Abstract
The post-translational modification of intracellular proteins through O-linked β-N-acetylglucosamine (O-GlcNAc) is a conserved regulatory mechanism in multicellular organisms. Catalyzed by O-GlcNAc transferase (OGT), this dynamic modification has an essential role in signal transduction, gene expression, organelle function and systemic physiology. Here, we present Opto-OGT, an optogenetic probe that allows for precise spatiotemporal control of OGT activity through light stimulation. By fusing a photosensitive cryptochrome protein to OGT, Opto-OGT can be robustly and reversibly activated with high temporal resolution by blue light and exhibits minimal background activity without illumination. Transient activation of Opto-OGT results in mTORC activation and AMPK suppression, which recapitulate nutrient-sensing signaling. Furthermore, Opto-OGT can be customized to localize to specific subcellular sites. By targeting OGT to the plasma membrane, we demonstrate the downregulation of site-specific AKT phosphorylation and signaling outputs in response to insulin stimulation. Thus, Opto-OGT is a powerful tool for defining the role of O-GlcNAcylation in cell signaling and physiology.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The MS proteomics data have been deposited to the JPOST repository with the dataset identifier JPST002053. Further information on the MS data and AlphaFold simulation is available in the Supplementary Information. Data supporting the findings of this study are available in the Article, Supplementary Videos and Supplementary Information. Source data are provided with this paper.
References
Haltiwanger, R. S., Holt, G. D. & Hart, G. W. Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N-acetylglucosamine:peptide β-N-acetylglucosaminyltransferase. J. Biol. Chem. 265, 2563–2568 (1990).
Hart, G. W., Housley, M. P. & Slawson, C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022 (2007).
Yang, X. & Qian, K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 18, 452–465 (2017).
Lazarus, M. B., Nam, Y., Jiang, J., Sliz, P. & Walker, S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature 469, 564–569 (2011).
Elsen, N. L. et al. Insights into activity and inhibition from the crystal structure of human O-GlcNAcase. Nat. Chem. Biol. 13, 613–615 (2017).
Bond, M. R. & Hanover, J. A. A little sugar goes a long way: the cell biology of O-GlcNAc. J. Cell Biol. 208, 869–880 (2015).
Nagel, A. K. & Ball, L. E. O-GlcNAc transferase and O-GlcNAcase: achieving target substrate specificity. Amino Acids 46, 2305–2316 (2014).
Stephen, H. M., Adams, T. M. & Wells, L. Regulating the regulators: mechanisms of substrate selection of the O-GlcNAc cycling enzymes OGT and OGA. Glycobiology 2021, 1–10 (2021).
Bullen, J. W. et al. Cross-talk between two essential nutrient-sensitive enzymes: O-GlcNAc transferase (OGT) and AMP-activated protein kinase (AMPK). J. Biol. Chem. 289, 10592–10606 (2014).
Harwood, K. R. & Hanover, J. A. Nutrient-driven O-GlcNAc cycling—think globally but act locally. J. Cell Sci. 127, 1857–1867 (2014).
Bond, M. R. & Hanover, J. A. O- GlcNAc cycling: a link between metabolism and chronic disease. Annu. Rev. Nutr. 33, 205–229 (2013).
de Queiroz, R. M., Carvalho, E. & Dias, W. B. O-GlcNAcylation: the sweet side of the cancer. Front. Oncol. 4, 132 (2014).
Qian, K. et al. Transcriptional regulation of O-GlcNAc homeostasis is disrupted in pancreatic cancer. J Biol. Chem. 293, 13989–14000 (2018).
Ma, Z. & Vosseller, K. Cancer metabolism and elevated O-GlcNAc in oncogenic signaling. J. Biol. Chem. 289, 34457–34465 (2014).
Sodi, V. L. et al. Signal transduction mTOR/MYC axis regulates O-GlcNAc transferase expression and O-GlcNAcylation in breast cancer. Mol. Cancer Res. 13, 923–933 (2015).
Martínez-Viturro, C. M. et al. Diazaspirononane nonsaccharide inhibitors of O-GlcNAcase (OGA) for the treatment of neurodegenerative disorders. J. Med. Chem. 63, 14017–14044 (2020).
