Abstract
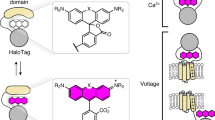
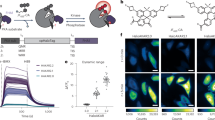
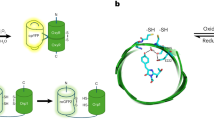
Monitoring H2O2 dynamics in conjunction with key biological interactants is critical for elucidating the physiological outcome of cellular redox regulation. Optogenetic hydrogen peroxide sensor with HaloTag with JF635 (oROS-HT635) allows fast and sensitive chemigenetic far-red H2O2 imaging while overcoming drawbacks of existing red fluorescent H2O2 indicators, including oxygen dependency, high pH sensitivity, photoartifacts and intracellular aggregation. The compatibility of oROS-HT635 with blue-green-shifted optical tools allows versatile optogenetic dissection of redox biology. In addition, targeted expression of oROS-HT635 and multiplexed H2O2 imaging enables spatially resolved imaging of H2O2 targeting the plasma membrane and neighboring cells. Here we present multiplexed use cases of oROS-HT635 with other green fluorescence reporters by capturing acute and real-time changes in H2O2 with intracellular redox potential and Ca2+ levels in response to auranofin, an inhibitor of antioxidative enzymes, via dual-color imaging. oROS-HT635 enables detailed insights into intricate intracellular and intercellular H2O2 dynamics, along with their interactants, through spatially resolved, far-red H2O2 imaging in real time.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The complete minimal raw dataset from the experiments, representative images, downstream analysis and visualizations generated for this article are available on figshare at https://doi.org/10.6084/m9.figshare.28306691 (ref. 96). Plasmids for oROS-HT and its loss-of-function (C199S) and subcellular targeting variants described in this paper are available through Addgene at pC1-lifeact-oROS-HT (216420), pC1-IMS-oROS-HT (216419), pC1-dmito-oROS-HT (216418), pC1-oROS-HT-CaaX (216417), pDisplay-oROS-HT (216416), AAV2_CAG_oROS-HT(C199S)_WPRE (216415), AAV2_CAG_oROS-HT_WPRE (216414), pC1_oROS-HT (216413) and pC1_oROS-HT_LF(C199S) (216412). We will also provide plasmids upon request. The study accessed the PDB database (1I6A, 1I69 and 6U2M) for structural analysis. Source data are provided with this paper.
Code availability
Source code for simulation, data extraction, analysis and visualization is available on figshare at https://doi.org/10.6084/m9.figshare.28306691 (ref. 96).
References
Forman, H. J. & Zhang, H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 20, 689–709 (2021).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383 (2020).
Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 11, 613–619 (2017).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462 (2014).
Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 194, 7–15 (2011).
D’Autréaux, B. & Toledano, M. B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824 (2007).
Ren, X. et al. Redox signaling mediated by thioredoxin and glutathione systems in the central nervous system. Antioxid. Redox Signal. 27, 989–1010 (2017).
Marinho, H. S., Real, C., Cyrne, L., Soares, H. & Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2, 535–562 (2014).
Johnson, F. & Giulivi, C. Superoxide dismutases and their impact upon human health. Mol. Aspects Med. 26, 340–352 (2005).
Martin, C., Binda, C., Fraaije, M. W. & Mattevi, A. The multipurpose family of flavoprotein oxidases. Enzymes 47, 63–86 (2020).
Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 25, 13–33 (2024).
Kishi, S., Nagasu, H., Kidokoro, K. & Kashihara, N. Oxidative stress and the role of redox signalling in chronic kidney disease. Nat. Rev. Nephrol. 20, 101–119 (2024).
Chun, H. et al. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H2O2– production. Nat. Neurosci. 23, 1555–1566 (2020).
Pak, V. V. et al. Ultrasensitive genetically encoded indicator for hydrogen peroxide identifies roles for the oxidant in cell migration and mitochondrial function. Cell Metab. 31, 642–653 (2020).
