM7PRY-SPRY.
Extended Data Fig. 2 Structural features of the TRIM7PRY-SPRY-2C peptide complex.
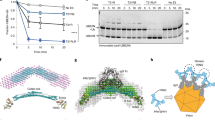
a, The overall structures of TRIM7PRY-SPRY-2C peptide complex solved in different space groups (top). Bottom, 2Fo-Fc electron density map of the 2C fragments contoured at 1.0 σ. b, Structural comparison of the three TRIM7PRY-SPRY-2C peptide structures and a previously published substrate-free structure (PDB ID: 6UMA). c, ITC measurement of TRIM7PRY-SPRY-2CΔN association in a high salt buffer containing 500 mM NaCl. d, Conservation scores across different species were mapped to the TRIM7 structure. Multiple sequence alignment of human TRIM7 and orthologs in other mammals, birds and amphibians were performed with Clustal Omega. The multiple sequence alignment and the resolved structure of TRIM7PRY-SPRY were used as inputs for conservation analysis using the ConSurf web server (https://consurf.tau.ac.il/).
Extended Data Fig. 3 Quantification of the binding affinity between TRIM7 and CVB3 2C mutations.
Thermodynamic analysis of the interaction between TRIM7PRY-SPRY and 2C mutants (T323G, T323S, T323I).
Extended Data Fig. 4 MD simulations and ITC measurements of the interaction between TRIM7PRY-SPRY and 2C variants.
a, (left) Structural comparison between TRIM2-2C and the calculated TRIM7-2C (Q329E). (right) Effects of simulations on the Q329E mutation. Molecular dynamics simulation suggested that Q329E mutation eliminates the interactions between the residue 329 and TRIM7, especially the interactions with Arg385, Asn438, and Ser499 in TRIM7 (Supplementary Table 2). Consequently, the C-terminal carboxylate group of 2C at residue 329 is more exposed to solvent, leading to a further reduced TRIM7-2C interaction and increased 2C C-terminal solvation. b, The root-mean-square deviation (RMSD) change of the 2C peptide structures in MD simulation (left). The RMSD values of WT (black) and Q329E (red) 2C peptide structures with reference to the first frame of the trajectory in MD simulation were calculated and plotted. While the WT 2C peptide gradually converged to the original position, the Q329E mutant drifted away as indicated by the increasing RMSD over MD simulation. Right, the Q329E mutation led to increased hydrogen bonding with water. The number of hydrogen-bonded water molecules to the side chain amide of Q329 in WT 2C (black) and the side chain carboxyl of E329 in the mutant (red) were plotted over MD simulation.
Extended Data Fig. 5 NSP proteins from SARS-CoV-2 counteract the IFNB1 promoter activity.
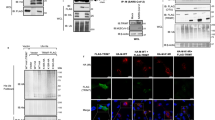
a, Co-immunoprecipitation of TRIM7 with NSP5 and NSP8. FLAG-tagged NSP5, NSP8 or empty vector was co-transfected with HA-TRIM7 in HEK293T cells. Cells were lysed and immunoprecipitated with anti-HA antibody and detected by anti-FLAG antibody. HA-TRIM7-Mut, TRIM7 catalytic dead mutant (C29A/C31A); NSP5/NSP8-Q/A, the alanine replacement of the C-terminal glutamine in NSP5/8. b, Crystal structure of TRIM7PRY-SPRY in complex with NSP12 peptide (aa 928-932). c, 2Fo-Fc electron density map of the TRIM7-bound NSP peptides contoured at 1.0 σ. d, Luciferase activity of IFNB1 promoter reporter in HEK293T cells transfected with indicated NSP proteins and RIG-I CARD expression plasmids. Relative luciferase activity was quantified 24 h post-transfection. Data are presented as mean ± SEM; n = 6, biologically independent samples were examined over three independent experiments. Statistical significance was determined by comparing with ‘Vector’ group using one-way ANOVA with Tukey’s correction. e, f, Analysis of TBK1 phosphorylation in the presence of NSP5 (e) or NSP8 (f).
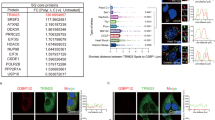
Extended Data Fig. 6 ITC measurements of TRIM7PRY-SPRY with multiple cellular proteins functionally linked to TRIM7.
a, TRIM7PRY-SPRY binds with GN1 and RACO-1 C-terminus peptide fragments. b, Thermodynamic analysis of the interaction between TRIM7PRY-SPRY and BRMS1 peptide (left panel), DUSP6 peptide (middle panel) and STING protein (right panel).
Supplementary information
Supplementary Information
Supplementary Figs 1–11, Supplementary Tables 1–3 and uncropped gel images.
Source data
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, X., Xiao, J., Li, X. et al. A C-terminal glutamine recognition mechanism revealed by E3 ligase TRIM7 structures. Nat Chem Biol 18, 1214–1223 (2022). https://doi.org/10.1038/s41589-022-01128-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41589-022-01128-x
This article is cited by
-
TRIM7 suppresses transmissible gastroenteritis virus replication by targeting the degradation of N protein and activating RIG-I-mediated type I IFN antiviral response
Veterinary Research (2025)
-
Degrons and degradation signals beyond short linear motifs
Nature Chemical Biology (2025)
-
Degrons: defining the rules of protein degradation
Nature Reviews Molecular Cell Biology (2025)
-
TRIM7 ubiquitinates SARS-CoV-2 membrane protein to limit apoptosis and viral replication
Nature Communications (2024)
-
TRIM-away via Gln/C-degrons
Nature Chemical Biology (2022)