Abstract
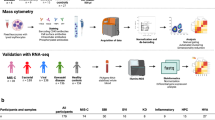
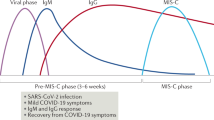
Recent reports highlight a new clinical syndrome in children related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1—multisystem inflammatory syndrome in children (MIS-C)—which comprises multiorgan dysfunction and systemic inflammation2,3,4,5,6,7,8,9,10,11,12,13. We performed peripheral leukocyte phenotyping in 25 children with MIS-C, in the acute (n = 23; worst illness within 72 h of admission), resolution (n = 14; clinical improvement) and convalescent (n = 10; first outpatient visit) phases of the illness and used samples from seven age-matched healthy controls for comparisons. Among the MIS-C cohort, 17 (68%) children were SARS-CoV-2 seropositive, suggesting previous SARS-CoV-2 infections14,15, and these children had more severe disease. In the acute phase of MIS-C, we observed high levels of interleukin-1β (IL-1β), IL-6, IL-8, IL-10, IL-17, interferon-γ and differential T and B cell subset lymphopenia. High CD64 expression on neutrophils and monocytes, and high HLA-DR expression on γδ and CD4+CCR7+ T cells in the acute phase, suggested that these immune cell populations were activated. Antigen-presenting cells had low HLA-DR and CD86 expression, potentially indicative of impaired antigen presentation. These features normalized over the resolution and convalescence phases. Overall, MIS-C presents as an immunopathogenic illness1 and appears distinct from Kawasaki disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
We have provided all of the deidentified raw data used in this report in the Supplementary Data, representing data in the figures and tables reported in the manuscript.
Change history
20 August 2020
In the supplementary information originally posted for this article, Supplementary Tables 1–4 and Supplementary Figs. 1–3 were missing. The error has been corrected online.
References
Rowley, A. H. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat. Rev. Immunol. 20, 453–454 (2020).
Verdoni, L. et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 395, 1771–1778 (2020).
Riphagen, S., Gomez, X., Gonzalez-Martinez, C., Wilkinson, N. & Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 395, 1607–1608 (2020).
Cabrero-Hernandez, M. et al. Severe SARS-CoV-2 infection in children with suspected acute abdomen: a case series from a tertiary hospital in Spain. Pediatr. Infect. Dis. J. 39, e195–e198 (2020).
Belhadjer, Z. et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation 142, 429–436 (2020).
Chiotos, K. et al. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: a case series. J. Pediatr. Infect. Dis. Soc. 9, 393–398 (2020).
Whittaker, E. et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 324, 259–269 (2020).
Ramcharan, T. et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr. Cardiol. 2020, 1–11 (2020).
Kaushik, S. et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection: a multi-institutional study from New York City. J. Pediatr. https://doi.org/10.1016/j.jpeds.2020.06.045 (2020).
Capone, C. A. et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood (MIS-C) associated with SARS-CoV-2 infection. J. Pediatr. https://doi.org/10.1016/j.jpeds.2020.06.044 (2020).
Toubiana, J. et al. Kawasaki-like multisystem inflammatory syndrome in children during the COVID-19 pandemic in Paris, France: prospective observational study. BMJ 369, m2094 (2020).
Feldstein, L. R. et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 383, 334–346 (2020).
Dufort, E. M. et al. Multisystem inflammatory syndrome in children in New York State. N. Engl. J. Med. 383, 347–358 (2020).
Sun, B. et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg. Microbes Infect. 9, 940–948 (2020).
Long, Q. X. et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 26, 845–848 (2020).
Guidance—Paediatric Multisystem Inflammatory Syndrome Temporally Associated with COVID-19 (PIMS) (Royal College of Paediatrics and Child Health, 2020); https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19
Dallaire, F. & Dahdah, N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J. Am. Soc. Echocardiogr. 24, 60–74 (2011).
Newburger, J. W., Takahashi, M. & Burns, J. C. Kawasaki disease. J. Am. Coll. Cardiol. 67, 1738–1749 (2016).
Rowley, A. H. & Shulman, S. T. The epidemiology and pathogenesis of Kawasaki disease. Front Pediatr. 6, 374 (2018).