Ermakova, Y. G. et al. Red fluorescent genetically encoded indicator for intracellular hydrogen peroxide. Nat. Commun. 5, 5222 (2014).
Pang, Y. et al. SHRIMP: genetically encoded mScarlet-derived red fluorescent hydrogen peroxide sensor with high brightness and minimal photoactivation. Preprint at bioRxiv https://doi.org/10.1101/2023.08.09.552302 (2023).
Morgan, B. et al. Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nat. Chem. Biol. 12, 437–443 (2016).
Gutscher, M. et al. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J. Biol. Chem. 284, 31532–31540 (2009).
Eid, M., Barayeu, U., Sulková, K., Aranda-Vallejo, C. & Dick, T. P. Using the heme peroxidase APEX2 to probe intracellular H2O2 flux and diffusion. Nat. Commun. 15, 1239 (2024).
Murphy, M. P. et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 4, 651–662 (2022).
Patriarchi, T. et al. An expanded palette of dopamine sensors for multiplex imaging in vivo. Nat. Methods 17, 1147–1155 (2020).
Dana, H. et al. Sensitive red protein calcium indicators for imaging neural activity. eLife 5, e12727 (2016).
Wu, J. et al. Improved orange and red Ca2+ indicators and photophysical considerations for optogenetic applications. ACS Chem. Neurosci. 4, 963–972 (2013).
Ning, L. et al. A bright, nontoxic, and non-aggregating red fluorescent protein for long-term labeling of fine structures in neurons. Front. Cell Dev. Biol. 10, 893468 (2022).
Pedre, B. et al. Structural snapshots of OxyR reveal the peroxidatic mechanism of H2O2 sensing. Proc. Natl Acad. Sci. USA 115, E11623–E11632 (2018).
Åslund, F., Zheng, M., Beckwith, J. & Storz, G. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc. Natl Acad. Sci. USA 96, 6161–6165 (1999).
Tao, K. In vivo oxidation–reduction kinetics of OxyR, the transcriptional activator for an oxidative stress-inducible regulon in Escherichia coli. FEBS Lett. 457, 90–92 (1999).
Lee, C. et al. Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path. Nat. Struct. Mol. Biol. 11, 1179–1185 (2004).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Lee, J. D. et al. Structure-guided engineering of a fast genetically encoded sensor for real-time H2O2 monitoring. Preprint at bioRxiv https://doi.org/10.1101/2024.01.31.578117 (2024).
Choi, H. et al. Structural basis of the redox switch in the OxyR transcription factor. Cell 105, 103–113 (2001).
Jo, I. et al. Structural details of the OxyR peroxide-sensing mechanism. Proc. Natl Acad. Sci. USA 112, 6443–6448 (2015).
Fenno, L. E. et al. Comprehensive dual- and triple-feature intersectional single-vector delivery of diverse functional payloads to cells of behaving mammals. Neuron 107, 836–853 (2020).
Deo, C. et al. The HaloTag as a general scaffold for far-red tunable chemigenetic indicators. Nat. Chem. Biol. 17, 718–723 (2021).
Grimm, J. B. et al. A general method to fine-tune fluorophores for live-cell and in vivo imaging. Nat. Methods 14, 987–994 (2017).
Jan, Y.-H. et al. Vitamin K3 (menadione) redox cycling inhibits cytochrome P450-mediated metabolism and inhibits parathion intoxication. Toxicol. Appl. Pharmacol. 288, 114–120 (2015).
Loor, G. et al. Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Radic. Biol. Med. 49, 1925–1936 (2010).
Criddle, D. N. et al. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J. Biol. Chem. 281, 40485–40492 (2006).
Tongul, B. & Tarhan, L. The effect of menadione-induced oxidative stress on the in vivo reactive oxygen species and antioxidant response system of Phanerochaete chrysosporium. Process Biochem. 49, 195–202 (2014).
Lim, J. B., Langford, T. F., Huang, B. K., Deen, W. M. & Sikes, H. D. A reaction–diffusion model of cytosolic hydrogen peroxide. Free Radic. Biol. Med. 90, 85–90 (2016).