Selnick, H. G. et al. Discovery of MK-8719, a potent O-GlcNAcase inhibitor as a potential treatment for tauopathies. J. Med. Chem. 62, 10062–10097 (2019).
Ryan, J. M. et al. O1-12-05: Phase 1 study in healthy volunteers of the O-Glcnacase inhibitor ASN120290 as a novel therapy for progressive supranuclear palsy and related tauopathies. Alzheimer’s Dement. 14, P251 (2018).
Leney, A. C., El Atmioui, D., Wu, W., Ovaa, H. & Heck, A. J. R. Elucidating crosstalk mechanisms between phosphorylation and O-GlcNAcylation. Proc. Natl Acad. Sci. USA 114, E7255–E7261 (2017).
Ruan, H. B., Nie, Y. & Yang, X. Regulation of protein degradation by O-GlcNAcylation: crosstalk with ubiquitination. Mol. Cell. Proteom. 12, 3489–3497 (2013).
Ong, Q., Han, W. & Yang, X. O-GlcNAc as an integrator of signaling pathways. Front. Endocrinol. 9, 599 (2018).
Tischer, D. & Weiner, O. D. Illuminating cell signalling with optogenetic tools. Nat. Rev. Mol. Cell Biol. 15, 551–558 (2014).
Kwon, E. & Heo, W. D. Optogenetic tools for dissecting complex intracellular signaling pathways. Biochem. Biophys. Res. Commun. 527, 331–336 (2020).
Zhang, K. & Cui, B. Optogenetic control of intracellular signaling pathways. Trends Biotechnol. 33, 92–100 (2015).
Wu, Y. I. et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461, 104–108 (2009).
Kennedy, M. J. et al. Rapid blue-light-mediated induction of protein interactions in living cells. Nat. Methods 7, 973–975 (2010).
Toettcher, J. E., Voigt, C. A., Weiner, O. D. & Lim, W. A. The promise of optogenetics in cell biology: interrogating molecular circuits in space and time. Nat. Methods 8, 35–38 (2011).
Duan, L. et al. Optical activation of TrkA signaling. ACS Synth. Biol. 7, 1685–1693 (2018).
Huang, P. et al. Optical activation of TrkB signaling. J. Mol. Biol. 432, 3761–3770 (2020).
Zhang, K. et al. Light-mediated kinetic control reveals the temporal effect of the Raf/MEK/ERK pathway in PC12 cell neurite outgrowth. PLoS ONE 9, e92917 (2014).
Toettcher, J. E., Weiner, O. D. & Lim, W. A. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell 155, 1422–1434 (2013).
Ong, Q. et al. The timing of Raf/ERK and AKT activation in protecting PC12 cells against oxidative stress. PLoS ONE 11, e0153487 (2016).
Paonessa, F. et al. Regulation of neural gene transcription by optogenetic inhibition of the RE1-silencing transcription factor. Proc. Natl Acad. Sci. USA 113, E91–E100 (2016).
Polesskaya, O. et al. Optogenetic regulation of transcription. BMC Neurosci. 19, 12 (2018).
Lan, T. H., He, L., Huang, Y. & Zhou, Y. Optogenetics for transcriptional programming and genetic engineering. Trends Genet. 38, 1253–1270 (2022).
Nguyen, M. K. et al. Optogenetic oligomerization of Rab GTPases regulates intracellular membrane trafficking. Nat. Chem. Biol. 12, 431–436 (2016).
Inaba, H., Miao, Q. & Nakata, T. Optogenetic control of small GTPases reveals RhoA mediates intracellular calcium signaling. J. Biol. Chem. 296 100290 (2021).
Bryant, P., Pozzati, G. & Elofsson, A. Improved prediction of protein−protein interactions using AlphaFold2. Nat. Commun. 13, 1–11 (2022).
Kozakov, D. et al. The ClusPro web server for protein–protein docking. Nat. Protoc. 12, 255–278 (2017).
