Abstract
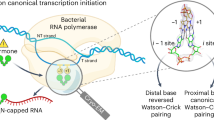
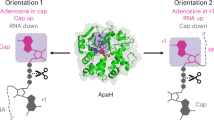
Stress-induced dinucleoside tetraphosphates (Np4Ns, where N is adenosine, guanosine, cytosine or uridine) are ubiquitous in living organisms, yet their function has been largely elusive for over 50 years. Recent studies have revealed that RNA polymerase can influence the cellular lifetime of transcripts by incorporating these alarmones into RNA as 5′-terminal caps. Here we present structural and biochemical data that reveal the molecular basis of noncanonical transcription initiation from Np4As by Escherichia coli and Thermus thermophilus RNA polymerases. Our results show the influence of the first two nucleotide incorporation steps on capping efficiency and the different interactions of Np4As with transcription initiation complexes. These data provide critical insights into the substrate selectivity that dictates levels of Np4 capping in bacterial cells.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Coordinates of the structures and the cryo-EM density maps were deposited to the PDB and the EM Data Bank under the following accession codes: 9UKN and EMD-64250, Apo(−1dC); 9UJK and EMD-64215, ATP(−1dC); 9UJL and EMD-64216, Ap4A(−1dC); 9UPW and EMD-64404, Gp4A(−1dC); 9UKS and EMD-64253, Gp4A(−1dA); 9UJP and EMD-64219, Up4A(−1dA); 9UJN and EMD-64218, ATP-C(−1dC); 9UKP and EMD-64252; Ap4A-C(−1dC); 9UKU and EMD-64255, Gp4A-C(−1dC); 9UKO and EMD-64251, Ap4A-C(−1dA); 9UMA and EMD-64271, Gp4A-C(−1dA); 9ULS and EMD-64266, Up4A-C*(−1dA); 9UKT and EMD-64254, Gp4A-C-U(−1dC); 9ULT and EMD-64267, Up4A-C(−1dA); 9V4M, Ap4G(−1dG). The MD simulation data used in this study are available from Zenodo (https://doi.org/10.5281/zenodo.15493272)72. Source data are provided with this paper.
References
Ferguson, F., McLennan, A. G., Urbaniak, M. D., Jones, N. J. & Copeland, N. A. Re-evaluation of diadenosine tetraphosphate Ap4A from a stress metabolite to bona fide secondary messenger. Front. Mol. Biosci. 7, 606807 (2020).
Zamecnik, P. C., Stephenson, M. L., Janeway, C. M. & Randerath, K. Enzymatic synthesis of diadenosine tetraphosphate and diadenosine triphosphate with a purified lysyl-sRNA synthetase. Biochem. Biophys. Res. Commun. 24, 91–97 (1966).
Finamore, F. J. & Warner, A. H. The occurrence of P1, P4-diguanosine 5′-tetraphosphate in brine shrimp eggs. J. Biol. Chem. 238, 344–348 (1963).
Coste, H., Brevet, A., Plateau, P. & Blanquet, S. Non-adenylylated bis(5’-nucleosidyl) tetraphosphates occur in Saccharomyces cerevisiae and in Escherichia coli and accumulate upon temperature shift or exposure to cadmium. J. Biol. Chem. 262, 12096–12103 (1987).
Baker, J. C. & Jacobson, M. K. Alteration of adenyl dinucleotide metabolism by environmental stress. Proc. Natl Acad. Sci. USA 83, 2350–2352 (1986).
Lee, P. C., Bochner, B. R. & Ames, B. N. AppppA, heat-shock stress, and cell oxidation. Proc. Natl Acad. Sci. USA 80, 7496–7500 (1983).
Brevet, A., Plateau, P., Best-Belpomme, M. & Blanquet, S. Variation of Ap4A and other dinucleoside polyphosphates in stressed Drosophila cells. J. Biol. Chem. 260, 15566–15570 (1985).
Kisselev, L. L., Justesen, J., Wolfson, A. D. & Frolova, L. Y. Diadenosine oligophosphates (ApnA), a novel class of signalling molecules?. FEBS Lett. 427, 157–163 (1998).