Fernandez-Cooke, E. et al. Epidemiological and clinical features of Kawasaki disease in Spain over 5 years and risk factors for aneurysm development. (2011–2016): KAWA-RACE study group. PLoS ONE 14, e0215665 (2019).
Tacke, C. E. et al. Five years of Kawasaki disease in the Netherlands: a national surveillance study. Pediatr. Infect. Dis. J. 33, 793–797 (2014).
Long, Q. X. et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 26, 1200–1204 (2020).
Wang, Y. & Jonsson, F. Expression, role, and regulation of neutrophil fcγ receptors. Front. Immunol. 10, 1958 (2019).
Marini, O. et al. Mature CD10+ and immature CD10− neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood 129, 1343–1356 (2017).
Costa, S., Bevilacqua, D., Cassatella, M. A. & Scapini, P. Recent advances on the crosstalk between neutrophils and B or T lymphocytes. Immunology 156, 23–32 (2019).
Zanoni, I. & Granucci, F. Role of CD14 in host protection against infections and in metabolism regulation. Front. Cell Infect. Microbiol. 3, 32 (2013).
Krutmann, J. et al. Cross-linking Fc receptors on monocytes triggers IL-6 production. Role in anti-CD3-induced T cell activation. J. Immunol. 145, 1337–1342 (1990).
Tanaka, M. et al. Activation of FcγRI on monocytes triggers differentiation into immature dendritic cells that induce autoreactive T cell responses. J. Immunol. 183, 2349–2355 (2009).
Freer, G. & Matteucci, D. Influence of dendritic cells on viral pathogenicity. PLoS Pathog. 5, e1000384 (2009).
Maecker, H. T., McCoy, J. P. & Nussenblatt, R. Standardizing immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 12, 191–200 (2012).
Van den Broek, T., Borghans, J. A. M. & van Wijk, F. The full spectrum of human naive T cells. Nat. Rev. Immunol. 18, 363–373 (2018).
Zheng, J., Liu, Y., Lau, Y. L. & Tu, W. γδ-T cells: an unpolished sword in human anti-infection immunity. Cell. Mol. Immunol. 10, 50–57 (2013).
Vignali, D. A., Collison, L. W. & Workman, C. J. How regulatory T cells work. Nat. Rev. Immunol. 8, 523–532 (2008).
Dono, M., Cerruti, G. & Zupo, S. The CD5+ B-cell. Int. J. Biochem. Cell Biol. 36, 2105–2111 (2004).
Mathew, D. et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science https://doi.org/10.1126/science.abc8511 (2020).
Laing, A. G. et al. A consensus COVID-19 immune signature combines immuno-protection with discrete sepsis-like traits associated with poor prognosis. Preprint at medRxiv https://doi.org/10.1101/2020.06.08.20125112 (2020).
Matsuguma, C. et al. Dynamics of immunocyte activation during intravenous immunoglobulin treatment in Kawasaki disease. Scand. J. Rheumatol. 48, 491–496 (2019).
Lamers, M. M. et al. SARS-CoV-2 productively infects human gut enterocytes. Science 369, 50–54 (2020).
Ellinghaus, D. et al. Genomewide association study of severe Covid-19 with respiratory failure. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2020283 (2020).
Wan, Y. et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J. Virol. 94, e02015-19 (2020).
Liu, L. et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 4, e123158 (2019).
Davies, P. et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc. Health https://doi.org/10.1016/S2352-4642(20)30215-7 (2020).
Matics, T. J. & Sanchez-Pinto, L. N. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the Sepsis-3 definitions in critically Ill children. JAMA Pediatr. 171, e172352 (2017).
Cherian, T. et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull. World Health Organ. 83, 353–359 (2005).
Pickering, S. et al. Comparative assessment of multiple COVID-19 serological technologies supports continued evaluation of point-of-care lateral flow assays in hospital and community healthcare settings. Preprint at medRxiv https://doi.org/10.1101/2020.06.02.20120345 (2020).
Grehan, K., Ferrara, F. & Temperton, N. An optimised method for the production of MERS-CoV spike expressing viral pseudotypes. MethodsX 2, 379–384 (2015).
Seow, J. et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. Preprint at medRxiv https://doi.org/10.1101/2020.07.09.20148429 (2020).
Wickham, H. et al. Welcome to the Tidyverse. J. Open Source Softw. 4, 1686 (2019).
