Abstract
PD-1 is a key negative regulator of CD8+ T cell activation and is highly expressed by exhausted T cells in cancer and chronic viral infection. Although PD-1 blockade can improve viral and tumor control, physiological PD-1 expression prevents immunopathology and improves memory formation. The mechanisms driving high PD-1 expression in exhaustion are not well understood and could be critical to disentangling its beneficial and detrimental effects. Here, we functionally interrogated the epigenetic regulation of PD-1 using a mouse model with deletion of an exhaustion-specific PD-1 enhancer. Enhancer deletion exclusively alters PD-1 expression in CD8+ T cells in chronic infection, creating a ‘sweet spot’ of intermediate expression where T cell function is optimized compared to wild-type and Pdcd1-knockout cells. This permits improved control of chronic infection without additional immunopathology. Together, these results demonstrate that tuning PD-1 via epigenetic editing can reduce CD8+ T cell dysfunction while avoiding excess immunopathology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
scRNA-seq, ATAC-seq and bulk RNA-seq data have been deposited in the Gene Expression Omnibus under accession number GSE212507. scRNA-seq and additional data can also be found on the Single Cell Portal under accession number SCP1772. Custom gene signatures for exhausted subsets were generated from published bulk RNA-seq data10. The gene sets analyzed with GSEA are available through the Broad Institute Molecular Signatures Database found at https://www.gsea-msigdb.org/gsea/msigdb/index.jsp and were accessed through the msigdbr package in R. The transcription factor motifs analyzed with TOBIAS are available through the JASPAR Database found at https://jaspar.elixir.no/. The GRCM39 genome assembly was used for bulk RNA-seq alignment and can be found at https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000001635.27/. Source data are provided with this paper.
Code availability
Code to regenerate all genomics analyses and figures is hosted publicly on GitHub at https://github.com/hms-sharpies/pd1-enhdel-omics.
References
Zajac, A. J. et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 (1998).
Wherry, E. J. et al. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927 (2003).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Jin, H.-T. et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl Acad. Sci. USA 107, 14733–14738 (2010).
Wherry, E. J. et al. Molecular signature of CD8 T cell exhaustion during chronic viral infection. Immunity 27, 824 (2007).
Alfei, F. et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 571, 265–269 (2019).
Khan, O. et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature 571, 211–218 (2019).
Scott, A. C. et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571, 270–274 (2019).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
Hudson, W. H. et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1+ stem-like CD8+ T cells during chronic infection. Immunity 51, 1043–1058 (2019).
Zander, R. et al. CD4+ T cell help is required for the formation of a cytolytic CD8+ T cell subset that protects against chronic infection and cancer. Immunity 51, 1028–1042 (2019).
Giles, J. R. et al. Shared and distinct biological circuits in effector, memory and exhausted CD8+ T cells revealed by temporal single-cell transcriptomics and epigenetics. Nat. Immunol. 23, 1600–1613 (2022).
Daniel, B. et al. Divergent clonal differentiation trajectories of T cell exhaustion. Nat. Immunol. 23, 1614–1627 (2022).
Iwai, Y. et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99, 12293–12297 (2002).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Reck, M. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833 (2016).
Ferris, R. L. et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 375, 1856–1867 (2016).
Nghiem, P. T. et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N. Engl. J. Med. 374, 2542–2552 (2016).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
Kelderman, S., Schumacher, T. N. & Kvistborg, P. Mismatch repair-deficient cancers are targets for anti-PD-1 therapy. Cancer Cell 28, 11–13 (2015).
Pauken, K. E. et al. The PD-1 pathway regulates development and function of memory CD8+ T cells following respiratory viral infection. Cell Rep. 31, 107827 (2020).
Kalia, V. et al. Metabolic regulation by PD-1 signaling promotes long-lived quiescent CD8 T cell memory in mice. Sci. Transl. Med. 13, eaba6006 (2021).
Odorizzi, P. M. et al. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J. Exp. Med. 212, 1125–1137 (2015).
Sen, D. R. et al. The epigenetic landscape of T cell exhaustion. Science 354, 1165–1169 (2016).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Pauken, K. E. et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165 (2016).
Yoshida, H. et al. The cis-regulatory atlas of the mouse immune system. Cell 176, 897–912 (2019).
Matloubian, M., Concepcion, R. J. & Ahmed, R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68, 8056–8063 (1994).