McLennan, A. G. Dinucleoside polyphosphates—friend or foe?. Pharmacol. Ther. 87, 73–89 (2000).
Farr, S. B., Arnosti, D. N., Chamberlin, M. J. & Ames, B. N. An apaH mutation causes AppppA to accumulate and affects motility and catabolite repression in Escherichia coli. Proc. Natl Acad. Sci. USA. 86, 5010–5014 (1989).
Leveque, F., Blanchin-Roland, S., Fayat, G., Plateau, P. & Blanquet, S. Design and characterization of Escherichia coli mutants devoid of Ap4N-hydrolase activity. J. Mol. Biol. 212, 319–329 (1990).
Cartwright, J. L., Britton, P., Minnick, M. F. & McLennan, A. G. The IalA invasion gene of Bartonella bacilliformis encodes a (di)nucleoside polyphosphate hydrolase of the MutT motif family and has homologs in other invasive bacteria. Biochem. Biophys. Res. Commun. 256, 474–479 (1999).
Brevet, A., Chen, J., Leveque, F., Plateau, P. & Blanquet, S. In vivo synthesis of adenylylated bis(5′-nucleosidyl) tetraphosphates (Ap4N) by Escherichia coli aminoacyl-tRNA synthetases. Proc. Natl Acad. Sci. USA 86, 8275–8279 (1989).
Luciano, D. J., Levenson-Palmer, R. & Belasco, J. G. Stresses that raise Np4A levels induce protective nucleoside tetraphosphate capping of bacterial RNA. Mol. Cell 75, 957–966 (2019).
Shatalin, K. et al. Inhibitors of bacterial H2S biogenesis targeting antibiotic resistance and tolerance. Science 372, 1169–1175 (2021).
Zegarra, V., Mais, C. N., Freitag, J. & Bange, G. The mysterious diadenosine tetraphosphate Ap4A. Microlife 4, uqad016 (2023).
Lee, Y. N., Nechushtan, H., Figov, N. & Razin, E. The function of lysyl-tRNA synthetase and Ap4A as signaling regulators of MITF activity in FcεRI-activated mast cells. Immunity 20, 145–151 (2004).
Guerra, J. et al. Lysyl-tRNA synthetase produces diadenosine tetraphosphate to curb STING-dependent inflammation. Sci. Adv. 6, eaax3333 (2020).
Giammarinaro, P. I. et al. Diadenosine tetraphosphate regulates biosynthesis of GTP in Bacillus subtilis. Nat. Microbiol 7, 1442–1452 (2022).
Hudecek, O. et al. Dinucleoside polyphosphates act as 5′-RNA caps in bacteria. Nat. Commun. 11, 1052 (2020).
Luciano, D. J. & Belasco, J. G. Np4A alarmones function in bacteria as precursors to RNA caps. Proc. Natl Acad. Sci. USA 117, 3560–3567 (2020).
Frantisek Potuznik, J. et al. Diadenosine tetraphosphate (Ap4A) serves as a 5′ RNA cap in mammalian cells. Angew. Chem. Int. Ed. Engl. 63, e202314951 (2024).
Malygin, A. G. & Shemyakin, M. F. Adenosine, NAD and FAD can initiate template-dependent RNA synthesis catalyzed by Escherichia coli RNA polymerase. FEBS Lett. 102, 51–54 (1979).
Julius, C. & Yuzenkova, Y. Bacterial RNA polymerase caps RNA with various cofactors and cell wall precursors. Nucleic Acids Res. 45, 8282–8290 (2017).
Bird, J. G. et al. The mechanism of RNA 5′ capping with NAD+, NADH and desphospho-CoA. Nature 535, 444–447 (2016).
Chen, Y. G., Kowtoniuk, W. E., Agarwal, I., Shen, Y. & Liu, D. R. LC/MS analysis of cellular RNA reveals NAD-linked RNA. Nat. Chem. Biol. 5, 879–881 (2009).
Kowtoniuk, W. E., Shen, Y., Heemstra, J. M., Agarwal, I. & Liu, D. R. A chemical screen for biological small molecule–RNA conjugates reveals CoA-linked RNA. Proc. Natl Acad. Sci. USA 106, 7768–7773 (2009).