Heppert, J. K. et al. Comparative assessment of fluorescent proteins for in vivo imaging in an animal model system. Mol. Biol. Cell 27, 3385–3394 (2016).
Campbell, B. C. et al. mGreenLantern: a bright monomeric fluorescent protein with rapid expression and cell filling properties for neuronal imaging. Proc. Natl Acad. Sci. USA 117, 30710–30721 (2020).
Schweikhard, V. et al. Application note: the Power HyD family of detectors. Nat. Methods https://www.nature.com/articles/d42473-020-00398-0 (2020).
Lambert, T. J. FPbase: a community-editable fluorescent protein database. Nat. Methods 16, 277–278 (2019).
Yamada, Y. & Mikoshiba, K. Quantitative comparison of novel GCaMP-type genetically encoded Ca2+ indicators in mammalian neurons. Front. Cell Neurosci. 6, 41 (2012).
Han, L. et al. RFP tags for labeling secretory pathway proteins. Biochem. Biophys. Res. Commun. 447, 508–512 (2014).
Abdelfattah, A. S. et al. A bright and fast red fluorescent protein voltage indicator that reports neuronal activity in organotypic brain slices. J. Neurosci. 36, 2458–2472 (2016).
Costantini, L. M., Fossati, M., Francolini, M. & Snapp, E. L. Assessing the tendency of fluorescent proteins to oligomerize under physiologic conditions: fluorescent protein oligomerization assay. Traffic 13, 643–649 (2012).
Belousov, V. V. et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 3, 281–286 (2006).
Taniguchi, J. et al. Comment on ‘Accumbens cholinergic interneurons dynamically promote dopamine release and enable motivation’. eLife 13, e95694 (2024).
Heim, R., Prasher, D. C. & Tsien, R. Y. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc. Natl Acad. Sci. USA 91, 12501–12504 (1994).
Ma, Y., Sun, Q. & Smith, S. C. The mechanism of oxidation in chromophore maturation of wild-type green fluorescent protein: a theoretical study. Phys. Chem. Chem. Phys. 19, 12942–12952 (2017).
Takahashi, E. et al. Genetic oxygen sensor: GFP as an indicator of intracellular oxygenation. Adv. Exp. Med. Biol. 566, 39–44 (2005).
Ishii, T., Warabi, E. & Mann, G. E. Mechanisms underlying NRF2 nuclear translocation by non-lethal levels of hydrogen peroxide: p38 MAPK-dependent neutral sphingomyelinase2 membrane trafficking and ceramide/PKCζ/CK2 signaling. Free Radic. Biol. Med. 191, 191–202 (2022).
Covas, G., Marinho, H. S., Cyrne, L. & Antunes, F. Activation of NRF2 by H2O2: de novo synthesis versus nuclear translocation. Methods Enzymol. 528, 157–171 (2013).
Görlach, A., Bertram, K., Hudecova, S. & Krizanova, O. Calcium and ROS: a mutual interplay. Redox Biol. 6, 260–271 (2015).
Nikolaienko, R., Bovo, E. & Zima, A. V. Redox dependent modifications of ryanodine receptor: basic mechanisms and implications in heart diseases. Front. Physiol. 9, 1775 (2018).
Johnstone, V. P. A. & Hool, L. C. Glutathionylation of the L-type Ca2+ channel in oxidative stress-induced pathology of the heart. Int. J. Mol. Sci. 15, 19203–19225 (2014).
Gonnot, F. et al. SERCA2 phosphorylation at serine 663 is a key regulator of Ca2+ homeostasis in heart diseases. Nat. Commun. 14, 3346 (2023).
Goodman, J. B. et al. Redox-resistant SERCA [sarco(endo)plasmic reticulum calcium ATPase] attenuates oxidant-stimulated mitochondrial calcium and apoptosis in cardiac myocytes and pressure overload-induced myocardial failure in mice. Circulation 142, 2459–2469 (2020).
Akaike, T. et al. A sarcoplasmic reticulum localized protein phosphatase regulates phospholamban phosphorylation and promotes ischemia reperfusion injury in the heart. JACC Basic Transl. Sci. 2, 160–180 (2017).
