Abstract
Semaglutide reduces albuminuria and the risk of kidney disease progression in patients with type 2 diabetes and chronic kidney disease (CKD). We conducted a randomized placebo-controlled double-blind clinical trial in adults with CKD (estimated glomerular filtration rate (eGFR) ≥25 ml min−1 1.73 m−2 and urine albumin-to-creatinine ratio (UACR) ≥30 and <3,500 mg g−1) and body mass index ≥27 kg m−2. Participants were randomized to semaglutide 2.4 mg per week or placebo. The primary endpoint was percentage change from baseline in UACR at week 24. Safety was monitored throughout. Overall, 125 participants were screened, of whom 101 were randomized to semaglutide (n = 51) or placebo (n = 50). Mean age was 55.8 (s.d. 12) years; 40 participants (39.6%) were female; median UACR was 251 mg g−1 (interquartile range 100, 584); mean eGFR was 65.0 (s.d. 25) ml min−1 1.73 m−2; and mean body mass index was 36.2 (s.d. 5.6) kg m−2. Chronic glomerulonephritis (n = 25) and hypertensive CKD (n = 27) were the most common CKD etiologies. Treatment for 24 weeks with semaglutide compared to placebo reduced UACR by −52.1% (95% confidence interval −65.5, −33.4; P < 0.0001). Gastrointestinal adverse events were more often reported with semaglutide (n = 30) than with placebo (n = 15). Semaglutide treatment for 24 weeks resulted in a clinically meaningful reduction in albuminuria in patients with overweight/obesity and non-diabetic CKD. ClinicalTrials.gov registration: NCT04889183.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data of this clinical trial are accessible through the University of Groningen Datalogue catalogues (https://umcgresearchdatacatalogue.nl/l).
Code availability
Software code used for the statistical analysis is accessible through the University of Groningen Datalogue catalogues (https://umcgresearchdatacatalogue.nl/).
References
World Health Organization. Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2024).
Non-Communicable Disease Risk Factor Collaboration. Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 403, 1027–1050 (2024).
Global Burden Disease Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402, 203–234 (2023).
Kovesdy, C. P., Furth, S. L. & Zoccali, C & World Kidney Day Steering Committee. Obesity and kidney disease: hidden consequences of the epidemic. Kidney Int. 91, 260–262 (2017).
Garofalo, C. et al. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 91, 1224–1235 (2017).
Jiang, Z. et al. Obesity and chronic kidney disease. Am. J. Physiol. Endocrinol. Metab. 324, E24–E41 (2023).
Hall, J. E., do Carmo, J. M., da Silva, A. A., Wang, Z. & Hall, M. E. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat. Rev. Nephrol. 15, 367–385 (2019).
Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the evaluation and management of chronic kidney disease. Kidney Int. 105, S117–S314 (2024).
Kidney Disease: Improving Global Outcomes Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for diabetes management in chronic kidney disease. Kidney Int. 102, S1–S127 (2022).
Oshima, M. et al. Early change in albuminuria with canagliflozin predicts kidney and cardiovascular outcomes: a post hoc analysis from the CREDENCE trial. J. Am. Soc. Nephrol. 31, 2925–2936 (2020).
de Zeeuw, D. et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 65, 2309–2320 (2004).
Heerspink, H. J. et al. Is a reduction in albuminuria associated with renal and cardiovascular protection? A post hoc analysis of the ALTITUDE trial. Diabetes Obes. Metab. 18, 169–177 (2016).
Michos, E. D., Bakris, G. L., Rodbard, H. W. & Tuttle, K. R. Glucagon-like peptide-1 receptor agonists in diabetic kidney disease: a review of their kidney and heart protection. Am. J. Prev. Cardiol. 14, 100502 (2023).
Muskiet, M. H. A. et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat. Rev. Nephrol. 13, 605–628 (2017).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Lincoff, A. M. et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 389, 2221–2232 (2023).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 391, 109–121 (2024).
Colhoun, H. M. et al. Long-term kidney outcomes of semaglutide in obesity and cardiovascular disease in the SELECT trial. Nat. Med. 30, 2058–2066 (2024).
Heerspink, H. J. L. et al. Effects of semaglutide on albuminuria and kidney function in people with overweight or obesity with or without type 2 diabetes: exploratory analysis from the STEP 1, 2, and 3 trials. Diabetes Care 46, 801–810 (2023).
Shaman, A. M. et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation 145, 575–585 (2022).
Apperloo, E. M. et al. Effect of semaglutide on kidney function across different levels of baseline HbA1c, blood pressure, body weight and albuminuria in SUSTAIN 6 and PIONEER 6. Nephrol. Dial. Transplant. gfae150 (2024).
Tuttle, K. R. et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 6, 605–617 (2018).
Heerspink, H. J. L. et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 7, 128–139 (2019).
Mann, J. F. E. et al. Effects of semaglutide with and without concomitant SGLT2 inhibitor use in participants with type 2 diabetes and chronic kidney disease in the FLOW trial. Nat. Med. https://doi.org/10.1038/s41591-024-03133-0 (2024).