Chen, Z. et al. TCF-1-centered transcriptional network drives an effector versus exhausted CD8 T cell-fate decision. Immunity 51, 840–855 (2019).
Beltra, J.-C. et al. Developmental relationships of four exhausted CD8+ T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity 52, 825–841 (2020).
Bentsen, M. et al. ATAC-seq footprinting unravels kinetics of transcription factor binding during zygotic genome activation. Nat. Commun. 11, 4267 (2020).
Castro-Mondragon, J. A. et al. JASPAR 2022: the 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 50, D165–D173 (2022).
Chen, J. et al. NR4A transcription factors limit CAR T cell function in solid tumours. Nature 567, 530–534 (2019).
Doering, T. A. et al. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity 37, 1130–1144 (2012).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
Frebel, H. et al. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J. Exp. Med. 209, 2485–2499 (2012).
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367, eaba7365 (2020).
Wang, Z. et al. Phase I study of CAR-T cells with PD-1 and TCR disruption in mesothelin-positive solid tumors. Cell. Mol. Immunol. 18, 2188–2198 (2021).
Schumann, K. et al. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc. Natl Acad. Sci. USA 112, 10437–10442 (2015).
Kalinin, R. S. et al. Engineered removal of PD-1 from the surface of CD19 CAR-T cells results in increased activation and diminished survival. Front. Mol. Biosci. 8, 745286 (2021).
LaFleur, M. W. et al. PTPN2 regulates the generation of exhausted CD8+ T cell subpopulations and restrains tumor immunity. Nat. Immunol. 20, 1335–1347 (2019).
Guo, M. et al. EZH2 represses the B cell transcriptional program and regulates antibody-secreting cell metabolism and antibody production. J. Immunol. 200, 1039–1052 (2018).
Corces, M. R. et al. Lineage-specific and single-cell chromatin accessibility charts human hematopoiesis and leukemia evolution. Nat. Genet. 48, 1193–1203 (2016).
Villani, A. C. et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 356, eaah4573 (2017).
Gonye, A. L. K. et al. Protocol for bulk RNA sequencing of enriched human neutrophils from whole blood and estimation of sample purity. STAR Protoc. 4, 102125 (2023).
Acknowledgements
We thank past and present members of the W.N.H., A.H.S. and D.R.S. laboratories for thoughtful scientific discussions. We thank G. Freeman for the generous gift of anti-PD-L1 and isotype antibody. We thank the Dana-Farber Flow Cytometry Core, the Dana-Farber Animal Research Facility and the Harvard Center for Comparative Medicine for their assistance. A.H.S. was supported by NIH P01 AI56299 and funding from the Evergrande Center for Immunologic Diseases. W.N.H. was supported by funding from the Parker Institute for Cancer Immunotherapy. D.R.S. was supported by funding from the Karin Grunebaum Cancer Research Foundation, the Melanoma Research Alliance, the V Foundation for Cancer Research, NIAID DP2 AI176139 and NIAID U19 AI082630. S.A.W. was supported by NIGMS T32 GM007753, NIGMS T32 GM144273 and NIH NCI T32 CA207021. T.J.L. was supported by NIGMS T32 GM144273. B.C.M. was supported by NIH K08 CA248960. A.P.R.B., D.K.N., C.D.S. and J.M.B. were supported by NIH R01 AI113021.
Author information
Authors and Affiliations
Contributions
S.A.W., D.R.S., W.N.H. and A.H.S. conceived the study and designed the experiments. S.A.W., A.Y.H., M.E.F., D.M., A.C.Y.C., T.J.L., B.C.M., M.H., T.H.N., J.H.R., N.S., C.M.-A.N., D.G.P., J.F.O. and R.A.D. performed the experiments and/or data analysis. A.P.R.B., D.K.N., C.D.S. and J.M.B. provided ATAC-seq data. S.A.W., D.R.S. and A.H.S. wrote the manuscript. M.A.S., M.W.L., J.G.D. and W.N.H. contributed key discussions. All authors reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A.H.S. has patents/pending royalties on the PD-1 pathway from Roche and Novartis and has research funding from IOME, AbbVie, Taiwan Bio and Calico unrelated to the submitted work. A.H.S. serves on advisory boards for Elpiscience, Monopteros, Alixia, IOME, Corner Therapeutics, BioEntre, GlaxoSmithKline, Amgen and Janssen and is also on scientific advisory boards for the Massachusetts General Cancer Center, Program in Cellular and Molecular Medicine at Boston Children’s Hospital, the Human Oncology and Pathogenesis Program at Memorial Sloan Kettering Cancer Center, Perlmutter Cancer Center at New York University, the Gladstone Institutes and the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins. A.H.S. is an academic editor for the Journal of Experimental Medicine. W.N.H. and D.R.S. have a patent application on T cell exhaustion-specific enhancers held by the Dana-Farber Cancer Institute. W.N.H. is a cofounder, employee of and equity holder in Arsenal Biosciences. W.N.H. holds equity in Tango Therapeutics and Arsenal Biosciences. B.C.M. has consulted for Cellarity, LifeOmic and Telix Pharmaceuticals. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Bertram Bengsch and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ioana Staicu, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Creation and characterization of uninfected enhancer-deleted mice.