Kassambara, K. & Mundt, F. factoextra: Extract and visualize the results of multivariate data analyses. R package version 1.0.7 https://cran.r-project.org/web/packages/factoextra/index.html (2020).
Kolde, R. pheatmap: Pretty heatmaps. R package version 1.0.12 https://cran.r-project.org/web/packages/pheatmap/index.html (2019).
RStudio Team RStudio: Integrated Development for R (RStudio, 2020).
R Development Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2020); http://www.R-project.org/
Acknowledgements
We thank the parents and children who agreed to take part in this work. We thank J. Irons and the Evelina DIAMONDS Study team, the Evelina PIMS-TS Working Group and the Evelina Paediatric Research Team. We thank the flow cytometry core at the King’s College London (KCL) NIHR Biomedical Research Centre, and for logistical support we thank M. Brown, R. Ellis, S. Cochrane and C. Trouillet. We thank F. Krammer (Icahn School of Medicine at Mount Sinai) for provision of the RBD expression plasmid, P. Brouwer, M. van Gils and R. Sanders (University of Amsterdam) for the spike protein expression plasmid, and L. James, J. Luptak and L. Kiss (LMB, Cambridge) for the provision of purified nucleocapsid protein. The development of SARS-CoV-2 reagents (RBD) was partially supported by the NIAID Centers of Excellence for Influenza Research and Surveillance (CEIRS) contract HHSN272201400008C. The study was supported by the King’s Together COVID-19 seed funds (M.J.C., M.S.H., K.J.D., S.N. and M.H.M.). M.J.C. is supported by a National Institute for Health Research (NIHR) Academic Clinical Lectureship. M.F. is supported by a National Institute of Academic Anesthesia BJA/RCoA fellowship (WKRO-2018-0047). A.J. is supported by PhD funds from the Critical Care Department at Guy’s and St Thomas’ Hospital. S.A. was supported by an MRC-KCL Doctoral Training Partnership in Biomedical Sciences Industrial Collaborative Award in Science and Engineering (iCASE) in partnership with Orchard Therapeutics (MR/R015643/1). C.G. is supported by the MRC-KCL Doctoral Training Partnership in Biomedical Sciences (MR/N013700/1). M.S.-H. is supported by the NIHR Clinician Scientist Award (CS-2016-16-011). The views expressed in this publication are those of the authors and not necessarily those of the NHS, NIHR or Department of Health and Social Care. This research was funded/supported by the NIHR Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and KCL and/or the NIHR Clinical Research Facility. The funding sources did not have any role in the design, conduct or interpretation of the study results.
Author information
Authors and Affiliations
Contributions
M.J.C., P.W., J.K., M.F., A.J., S.M.T. and M.S.-H. had full access to all of the data in the study and take responsibility for the integrity of the data. K.J.D., S.A., J.S. and C.G. performed the ELISA and viral neutralization assays. M.F., A.J. and E.T. performed all of the flow cytometry optimization and analyses. M.J.C., M.F. and M.S.-H. take responsibility for the accuracy of the data analyses. M.J.C. and M.S.-H. conceived of and designed the study. M.J.C., M.F. and M.S.-H. performed the statistical analysis. M.J.C., M.F., A.J. and M.S.-H. drafted the manuscript. All authors contributed to acquisition, analysis and interpretation of the data and critical revision of the manuscript for important intellectual content. M.J.C., S.M.T. and M.S.-H. obtained the funding. S.M.T. and M.S.-H. provided administrative, technical or material support. M.S.-H. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Saheli Sadanand was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Context of the study cohort.
a, Epidemiology of SARS-CoV-2 infection in England prior to, and during the study period by region. Evelina London Children’s Hospital is in central London and provides tertiary immunology, infectious diseases, cardiology and intensive care to children in south-east England in collaboration with other tertiary centers (referred to ‘approximate catchment’). Data from the UK Government (https://coronavirus.data.gov.uk/) accessed on 22nd June 2020. ‘Index case’ denotes the first case of PIMS-TS identified at Evelina London Children’s Hospital. b, Flow chart of recruitment to the study cohort. c, Heat map of prevalence of clinical features in MIS-C (PIMS-TS in UK terminology, and Kawasaki-like disease in some continental European cohorts (Supplementary Table 4)) and two recent European Kawasaki disease (KD) cohorts. d, Clinical features of MIS-C / PIMS-TS in comparison with two recent European KD cohorts.