Varghese, E. & Büsselberg, D. Auranofin, an anti-rheumatic gold compound, modulates apoptosis by elevating the intracellular calcium concentration ([Ca2+]i) in MCF-7 breast cancer cells. Cancers 6, 2243–2258 (2014).
Harper, M. T. Auranofin, a thioredoxin reductase inhibitor, causes platelet death through calcium overload. Platelets 30, 98–104 (2019).
Kernik, D. C. et al. A computational model of induced pluripotent stem-cell derived cardiomyocytes incorporating experimental variability from multiple data sources. J. Physiol. 597, 4533–4564 (2019).
Lee, Y.-K. et al. Calcium homeostasis in human induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Rev. Rep. 7, 976–986 (2011).
Tu, C., Chao, B. S. & Wu, J. C. Strategies for improving the maturity of human induced pluripotent stem cell-derived cardiomyocytes. Circ. Res. 123, 512–514 (2018).
Goversen, B., van der Heyden, M. A. G., van Veen, T. A. B. & de Boer, T. P. The immature electrophysiological phenotype of iPSC-CMs still hampers in vitro drug screening: special focus on IK1. Pharmacol. Ther. 183, 127–136 (2018).
Itzhaki, I. et al. Calcium handling in human induced pluripotent stem cell derived cardiomyocytes. PLoS ONE 6, e18037 (2011).
Koivumäki, J. T. et al. Structural immaturity of human iPSC-derived cardiomyocytes: in silico investigation of effects on function and disease modeling. Front. Physiol. 9, 80 (2018).
Kritsiligkou, P. et al. Proteome-wide tagging with an H2O2 biosensor reveals highly localized and dynamic redox microenvironments. Proc. Natl Acad. Sci. USA 120, e2314043120 (2023).
Montiel, V. et al. Inhibition of aquaporin-1 prevents myocardial remodeling by blocking the transmembrane transport of hydrogen peroxide. Sci. Transl. Med. 12, eaay2176 (2020).
Schattauer, S. S. et al. Peroxiredoxin 6 mediates Gαi protein-coupled receptor inactivation by cJun kinase. Nat. Commun. 8, 743 (2017).
Schattauer, S. S. et al. Reactive oxygen species (ROS) generation is stimulated by κ opioid receptor activation through phosphorylated c-Jun N-terminal kinase and inhibited by p38 mitogen-activated protein kinase (MAPK) activation. J. Biol. Chem. 294, 16884–16896 (2019).
Terzi, A. & Suter, D. M. The role of NADPH oxidases in neuronal development. Free Radic. Biol. Med. 154, 33–47 (2020).
Ma, M. W. et al. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 12, 7 (2017).
Schröder, K. NADPH oxidases: current aspects and tools. Redox Biol. 34, 101512 (2020).
Sies, H. et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 23, 499–515 (2022).
Miller, E. W., Dickinson, B. C. & Chang, C. J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl Acad. Sci. USA 107, 15681–15686 (2010).
Thiagarajah, J. R., Chang, J., Goettel, J. A., Verkman, A. S. & Lencer, W. I. Aquaporin-3 mediates hydrogen peroxide-dependent responses to environmental stress in colonic epithelia. Proc. Natl Acad. Sci. USA 114, 568–573 (2017).
Haskew-Layton, R. E. et al. Controlled enzymatic production of astrocytic hydrogen peroxide protects neurons from oxidative stress via an NRF2-independent pathway. Proc. Natl Acad. Sci. USA 107, 17385–17390 (2010).
Niemczyk, E. et al. A possible involvement of plasma membrane NAD(P)H oxidase in the switch mechanism of the cell death mode from apoptosis to necrosis in menadione-induced cell injury. Acta Biochim. Pol. 51, 1015–1022 (2004).
Thor, H. et al. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J. Biol. Chem. 257, 12419–12425 (1982).