(a) Sanger sequencing trace of enhancer-deleted mice at site of enhancer deletion (bottom) with base-pair annotation (middle) compared to wildtype sequence (top) with regions of homology highlighted (red, blue boxes) as well as site of sgRNA binding noted (black boxes). (b) Gating strategy for baseline immune characterization, panel 1 (top row, T cell subsets) and panel 2 (bottom row), non-T cell lymphocytes. (c) Percentage of the indicated immune cell type of Live CD45+ cells in spleen (left) and liver (right) at baseline in EnhDel (n = 8) and WT (n = 6) mice. One of two representative experiments shown, significance calculated using an unpaired Student’s t-test. Errors bars represent mean and standard deviation. (d) Percentage of the indicated immune cell type expressing PD-1 in the spleen at baseline in EnhDel (n = 8) and WT (n = 6) mice. One of two representative experiments shown, significance calculated using an unpaired Student’s t-test. Errors bars represent mean and standard deviation. (e) Representative flow cytometry plot of PD-1 expression in CD8+ T cells and CD4+ Treg cells from EnhDel and WT mice. (f) Representative flow cytometry plots of CD25 (left), CX3CR1 (center), CD62L and CD44 (right) in CD8+ T cells from the spleen at baseline. (g) Chromatin accessibility tracks from ImmGen from immune populations in the spleen without stimulation. Enhancer of interest (−23.8 kb) highlighted in blue box. (h) Representative flow cytometry plots of Cell Trace Violet (CTV) following 72 hours of in vitro stimulation (left), and quantification of division number by CTV dilution (right) in EnhDel and WT cells. Dots represent individual mice; n = 8 mice for EnhDel, n = 6 mice for WT. One of two representative experiments shown, significance calculated using an unpaired Student’s t-test. Bar height represents mean, error bars represent standard deviation. Asterisks used to indicate significance correspond to the following: ns, not significant (P > 0.05), *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001.
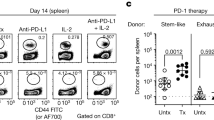
Extended Data Fig. 2 PD-1 expression dynamics impact EnhDel exhausted subset distribution and cell number in chronic infection.