Neuen, B. L. et al. Cardiovascular, kidney and safety outcomes with GLP-1 receptor agonists alone and in combination with SGLT2 inhibitors in type 2 diabetes: a systematic review and meta-analysis. Circulation https://doi.org/10.1161/CIRCULATIONAHA.124.071689 (2024).
Apperloo, E. M. et al. Efficacy and safety of SGLT2 inhibitors with and without glucagon-like peptide 1 receptor agonists: a SMART-C collaborative meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 12, 545–557 (2024).
Heerspink, H. J. L. et al. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 10, 774–785 (2022).
Tuttle, K. R. et al. Body weight and eGFR during dulaglutide treatment in type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7). Diabetes Obes. Metab. 21, 1493–1497 (2019).
Heerspink, H. J. L. et al. Effects of tirzepatide versus insulin glargine on cystatin c-based kidney function: a SURPASS-4 post hoc analysis. Diabetes Care 46, 1501–1506 (2023).
Diabetes Care (American Diabetes Association, accessed 1 September 2024); https://diabetesjournals.org/diabetes/issue/73/Supplement_1
Li, K. et al. Effects of bariatric surgery on renal function in obese patients: a systematic review and meta analysis. PLoS ONE 11, e0163907 (2016).
Bilha, S. C. et al. The effects of bariatric surgery on renal outcomes: a systematic review and meta-analysis. Obes. Surg. 28, 3815–3833 (2018).
Clerte, M. et al. The measured glomerular filtration rate (mGFR) before and 6 months after bariatric surgery: a pilot study. Nephrol. Ther. 13, 160–167 (2017).
Fawaz, S. et al. Adiponectin reduces glomerular endothelial glycocalyx disruption and restores glomerular barrier function in a mouse model of type 2 diabetes. Diabetes 73, 964–976 (2024).
Sasson, A. N. & Cherney, D. Z. Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J. Diabetes 3, 1–6 (2012).
Chagnac, A. & Friedman, A. N. Measuring albuminuria in individuals with obesity: pitfalls of the urinary albumin-creatinine ratio. Kidney Med. 6, 100804 (2024).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Levey, A. S. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 130, 461–470 (1999).
Stevens, J. et al. UHPLC-MS/MS method for iohexol determination in human EDTA and lithium-heparin plasma, human urine and in goat- and pig EDTA plasma. Bioanalysis 12, 981–990 (2020).
Tuttle, K. R. et al. Efficacy and safety of aldosterone synthase inhibition with and without empagliflozin for chronic kidney disease: a randomised, controlled, phase 2 trial. Lancet 403, 379–390 (2024).
de Zeeuw, D. et al. Efficacy of a novel inhibitor of vascular adhesion protein-1 in reducing albuminuria in patients with diabetic kidney disease (ALBUM): a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 6, 925–933 (2018).
Heerspink, H. J. L. et al. Zibotentan in combination with dapagliflozin compared with dapagliflozin in patients with chronic kidney disease (ZENITH-CKD): a multicentre, randomised, active-controlled, phase 2b, clinical trial. Lancet 402, 2004–2017 (2023).
Heerspink, H. J. L. et al. Effect of avenciguat on albuminuria in patients with CKD: two randomized placebo-controlled trials. J. Am. Soc. Nephrol. 25, 1227–1239 (2024).
Heerspink, H. J. L. et al. Sparsentan in patients with IgA nephropathy: a prespecified interim analysis from a randomised, double-blind, active-controlled clinical trial. Lancet 401, 1584–1594 (2023).
Acknowledgements
We express our gratitude to all patients who participated in this trial. The authors thank J. Stevens, S. Galgey, H. Kamp-Nijmeijer, J. Beernink and B. Haandrikman for their continuous support in the conduct of this trial. D.Z.I.C. is supported by a Department of Medicine, University of Toronto Merit Award and receives support from the Canadian Institutes of Health Research (CIHR), Diabetes Canada and the Heart & Stroke/Richard Lewar Centre of Excellence in Cardiovascular Research. D.Z.I.C. is also the recipient of a 5-year CIHR-Kidney Foundation of Canada Team Grant award. The study was funded through a research grant from Novo Nordisk to the University Medical Center Groningen.
Author information
Authors and Affiliations
Contributions
E.M.A. and H.J.L.H. drafted the paper. N.J. conducted the analyses. All authors were involved in the study design, collection of the data and interpretation of the data and critically reviewed and edited the draft. The decision to submit the paper for publication was made jointly by all authors.