Wang, J., et al. Quantifying the RNA cap epitranscriptome reveals novel caps in cellular and viral RNA. Nucleic Acids Res. 47, e130 (2019).
Sherwood, A. V. et al. Hepatitis C virus RNA is 5′-capped with flavin adenine dinucleotide. Nature 619, 811–818 (2023).
Doamekpor, S. K. et al. DXO/Rai1 enzymes remove 5′-end FAD and dephospho-CoA caps on RNAs. Nucleic Acids Res. 48, 6136–6148 (2020).
Jiao, X. et al. 5′ end nicotinamide adenine dinucleotide cap in human cells promotes RNA decay through DXO-mediated deNADding. Cell 168, 1015–1027 (2017).
Bird, J. G. et al. Highly efficient 5′ capping of mitochondrial RNA with NAD+ and NADH by yeast and human mitochondrial RNA polymerase. eLife 7, e42179 (2018).
Cahova, H., Winz, M. L., Hofer, K., Nubel, G. & Jaschke, A. NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature 519, 374–377 (2015).
Walters, R. W. et al. Identification of NAD+ capped mRNAs in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 114, 480–485 (2017).
Benoni, R., Culka, M., Hudecek, O., Gahurova, L. & Cahova, H. Dinucleoside polyphosphates as RNA building blocks with pairing ability in transcription initiation. ACS Chem. Biol. 15, 1765–1772 (2020).
Vvedenskaya, I. O. et al. CapZyme-Seq comprehensively defines promoter-sequence determinants for RNA 5′ capping with NAD+. Mol. Cell 70, 553–564 (2018).
Zhang, Y. et al. Structural basis of transcription initiation. Science 338, 1076–1080 (2012).
Zhang, Y. et al. GE23077 binds to the RNA polymerase ‘i’ and ‘i + 1’ sites and prevents the binding of initiating nucleotides. eLife 3, e02450 (2014).
Basu, R. S. et al. Structural basis of transcription initiation by bacterial RNA polymerase holoenzyme. J. Biol. Chem. 289, 24549–24559 (2014).
Campbell, E. A. et al. Structural, functional, and genetic analysis of sorangicin inhibition of bacterial RNA polymerase. EMBO J. 24, 674–682 (2005).
Campbell, E. A. et al. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 104, 901–912 (2001).
Murakami, K. S. Structural biology of bacterial RNA polymerase. Biomolecules 5, 848–864 (2015).
Bae, B., Feklistov, A., Lass-Napiorkowska, A., Landick, R. & Darst, S. A. Structure of a bacterial RNA polymerase holoenzyme open promoter complex. eLife 4, e08504 (2015).
Rajeswaran, W. et al. Optimization of benzoxazinorifamycins to improve Mycobacterium tuberculosis RNA polymerase inhibition and treatment of tuberculosis. ACS Infect. Dis. 8, 1422–1438 (2022).
Westover, K. D., Bushnell, D. A. & Kornberg, R. D. Structural basis of transcription: nucleotide selection by rotation in the RNA polymerase II active center. Cell 119, 481–489 (2004).
McClure, W. R., Cech, C. L. & Johnston, D. E. A steady state assay for the RNA polymerase initiation reaction. J. Biol. Chem. 253, 8941–8948 (1978).
Skalenko, K. S. et al. Promoter-sequence determinants and structural basis of primer-dependent transcription initiation in Escherichia coli. Proc. Natl Acad. Sci. USA 118, e2106388118 (2021).
Hao, Z. et al. Pre-termination transcription complex: structure and function. Mol. Cell 81, 281–292 (2021).
Studier, F. W. Protein production by auto-induction in high-density shaking cultures. Protein Expr. Purif. 41, 207–234 (2005).
Murakami, K. S., Masuda, S. & Darst, S. A. Crystallographic analysis of Thermus aquaticus RNA polymerase holoenzyme and a holoenzyme/promoter DNA complex. Methods Enzymol. 370, 42–53 (2003).