(a) Flow cytometry plot of splenic single lymphocytes from an uninfected, untreated control animal (left) and a CD4-depletion treated infected animal (right) at day 7 of infection. (b) Gating strategy for identification of live transferred EnhDel and WT CD8+ T cells. (c) Serum titer of LCMV Clone 13 at day 28–30 of infection in the setting of 1500 cell EnhDel/WT co-transfer (Expt.1: n = 4 mice, Expt. 2: n = 4 mice). (d) Percentage of indicated exhausted subset of P14 co-transferred EnhDel or WT cells at D7 (n = 15 mice) after Cl. 13 infection. (e) Diagram of P14 EnhDel/WT co-transfer system with LCMV Clone 13 for studies of PD-1 pathway blockade effect. Recipients were treated with anti-PD-L1 antibody or isotype control at the indicated days prior to analysis at D30. (f) Percentage of EnhDel (blue) or WT (grey) cells of all Live CD8b+ cells. Isotype (n = 10) or αPD-L1 (n = 11) treated. Two of two experiments combined. Error bars represent mean and standard deviation. For comparisons across treatment, significance was calculated using an unpaired two-sided Student’s t-test. For comparisons within animal, a paired two-sided Student’s t-test was used. (g) Percentage of SLAMF6+ progenitor-exhausted (left plot) or TIM3+ (right plot) of EnhDel (blue) or WT (grey) cells. Isotype (n = 10) or αPD-L1 (n = 11). Error bars represent mean and standard deviation. (h) Flow cytometry plot of full-minus-one (FMO) staining without BrdU-FITC, used for establishing BrdU+ gate. All transferred cells at day 7 of Cl. 13 infection shown. (i) Percentage BrdU+ of all EnhDel or all WT cells at the indicated timepoints (D7: n = 17, D15: n = 13, D29: n = 11). (j) Flow cytometry plot of full-minus-one (FMO) staining without Annexin V-FITC, used for establishing Annexin V+ gate. All transferred cells at day 29 of Cl. 13 infection shown. (k) Percentage Annexin V+ of all EnhDel or all WT cells at the indicated timepoints (D7: n = 17, D15: n = 17, D29: n = 11). (l) PD-1 expression by subset in EnhDel and WT cells at different timepoints of infection; left: D7 (n = 10 mice), right: D28 (n = 6 mice). (m) Representative flow cytometry plots of PD-1 staining comparing exhausted subsets (Prog. vs. Term.) from D7 EnhDel cells (left), D7 WT cells (left-center), D28 EnhDel cells (right-center), and D28 WT cells (right). (D) (G) (I) (K) (L) Two of two experiments combined, significance calculated using a paired two-sided Student’s t-test. Asterisks used to indicate significance correspond to the following: ns, not significant (P > 0.05), *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001.
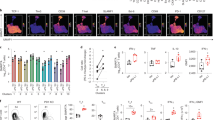
Extended Data Fig. 3 High quality scRNA-seq and ATAC-seq permits interrogation of transcription factor binding within exhaustion-specific enhancer.
(a) Violin plots of quality control metrics after filtering, by sample (genotype and replicate, n = 6 samples total). Left: number of reads per cell, center: number of unique reads per cell, right: percent mitochondrial reads per cell. (b) UMAP projection of cells separated by sample identity. Top: replicate 1, bottom: replicate 2; left: EnhDel, center: Pdcd1-KO, right: WT. (c) Top 25 differential genes (rows), organized by cluster, with color denoting scaled normalized expression in each cell (columns). (d) Exhausted subset signatures (left: progenitor-exhausted signature, middle: transitory-exhausted signature, right: terminally-exhausted signature) scored across all cells in each cluster (top row: Prog., second from top: Eff.-like, middle: Trans., second from bottom: Term., bottom: Div.). (e) TSS enrichment curves showing chromatin accessibility averaged across all TSS sites genome wide, by sorted genotype (left: EnhDel; center: Pdcd1-KO; right: WT) and exhausted subset (top: Prog., bottom: Term.). (f) Replicate concordance for ATAC-seq samples by sorted genotype (left: EnhDel, center: Pdcd1-KO, right: WT) and exhausted subset (top: Prog., bottom: Term.). (g) Venn diagram of the transcription factor motif sites within the exhaustion-associated Pdcd1 enhancer bound by the transcription factor as predicted by TOBIAS analysis of WT ATAC-seq tracks. Percentage and number for progenitor exhausted (left), terminally-exhausted (right), and both (center) displayed. Subset of predicted transcription factors bound in both Prog. and Term. listed. (h) Significance (p-value by Wilcoxon rank sum) of differential expression between WT Prog. and Term. cells by scRNA-seq for progenitor-specific predicted bound transcription factors (top), transcription factors predicted to be bound in both subsets (middle), and transcription factors without motifs in the exhaustion-associated Pdcd1 enhancer. Positive values denote higher expression in progenitor-exhausted cluster, negative values denote higher expression in terminal-exhausted cluster. Center line denotes mean and error bars denote standard deviation.
Extended Data Fig. 4 Transcriptional characterization of individual genes and pathways in EnhDel cells early and late in chronic infection.