Yamashoji, S., Ikeda, T. & Yamashoji, K. Extracellular generation of active oxygen species catalyzed by exogenous menadione in yeast cell suspension. Biochim. Biophys. Acta 1059, 99–105 (1991).
Suzuki, Y. & Ono, Y. Involvement of reactive oxygen species produced via NADPH oxidase in tyrosine phosphorylation in human B- and T-lineage lymphoid cells. Biochem. Biophys. Res. Commun. 255, 262–267 (1999).
Shu, X. et al. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 9, e1001041 (2011).
Barnett, M. E., Baran, T. M., Foster, T. H. & Wojtovich, A. P. Quantification of light-induced miniSOG superoxide production using the selective marker, 2-hydroxyethidium. Free Radic. Biol. Med. 116, 134–140 (2018).
Seo, M. J. et al. Dual inhibition of thioredoxin reductase and proteasome is required for auranofin-induced paraptosis in breast cancer cells. Cell Death Dis. 14, 42 (2023).
Renken, S. et al. Targeting of NRF2 improves antitumoral responses by human NK cells, TIL and CAR T cells during oxidative stress. J. Immunother. Cancer 10, e004458 (2022).
Koren, S. A. et al. All-optical spatiotemporal mapping of ROS dynamics across mitochondrial microdomains in situ. Nat. Commun. 14, 6036 (2023).
Woo, H. A. et al. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell 140, 517–528 (2010).
DeYulia, G. J. Jr, Cárcamo, J. M., Bórquez-Ojeda, O., Shelton, C. C. & Golde, D. W. Hydrogen peroxide generated extracellularly by receptor–ligand interaction facilitates cell signaling. Proc. Natl Acad. Sci. USA 102, 5044–5049 (2005).
Xu, C., Peng, B. & Liu, S. Using intra-brain drug infusion to investigate neural mechanisms underlying reward-seeking behavior in mice. STAR Protoc. 3, 101221 (2022).
Xavier, A. L. R. et al. Cannula implantation into the cisterna magna ofrodents. J. Vis. Exp. https://doi.org/10.3791/57378 (2018).
Farrants, H. et al. A modular chemigenetic calcium indicator for multiplexed in vivo functional imaging. Nat. Methods 21, 1916–1925 (2024).
Grimm, J. B. et al. A general method to optimize and functionalize red-shifted rhodamine dyes. Nat. Methods 17, 815–821 (2020).
Lee, J. D. et al. Data and code for “Monitoring in real time and far-red imaging of H2O2 dynamics with subcellular resolution”. figshare https://doi.org/10.6084/m9.figshare.28306691 (2025).
Lee, J. D. et al. FUSE: fluorescent signal engine. GitHub https://github.com/justindaholee/FUSE (2025).
García-Nafría, J., Watson, J. F. & Greger, I. H. IVA cloning: a single-tube universal cloning system exploiting bacterial in vivo assembly. Sci. Rep. 6, 27459 (2016).
Catapano, L. A., Arnold, M. W., Perez, F. A. & Macklis, J. D. Specific neurotrophic factors support the survival of cortical projection neurons at distinct stages of development. J. Neurosci. 21, 8863–8872 (2001).
Martin, D. L. Synthesis and release of neuroactive substances by glial cells. Glia 5, 81–94 (1992).
McKenna, M. et al. Organotypic whole hemisphere brain slice models to study the effects of donor age and oxygen-glucose-deprivation on the extracellular properties of cortical and striatal tissue. J. Biol. Eng. 16, 14 (2022).
Lian, X. et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 8, 162–175 (2013).
Bremner, S. B. et al. Full-length dystrophin deficiency leads to contractile and calcium transient defects in human engineered heart tissues. J. Tissue Eng. 13, 20417314221119628 (2022).
Tohyama, S. et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 12, 127–137 (2013).
Yoo, D. Studying the Role of Mechanical Contraction in Cardiac Muscle Development Using Genetically Engineered Non-Contractile Human Stem Cell-Derived Cardiomyocytes. PhD thesis, Univ. of Washington (2021).