Extended Data Fig. 2 Time course analysis of Spike, N, RBD and Pseudoviral neutralization assay (plaque reduction neutralization test) by serology to SARS-CoV-2 in patients with MIS-C at T1, T2 and T3 phases.
a, IgG to S; b, IgG to N; c, IgG to RBD; children with MIS-C at T1 (acute), T2 (resolution) and T3 (convalescence). Seronegative and seropositive children are plotted as blue and red respectively; a single PCR positive child was plotted as an open red circle with cross. Antibody concentrations were measured by ELISA and reported as optical density (absorbance). d, Pseudoviral neutralization assay at T1, T2 and T3. Abbreviations: ID50, 50% inhibitory dose. In figures, box and whisker plots, bottom border represents 25th percentile; line bisecting the shaded region of each box represents median; upper border of the box, 75th percentile. The whiskers represent extreme 1.5 times the 75th (highest) and 25th (lowest) values and markers beyond the extreme line are outliers. Dots are colored by serology result; positive (red) and negative (blue). No statistical testing was done between patients by phase of illness (T1 (n = 21 samples), T2 (n = 11 samples), T3 (n = 7 samples).
Extended Data Fig. 3 Clinical biomarkers in patients with MIS-C at T1, T2 and T3 phases.
a, Serum ferritin concentrations. decreased from acute and resolution illness phases (T1 and T2) to convalescence (T3; normal range shown as dashed lines, 22–275 μg/L). b, Serum troponin T concentrations were raised in most children in the acute illness phase (T1) and normalized by convalescence (T3; threshold for acute myocardial infarction in adults shown as dashed line, 30 ng/L). c, Blood platelet count increased significantly across time points from a relative thrombocytopenia in the acute phase (T1) to thrombocytosis by convalescence (T3; normal range shown as dashed lines, 150–450 ×109/L). d, Serum D-dimer concentrations were elevated in the acute and resolution illness phases (T1 and T2) and decreased in convalescence (T3; upper threshold of normal shown as dashed line, 0.55 mg/L FEU). In figures, box and whisker plots, bottom border represents 25th percentile; line bisecting the shaded region of each box represents median; upper border of the box, 75th percentile. The whiskers represent extreme 1.5 times the 75th (highest) and 25th (lowest) values and markers beyond the extreme line are outliers. Dots are coloured by serology result; positive (red) and negative (blue), with the red asterisk indicating the patient positive by PCR for SARS-CoV-2. Significance testing between patients by phase of illness (T1 (n = 25 samples), T2 (n = 15 samples), T3 (n = 6 samples) was using two-sided Wilcoxon rank sum tests, with correction for multiple comparisons. Note log10 scales for a and b. Abbreviations: FEU, fibrinogen equivalent units.
Extended Data Fig. 4 Additional information on Innate immune cell alterations in patients with MIS-C at T1, T2 and T3 phases compared to age-matched healthy controls.
Innate immune alterations persist in MIS-C. a, Monocyte CD14 (toll-like receptor 4 co-receptor) expression (MFI) was significantly reduced in patients compared with healthy controls. b, Monocyte CD64 (Fc gamma receptor 1) expression (MFI) was increased in acute illness phase (T1) compared with healthy controls and later illness phases (T2 and T3). c, Classical monocyte proportions remained unchanged relative to healthy controls and illness phases. d, Classical monocytes had significantly reduced CD86 (T cell co-stimulant) expression (MFI) in acute and resolution illness phases (T1 and T2) in comparison with healthy controls and convalescence (T3). Abbreviations: MFI, median fluorescence intensity. In figures, box and whisker plots, bottom border represents 25th percentile; line bisecting the shaded region of each box represents median; upper border of the box, 75th percentile. The whiskers represent extreme 1.5 times the 75th (highest) and 25th (lowest) values and markers beyond the extreme line are outliers. Dots are colored by serology result; positive (red) and negative (blue), with the red asterisk indicating the patient positive by RT-PCR for SARS-CoV-2. Healthy Controls are shaded as grey and patients with MIS-C as yellow. Significance testing between patients by phase of illness (T1 (n = 23 samples), T2 (n = 14 samples), T3 (n = 10 samples) was using two-sided Wilcoxon rank sum tests, with correction for multiple comparisons.