Chen, J., Noble, A. J., Kang, J. Y. & Darst, S. A. Eliminating effects of particle adsorption to the air/water interface in single-particle cryo-electron microscopy: bacterial RNA polymerase and CHAPSO. J. Struct. Biol. X 1, 100005 (2019).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Rice, W. J. et al. Routine determination of ice thickness for cryo-EM grids. J. Struct. Biol. 204, 38–44 (2018).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Scheres, S. H. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods 9, 853–854 (2012).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Maier, J. A. et al. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 (2015).
Galindo-Murillo, R. et al. Assessing the current state of Amber force field modifications for DNA. J. Chem. Theory Comput. 12, 4114–4127 (2016).
Zgarbova, M. et al. Refinement of the Cornell et al. nucleic acids force field based on reference quantum chemical calculations of glycosidic torsion profiles. J. Chem. Theory Comput. 7, 2886–2902 (2011).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007).
Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 52, 7189–7190 (1981).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Essmann, U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Abraham, M. J. et al. Gromacs: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Duan, W. et al. MD data for ‘Molecular basis for noncanonical transcription initiation from Np4A alarmones’. Zenodo https://doi.org/10.5281/zenodo.15493272 (2025).
Acknowledgements
This work was supported by the National Institutes of Health (NIH) grants T32 GM088118-09 to J.W.W., R35GM145359 and R01GM035769 to J.G.B., R01GM147652 to X.H. and R01GM112940 and R21GM151508 to A.S. The work was also supported by the Blavatnik Family Foundation and by the Howard Hughes Medical Institute (E.N.), the Hirschfelder Professorship Fund and the Research Forward Fund from the University of Wisconsin-Madison (X.H.) and the Croucher Fellowship for Postdoctoral Researchers from Croucher Foundation (I.C.U.). Some of the work was performed at NYU Langone Health’s Cryo-EM Laboratory (RRID: SCR_019202), which is partially supported by the Laura and Isaac Perlmutter Cancer Center Support Grant from the NIH National Cancer Institute (P30CA016087). The Laboratory for BioMolecular Structure is supported by the Department of Energy (DOE) Office of Biological and Environmental Research (KP1607011). The National Center for Cryo-EM Access and Training and the Simons EM Center located at the New York Structural Biology Center are supported by the NIH Common Fund Transformative High-Resolution Cryo-EM program (U24 GM129539) and by grants from the Simons Foundation (SF349247) and NY State Assembly Majority. This research used the Northeastern Collaborative Access Team beamlines, funded by the NIH (P30 GM124165), at the Advanced Photon Source, a US DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. This study used beamline 17-ID-2 (FMX) of the National Synchrotron Light Source II, a US DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704. The beamline operation is supported by the Center for BioMolecular Structure, funded by the NIH (P30GM133893) and the DOE Office of Biological and Environmental Research (FWP BO070). We thank L. Li for helping to purify Tt-RNAP and V. Epstein for discussions.
Author information
Authors and Affiliations
Contributions
W.D. carried out the structural studies on Tt-RNAP and contributed to the biochemical experiments. A.K. performed the in vitro transcription assays and contributed to the structural studies. I.C.U., Y.W. and X.H. conducted the MD simulations. J.W.W. conducted the structural studies on Ec-RNAP. B.W., W.J.R. and M.M.J.L. participated in the cryo-EM data collection and processing. W.D., J.G.B., D.J.L., X.H., E.N. and A.S. planned and interpreted the experiments. A.S. supervised the project. W.D., A.K., J.G.B. and A.S. wrote the paper with input from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Efficiency of Np4A incorporation during transcription initiation.