Young, J. E. et al. Elucidating molecular phenotypes caused by the SORL1 Alzheimer’s disease genetic risk factor using human induced pluripotent stem cells. Cell Stem Cell 16, 373–385 (2015).
Shin, Y. J. et al. Amyloid β peptides (Aβ) from Alzheimer’s disease neuronal secretome induce endothelial activation in a human cerebral microvessel model. Neurobiol. Dis. 181, 106125 (2023).
Knupp, A. et al. Depletion of the AD risk gene SORL1 selectively impairs neuronal endosomal traffic independent of amyloidogenic APP processing. Cell Rep. 31, 107719 (2020).
Stringer, C., Wang, T., Michaelos, M. & Pachitariu, M. Cellpose: a generalist algorithm for cellular segmentation. Nat. Methods 18, 100–106 (2021).
Pachitariu, M. & Stringer, C. Cellpose 2.0: how to train your own model. Nat. Methods 19, 1634–1641 (2022).
Plank, G. et al. The openCARP simulation environment for cardiac electrophysiology. Comput. Methods Prog. Biomed. 208, 106223 (2021).
Acknowledgements
J.D.L. was supported by 1F31DA056121-01A1 and the ISCRM Fellowship. A.B. was supported by the Brain Research Foundation, UW Royalty Research Fund, UW ISCRM IPA, NIGMS R01 GM139850-01, P30 DA048736-01-Pilot, NIMH RF1MH130391, NINDS U01NS128537, NIDA R21DA051193 and the McKnight Foundation’s Technologies in Neuroscience Award. S.J.W. was supported by the National Science Foundation DGE-2140004 and the Herbold Foundation. K.M.E. was supported by T32AG066574. A.A. was supported by the National Institute of General Medical Sciences grant RM1 GM131981, the National Institute of Arthritis and Musculoskeletal and Skin Diseases grant P30 AR074990, American Heart Association supplement grant AHA872208 and BCTP-NIH–NIBIB-5T32EB032787-02. We would like to thank the Janelia Materials program from Howard Hughes Medical Institute Janelia Research Campus for generous sharing of their Janelia Fluors essential for this study. This research received additional support from the Lynn and Mike Garvey Imaging Core, the UW NAPE Center, ISCRM Shared Equipment and Leica Center of Excellence for Cellular Imaging in Fred Hutch Cancer Research Center (H. West-Foyle and L. Schroeder). We also want to thank R. Moon for his support.
Author information
Authors and Affiliations
Contributions
J.D.L. and A.B. conceived the study design and oROS-HT635 engineering strategies, performed experiments, and analyzed the data. J.D.L, A.N., S.Z. and A.S. cloned and screened the sensor variants. J.D.L., Y.W., A.M. and V.C. constructed constructs for chemigenetic/optogenetic demonstration of subcellular H2O2 and performed analysis. J.D.L, S.J.W. and H.C. performed purified protein assays and analysis. J.D.L., C.E.G. and P.M.B. performed in silico hiPS cell-CM simulations and analysis. J.D.L. and Z.R.J. performed maturation studies under hypoxia, and brain slice imaging and analysis. K.M.E., A.A., S.B.B., I.K.A.P. and C.A.W. differentiated and prepared hiPS cell-derived cells. A.B., P.M.B., E.N. D.L.M., J.E.Y., D.B., M.R. and F.M.-H. supervised the preparation of materials, experiments, analyses and paper writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Marc Fransen, Celien Lismont and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
oROS-HT amino acid sequence, Supplementary Table 1 and Figs. 1–11.
Supplementary Data
Source data for the Supplementary figures.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, J.D., Nguyen, A., Gibbs, C.E. et al. Monitoring in real time and far-red imaging of H2O2 dynamics with subcellular resolution. Nat Chem Biol (2025). https://doi.org/10.1038/s41589-025-01891-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41589-025-01891-7
This article is cited by
-
Synthetic fluorophores for live-cell fluorescence microscopy and biosensing
Nature Chemical Biology (2025)
-
Lighting up hydrogen peroxide
Nature Chemical Biology (2025)