Extended Data Fig. 5 Additional information on adaptive immune system alterations in patients with MIS-C at T1, T2 and T3 phases compared to age-matched healthy controls.
a, HLA-DR expression (MFI) on total CD8+ T cells; b, CD8+ CCR7- T cells; and c, CD8+ CCR7+ T cells; d, The percentage of CD8+ CCR7+ T cells; e, The percentage of CD8+ CCR7+ HLA-DR+; f, HLA-DR expression (MFI) on CD4+ CCR7- T cells; g, The percentage of CD4+ CCR7+ T cells; h, The percentage of HLA-RDR+ T regulatory cells (Treg); i, HLA-DR expression (MFI) on total CD4+ T cells; j, HLA-DR MFI on gamma-delta T cells; k, HLA-DR MFI of CD4+ CCR7- T cells was not correlated with IgG to Spike protein; l, HLA-DR MFI of CD4+ CCR7+ and CD8+ CCR7+ T cells was highly correlated; m, Naïve B cell count; n, The percentage of transitional B cells; o, The percentage of plasmablasts; p, The percentage of CD5+ B cells. In figures, box and whisker plots, bottom border represents 25th percentile; line bisecting the shaded region of each box represents median; upper border of the box, 75th percentile. The whiskers represent extreme 1.5 times the 75th (highest) and 25th (lowest) values and markers beyond the extreme line are outliers. Dots are colored by serology result; positive (red) and negative (blue), with the red asterisk indicating the patient positive by RT-PCR for SARS-CoV-2. Healthy Controls are shaded as grey and patients with MIS-C as yellow. Spearman’s rank test was used for correlations. Significance testing between patients by phase of illness (T1 (n = 23 samples), T2 (n = 14 samples), T3 (n = 10 samples) was using two-sided Wilcoxon rank sum tests, with correction for multiple comparisons.
Extended Data Fig. 6 Unsupervised analysis of flow cytometry immune parameters in patients with MIS-C at T1, T2 and T3 phases compared to age-matched healthy controls.
Principal component analysis (PCA) was performed on flow cytometry variables. a, Patient samples are plotted on PC1 and PC2 colored by time point: healthy control, T1 (acute), T2 (resolution), and T3 (convalescent) phases of illness. Shape of point represents SARS-CoV-2 serological status: circle = healthy control, triangle = negative, square = positive. Arrows represent transition of the same patient between serial sampling time points. b, Patient samples are plotted on PC1 and PC2 colored by immunotherapy administered: healthy control, IVIg only, IVIg and steroids+/- biologic, none, and steroids only. Arrows represent transition of the same patient between serial sampling time points. c, Contributions of the top 20 variables within the dataset to PC1 and PC2. d, Hierarchal clustering of flow cytometry immune parameters in MIS-C and healthy controls. The left-hand y axes denote immunotherapy administered; time point (T1, T2 or T3) or healthy control; and serological status. The right-hand y axis denotes patient number (P; as in main Fig. 1a). Abbreviations: IVIg, intravenous immunoglobulin; P. Biologics administered included tocilizumab, infliximab and anakinra (see main Fig. 1a).
Supplementary information
Supplementary Information
Supplementary Tables 1–4, Figs. 1–3 and STROBE Reporting Summary.
Supplementary Data
All of the deidentified raw data used in this report, representing data in the figures and tables.
Rights and permissions
About this article
Cite this article
Carter, M.J., Fish, M., Jennings, A. et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med 26, 1701–1707 (2020). https://doi.org/10.1038/s41591-020-1054-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-020-1054-6
This article is cited by
-
In depth characterisation of the proteome of MIS-C and post COVID-19 infection in children reveals inflammatory pathway activation and evidence of tissue damage
Journal of Translational Medicine (2025)
-
Aging shapes infection profiles of influenza A virus and SARS-CoV-2 in human precision-cut lung slices
Respiratory Research (2025)
-
Neutrophil heterogeneity in Kawasaki disease and multisystem inflammatory syndrome in children
Pediatric Research (2025)
-
An Update on Multi-System Inflammatory Syndrome in Children
Current Rheumatology Reports (2025)
-
Differentiating Kawasaki Disease and Multisystem Inflammatory Syndrome in Children Using Blood Composite Scores: Insights into Clinical Outcomes and Predictive Indices
Pediatric Cardiology (2025)