a-c, Determination of the relative efficiencies of transcription initiation with Ap4A vs. ATP in the first transcription step. a, Representative gel showing the initial RNA products of transcription reactions performed in the presence of 0.75 mM Ap4A and 0.008-1.8 mM ATP as the initiating nucleotides and [α32P]-CTP as the extending nucleotide. The experiment was repeated 3 times with similar results, with quantification shown in panels (b) and (c). b, Graph showing the fraction of Ap4A-initiated RNA vs. the Ap4A/ATP ratio in transcription reactions conducted with tDNAs containing various -1 nucleotides. The curves are logarithmic regression fits using the three highest concentrations of ATP (0.52, 0.90, 1.80 mM) from panel (a). Each point is the mean ± SD of three independent experiments (n = 3). c, Relative efficiencies of transcription initiation with Ap4A vs. ATP [(kcat/Km, Ap4A)/(kcat/Km, ATP)] determined from panel (b). Each bar is the mean ± SD of three independent experiments (n = 3). d, Promoter sequences used to validate that DNA templates for cryo-EM studies of Tt-RNAP show expected differences in transcription efficiency from Np4A with purines or pyrimidines at the -1 position of tDNA. Nucleotides in orange differ from the promoter in Fig. 1e. e-f, Efficiency of Np4A incorporation by Tt-RNAP from the promoter described in panel (d). The efficiency was determined as in Fig. 1. e, A representative gel used for quantification. f, A bar graph showing data points from two independent experiments (n = 2).
Extended Data Fig. 2 X-ray crystal structure of the T. thermophilus transcription initiation complex loaded with Gp4A.
a, DNA template scaffold used for crystallization. b, The overall view of the structure is shown with a simulated annealing omit map contoured at 0.9 σ level (light blue mesh). Proteins and DNA are color-coded, as shown. Gp4A is shown in surface representation (magenta). c, Zoom-in view of the Gp4A and selected surrounding residues shown with a simulated annealing composite omit map contoured at 0.9 σ level. The view is similar to (b).
Extended Data Fig. 3 Cryo-EM maps and structures of the active site of T. thermophilus transcription initiation complexes containing Np4-capped di- and trinucleotide RNAs.
The figures focus on the transcription products (initiating Np4As in magenta and other nucleotides in pink) and tDNA (colored as in Fig. 2) shown with schematics of the RNA-template interactions (top), matching maps in mesh representation (middle) and zoomed-in top views of the maps and models of the cap moieties (bottom or right). a, ATP-C(-1dC) complex. b, Gp4A-C(-1dC) complex. c, Gp4A-C(-1dA) complex. d, Ap4A-C(-1dC) complex. e, Ap4A-C(-1dC) complex. f, Up4A-C(-1dA) complex. g, Gp4A-C-U(-1dC) complex. To show map quality with reduced background, the map contour level was set higher in the zoomed-in images (bottom) than in the overall views (top) by ~10 % (b, f) or ~20 % (d, e).
Extended Data Fig. 4 MD simulations of the modelled T. thermophilus transcription initiation complexes.
a-c, MD simulations of the structure-based models of Ap4A(-1dC) (a), Up4A(-1dA) (b), and Up4A-C(-1dC) (c), and the cryo-EM structure of Up4A-C(-1dCA) (d). The left panels show two-dimensional heatmap plots of the conformations represented by the +1 dT – A base-pair geometry. The middle panels show the plots for potential base pairing between the cap nucleotide and nucleotide -1 of the tDNA. A black star corresponds to the reference Watson-Crick base-pairing. A red cross depicts the initial structure at the start of the simulation. The right panels show representative structures from various parts of the heatmap indicated by colored triangles. Initial structures are in red. e, Base-pairing distance distribution in Up4A-C(-1dC) (left) and Up4A-C(-1dA) (right) systems. The X-axis corresponds to the base-pairing distance in (c) and (d), and the Y-axis shows the corresponding densities estimated via KDE. The dashed red line indicates the position of the initial model at ~9.0 Å. The percentage indicates the number of simulation frames that are close to the ideal base-pairing (Site 1), with the cutoff distance of 9.0 Å.
Supplementary information
Supplementary Information
Supplementary Tables 1–5 and Figs. 1–21.
Source data
Source Data Fig. 1
Unprocessed gels.
Source Data Fig. 4
Unprocessed gels.
Source Data Extended Data Fig. 1
Unprocessed gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duan, W., Kaushik, A., Unarta, I.C. et al. Molecular basis for noncanonical transcription initiation from Np4A alarmones. Nat Chem Biol (2025). https://doi.org/10.1038/s41589-025-02044-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41589-025-02044-6