Duan, L. et al. Understanding CRY2 interactions for optical control of intracellular signaling. Nat. Commun. 8, 547 (2017).
Sohn, J. W. & Ho, W. K. Cellular and systemic mechanisms for glucose sensing and homeostasis. Pflügers Arch. 472, 1547–1561 (2020).
Cork, G. K., Thompson, J. & Slawson, C. Real talk: the inter-play between the mTOR, AMPK and hexosamine biosynthetic pathways in cell signaling. Front. Endocrinol. 9, 522 (2018).
González, A., Hall, M. N., Lin, S. C. & Hardie, D. G. AMPK and TOR: the yin and yang of cellular nutrient sensing and growth control. Cell Metab. 31, 472–492 (2020).
Duan, L. et al. Optogenetic control of molecular motors and organelle distributions in cells. Chem. Biol. 22, 671–682 (2015).
Yang, X. et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature 451, 964–969 (2008).
Ruan, H. B., Singh, J. P., Li, M. D., Wu, J. & Yang, X. Cracking the O-GlcNAc code in metabolism. Trends Endocrinol. Metab. 24, 301–309 (2013).
Decourcelle, A., Loison, I., Baldini, S., Leprince, D. & Dehennaut, V. Evidence of a compensatory regulation of colonic O-GlcNAc transferase and O-GlcNAcase expression in response to disruption of O-GlcNAc homeostasis. Biochem. Biophys. Res. Commun. 521, 125–130 (2020).
Tan, Z. W. et al. O-GlcNAc regulates gene expression by controlling detained intron splicing. Nucleic Acids Res. 48, 5656–5669 (2021).
Xu, Q. et al. AMPK regulates histone H2B O-GlcNAcylation. Nucleic Acids Res. 42, 5594–5604 (2014).
Luo, B. et al. Chronic hexosamine flux stimulates fatty acid oxidation by activating AMP-activated protein kinase in adipocytes. J. Biol. Chem. 282, 7172–7180 (2007).
Latorre-Muro, P. et al. A cold-stress-inducible PERK/OGT axis controls TOM70-assisted mitochondrial protein import and cristae formation. Cell Metab. 33, 598–614 (2021).
Kreppel, L. K. & Hart, G. W. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J. Biol. Chem. 274, 32015–32022 (1999).
Iyer, S. P. N. & Hart, G. W. Roles of the tetratricopeptide repeat domain in O-GlcNAc transferase targeting and protein substrate specificity. J. Biol. Chem. 278, 24608–24616 (2003).
Iyer, S. P. N., Akimoto, Y. & Hart, G. W. Identification and cloning of a novel family of coiled-coil domain proteins that interact with O-GlcNac transferase. J. Biol. Chem. 278, 5399–5409 (2003).
Meek, R. W. et al. Cryo-EM structure provides insights into the dimer arrangement of the O-linked β-N-acetylglucosamine transferase OGT. Nat. Commun. 12, 6508 (2021).
Zhou, W. & Deiters, A. Chemogenetic and optogenetic control of post-translational modifications through genetic code expansion. Curr. Opin. Chem. Biol. 63, 123–131 (2021).
He, J. et al. Spatiotemporal activation of protein O-GlcNAcylation in living cells. J. Am. Chem. Soc. 144, 4289–4293 (2022).
Ge, Y. et al. Target protein deglycosylation in living cells by a nanobody-fused split O-GlcNAcase. Nat. Chem. Biol. 17, 593–600 (2021).
Ramirez, D. H. et al. Engineering a proximity-directed O-GlcNAc transferase for selective protein O-GlcNAcylation in cells. ACS Chem. Biol. 15, 1059–1066 (2020).
Zhu, Y. & Hart, G. W. Dual-specificity RNA aptamers enable manipulation of target-specific O-GlcNAcylation and unveil functions of O-GlcNAc on β-catenin. Cell 186, 428–445 (2023).
Lehtinen, K., Nokia, M. S. & Takala, H. Red light optogenetics in neuroscience. Front. Cell. Neurosci. 15, 532 (2022).