Corresponding author
Ethics declarations
Competing interests
E.M., F.W., J.M.C., M.L.-M., J.V., K.H. and A.v.d.A. report no conflicts of interest. J.L.G. declares funding to conduct clinical trials from AstraZeneca, Bayer, Boehringer Ingelheim and Novo Nordisk (all to the INCLIVA Research Institute); consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim and Novo Nordisk; and payment of honoraria for lectures from AstraZeneca, Novo Nordisk, Bayer, Menarini, Boehringer Ingelheim and Eli Lilly. N.J. received travel support from AstraZeneca. S.C.G. declares funding to conduct clinical trials from AstraZeneca, Bayer, Boehringer Ingelheim and Novo Nordisk (all to the FIDES Research Foundation) and payment of honoraria for lectures from AstraZeneca, Novo Nordisk, Baxter, Chiesi, ChemoCentrix, Clarion and Boehringer Ingelheim. M.J.P. received board speaker fees and travel expenses from AstraZeneca, NovoNordisk, Boehringer Ingelheim, Eli Lilly, Bayer and Menarini. G.D.L. received lecture fees from Sanofi, AstraZeneca and Janssen and has served as a consultant for AbbVie, Sanofi, Novo Nordisk, AstraZeneca, Boehringer Ingelheim and Merck Sharp & Dohme. A.v.B. declares being contracted via the University of Groningen (no personal payment) to undertake consultancy for Novo Nordisk, Eli Lilly and Boehringer Ingelheim. D.Z.I.C. has received honoraria from Boehringer Ingelheim, Eli Lilly, Merck, AstraZeneca, Sanofi, Mitsubishi-Tanabe, AbbVie, Janssen, Bayer, Prometic, Bristol Myers Squibb, Maze, Gilead, CSL-Behring, Otsuka, Novartis, Youngene and Novo Nordisk and has received operational funding for clinical trials from Boehringer Ingelheim, Eli Lilly, Merck, Janssen, Sanofi, AstraZeneca, CSL-Behring and Novo Nordisk. S.B.A. reports no conflicts of interest. She receives research support from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada. R.E.S. received grants to the institution from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim and NovoNordisk and speaker and advisor honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Servier and TAD. C.W. has received grants and served on steering committees for Boehringer Ingelheim; served on advisory boards for Boehringer Ingelheim, Merck Sharp & Dohme and Bayer; and received lecture fees from Boehringer Ingelheim, Eli Lilly, AstraZeneca, Merck Sharp & Dohme, Novo Nordisk and Bayer. H.J.L.H. is a consultant for AstraZeneca, Alexion, Bayer, Boehringer Ingelheim, CSL-Behring, DIMERIX, Eli Lilly, Gilead, Janssen, Novartis, Novo Nordisk, Roche, Travere Pharmaceuticals and VIFOR Pharma. He received research support through his institution from AstraZeneca, Boehringer Ingelheim, Janssen and Novo Nordisk. He received lecture fees from AstraZeneca, Bayer and Novo Nordisk.
Peer review
Peer review information
Nature Medicine thanks Qiwei Li, Carel le Roux and Katherine Tuttle for their contribution to the peer review of this work. Primary Handling Editor: Sonia Muliyil, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
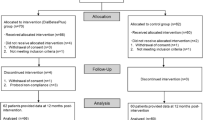
Extended Data Fig. 1 Patient disposition.
Of 125 participants assessed for eligibility, 101 were randomly assigned to semaglutide (n = 51) or placebo (n = 50). Of these, 47 participants in the semaglutide group and 46 participants in the placebo group completed the study.
Extended Data Fig. 2 Urinary Albumin Concentration over time.
Change from baseline in urinary albumin concentration over the study treatment period. Adjusted geometric mean change from baseline in the semaglutide and placebo group is shown. The error bars represent 95% confidence interval.
Extended Data Fig. 3 eGFR in subset of participants.
Change in eGFR in 47 participants with iohexol measured GFR. The effect of semaglutide compared to placebo on estimated GFR was calculated using a MMRM model with two-sided P-values and with fixed effects for treatment, visit, baseline log-transformed UACR, the interaction between treatment and visit, and the interaction between baseline and estimated GFR. Numbers in the lower panel represent the numbers contributing to the mean.
Extended Data Fig. 4 Diastolic blood pressure over time.
Change from baseline in diastolic blood pressure. The effect of semaglutide compared to placebo on estimated diastolic blood pressure was calculated using a MMRM model with two-sided P-values and with fixed effects for treatment, visit, baseline log-transformed UACR, the interaction between treatment and visit, and the interaction between baseline and diastolic blood pressure. Numbers in the lower panel represent the numbers contributing to the mean.
Extended Data Fig. 5 Correlation graphs.
Correlation between changes after 24 weeks treatment in body weight and creatinine eGFR (A), cystatin-C eGFR (B) and iohexol mGFR (C). The correlations were calculated with Spearman correlation. Two-side p-values are reported.
Supplementary information
Supplementary Information
SMART investigator teams.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Apperloo, E.M., Gorriz, J.L., Soler, M.J. et al. Semaglutide in patients with overweight or obesity and chronic kidney disease without diabetes: a randomized double-blind placebo-controlled clinical trial. Nat Med 31, 278–285 (2025). https://doi.org/10.1038/s41591-024-03327-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-024-03327-6