(a) Heatmap of the pseudobulk normalized expression per gene, averaged across sample replicates (n = 2 per genotype). Genes ordered by gene weight in axis 1 from the PCA model (PC2 loading). A curated set of genes is highlighted. (b) Violin plot of Car2 (left) and Cd7 (right) gene expression across cells grouped by cluster. The terminal cluster was used as reference; significance calculated using a Wilcoxon rank sum test. (c) Violin plot of Tox (left) and Ikzf2 (right) gene expression, cells grouped by cluster and genotype. Genotypes within a cluster were compared; significance calculated using a Wilcoxon rank sum test. (d) Pre-ranked GSEA of an apoptosis signature (HAMAI_APOPTOSIS_VIA_TRAIL_UP) in EnhDel versus WT (top) and EnhDel versus Pdcd1-KO (bottom) differentially expressed genes, analyzed separately by cluster (left: Prog., center-left: Eff.-like, center-right: Trans., right: Term.). Rank of genes at the bottom. (e) Violin plots of genes containing potential CRISPR off-target editing sites. Gene name indicated at column header. Cells grouped by genotype. Significance calculated using a Wilcoxon rank sum test. (f) Venn diagrams of overlap between D9 and D30 significant gene sets. Significant gene sets identified by pre-ranked GSEA analysis comparing EnhDel and WT cells. Top: Prog., bottom: Term. Number of gene sets in each Venn area. Significance of D9 vs. D30 overlap (Fisher’s exact test) listed. (g) Pre-ranked GSEA of an oxidative phosphorylation signature (HALLMARK_OXIDATIVE_PHOSPHORYLATION) in D9 EnhDel versus WT differentially expressed genes, analyzed separately by cluster (left: Prog., right: Term.). Rank of genes at the bottom. (h) Pre-ranked GSEA of an effector-versus-exhausted CD8+ T cell signature (GSE9650_EFFECTOR_VS_EXHAUSTED_CD8_TCELL_UP) in D9 EnhDel versus WT differentially expressed genes, analyzed separately by cluster (left: Prog., right: Term.). Rank of genes at the bottom. Asterisks used to indicate significance correspond to the following: ns, not significant (P > 0.05), *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001.
Extended Data Fig. 5 Flow phenotyping of co-transferred exhausted EnhDel, Pdcd1-KO, and WT cells.
(a) Gating strategy for identification of live transferred EnhDel and Pdcd1-KO CD8+ T cells. (b) Representative flow cytometry plots of SLAMF6 versus TIM3 gating (left) and CX3CR1 gating (right) for Pdcd1-KO cells. (c) Representative flow cytometry plot of TNF staining in EnhDel (top left) versus Pdcd1-KO (bottom left) co-transfer, and EnhDel (top right) versus WT (bottom right) co-transfer. (d) Percentage of cells expressing TNF within the IFNγ+ population, quantified by subset. (Left) comparison of EnhDel versus Pdcd1-KO (n = 7); (right) EnhDel versus WT (n = 10). Two of two experiments combined, significance calculated using a paired Student’s t-test. Paired samples connected with lines. (e) Representative flow cytometry plot of KLRG1 staining in EnhDel (top left) versus Pdcd1-KO (bottom left) co-transfer, and EnhDel (top right) versus WT (bottom right) co-transfer. (f) Representative flow cytometry plot of Annexin V staining in EnhDel (top left) versus Pdcd1-KO (bottom left) co-transfer, and EnhDel (top right) versus WT (bottom right) co-transfer.
Extended Data Fig. 6 Quality control of Cytokine Bead Array assay.
(a) Gating strategy for identification of arrayed cytokine beads with example bead standard. (b) Example cytokine bead array flow cytometry plot of experimental serum sample.
Supplementary information
Supplementary Information
Supplementary table legends.
Supplementary Tables 1–6
Combined supplementary tables.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Weiss, S.A., Huang, A.Y., Fung, M.E. et al. Epigenetic tuning of PD-1 expression improves exhausted T cell function and viral control. Nat Immunol 25, 1871–1883 (2024). https://doi.org/10.1038/s41590-024-01961-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41590-024-01961-3
This article is cited by
-
Epigenetic regulation of T cell exhaustion in cancer
Nature Reviews Cancer (2026)
-
Revitalizing T cells: breakthroughs and challenges in overcoming T cell exhaustion
Signal Transduction and Targeted Therapy (2026)
-
Regulators of CD8+ T cell exhaustion
Nature Reviews Immunology (2026)
-
PD-L1/PD-1 checkpoint pathway regulates astrocyte morphogenesis and myelination during brain development
Molecular Psychiatry (2025)
-
Glofitamab combined with tislelizumab in relapsed/refractory diffuse large B-cell lymphoma: a single-center pilot trial
Blood Cancer Journal (2025)