Carpenter, A. E. et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100 (2006).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Desta, I. T., Porter, K. A., Xia, B., Kozakov, D. & Vajda, S. Performance and its limits in rigid body protein−protein docking. Structure 28, 1071–1081 (2020).
Chuah, Y. H. et al. CAMK2D serves as a molecular scaffold for RNF8−MAD2 complex to induce mitotic checkpoint in glioma. Cell Death Differ. 30, 1973–1987 (2023).
Acknowledgements
This work was supported by the National Institutes of Health (R01DK089098, R01DK137467 and R01DK102648 to X.Y.), American Diabetes Association (1-19-IBS-119 to X.Y.), intramural support from the Agency for Science, Technology and Research (A*STAR) Biomedical Research Council core fund (to W.H.), A*STAR Strategic Research Program (the Brain-Body Initiative, iGrants no. 21718 to W.H.), an A*STAR Use-Inspired Basic Research award (to W.H.), the National Research Foundation Competitive Research Program (NRF-CRP23-2019-0004 to W.H.) and the National Medical Research Council Young Individual Research Grant (OFYIRG19nov-0045 to Q.O.). We thank P. L. Chia and H. Mao for proofreading the manuscript.
Author information
Authors and Affiliations
Contributions
Q.O., W.H. and X.Y. conceived the project. Q.O., Y.L. and X.Y. designed the experiments. Q.O., L.T.R.L., S.E.C., C.J.Y.L., V.K., J.Y. and T.T. performed the optogenetic experiments. Q.O., S.E.C., C.J.Y.L., S.-Y.K. and S.L.L. performed cloning experiments. Q.O. and C.G. performed the AlphaFold experiments. L.C.W and S.G.L. performed the MS experiments. Q.O., C.G., Y.L., V.K., T.T., L.C.W and S.G.L. analyzed the data. L.T.R.L., S.E.C., R.D., A.B. and R.S. offered technical advice. Q.O., A.B., W.H. and X.Y. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Luc Bertrand, Robert Hughes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
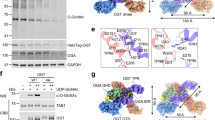
Extended Data Fig. 1 Comparison of nutrient sensing pathways when subjected to glucose concentration changes or Opto-OGT activation.
a, Representative Western blots of key nutrient sensing pathways when HEK293T cells are subjected to glucose concentration changes from 0 mM to 25 mM. Immunoblots shown are representative of two independent biological repeats. b, Representative Western blots of HEK293T cells transfected with OGT-mCherry-CRY2 undergoing 15 minutes of blue light. Key downstream signaling pathways are probed as labelled. Immunoblots shown are representative of two independent biological repeats. c, Representative Western blots of phosphor-ULK1 when HEK293T cells transfected with OGT-mCherry-CRY2 underwent 15 minutes of blue light. Immunoblots shown are representative of two independent biological repeats.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2, Figs. 1–11 and source data for supplementary figures.
Supplementary Video 1
Movie showing HEK293T cells transfected with OGT−mCherry−CRY2. The first 300 s are without blue light, while in the next 300 s, the cells are supplied with blue light at a 100-ms pulse every 5 s. No blue light is illuminated from 600−1,800 s and blue light is reapplied from 1,800−2,400 s at a 100-ms pulse every 5 s.
Supplementary Video 2
Movie showing COS-7 cells transfected with both OGT−mCherry−CRY2 (right) and CIBN−GFP−miro (left). Green and blue lights are continually supplied to the system at a 100-ms pulse every 15 s.
Supplementary Video 3
Movie showing HEK293T cells transfected with both OGT−mCherry−CRY2 (right) and CIBN−GFP−CAAX (left). Green and blue lights are continually supplied to the system at a 100-ms pulse every 5 s.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed western blots and MS statistical source data.
Source Data Fig. 2
Statistical source data
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ong, Q., Lim, L.T.R., Goh, C. et al. Spatiotemporal control of subcellular O-GlcNAc signaling using Opto-OGT. Nat Chem Biol 21, 300–308 (2025). https://doi.org/10.1038/s41589-024-01770-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41589-024-01770-7