Abstract
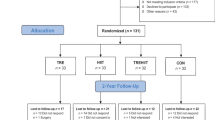
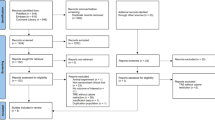
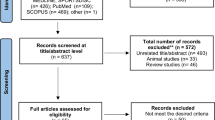
The optimal eating window for time-restricted eating (TRE) remains unclear, particularly its impact on visceral adipose tissue (VAT), which is associated with cardiometabolic morbidity and mortality. We investigated the effects of three TRE schedules (8 h windows in the early day, late day and participant-chosen times) combined with usual care (UC, based on education about the Mediterranean diet) versus UC alone over 12 weeks in adults with overweight or obesity. The primary outcome was VAT changes measured by magnetic resonance imaging. A total of 197 participants were randomized to UC (n = 49), early TRE (n = 49), late TRE (n = 52) or self-selected TRE (n = 47). No significant differences were found in VAT changes between early TRE (mean difference (MD): −4%; 95% confidence interval (CI), −12 to 4; P = 0.87), late TRE (MD: −6%; 95% CI, −13 to 2; P = 0.31) and self-selected TRE (MD: −3%; 95% CI, −11 to 5; P ≥ 0.99) compared with UC, nor among the TRE groups (all P ≥ 0.99). No serious adverse events occurred; five participants reported mild adverse events. Adherence was high (85–88%) across TRE groups. These findings suggest that adding TRE, irrespective of eating window timing, offers no additional benefit over a Mediterranean diet alone in reducing VAT. TRE appears to be a safe, well-tolerated and feasible dietary approach for adults with overweight or obesity. ClinicalTrials.gov registration: NCT05310721.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Due to privacy concerns, the datasets used in this study are not publicly available; however, researchers can request access to specific individual-level data for academic use only, within 36 months following the publication date, after de-identification. Proposals should be directed to corresponding authors J.R.R. and I.L. Upon proposal acceptance, requesters will be granted access to the data after signing a data access agreement.
Code availability
The R script for cleaning continuous glucose monitoring data is available via GitHub at https://github.com/DIVA-soft/CGManalyzer. The R script for analyzing data from accelerometers and continuous glucose monitors that have been time aligned are available via GitHub at https://github.com/DIVA-soft/GGIRmatcher. Future versions of these repositories have been migrated to https://github.com/PROFITH/cleanCGM and https://github.com/PROFITH/GGIRmatcher.
References
WHO. Obesity and overweight https://www.who.int/health-topics/obesity (2020).
NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 403, 1027–1050 (2024).
Bray, G. A. et al. The science of obesity management: an Endocrine Society scientific statement. Endocr. Rev. 39, 79–132 (2018).
Finkelstein, E. A., Trogdon, J. G., Cohen, J. W. & Dietz, W. Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff. 28, w822–w831 (2009).
Flanagan, E. W., Most, J., Mey, J. T. & Redman, L. M. Calorie restriction and aging in humans. Annu. Rev. Nutr. 40, 105–133 (2020).
Dote-Montero, M., Sanchez-Delgado, G. & Ravussin, E. Effects of intermittent fasting on cardiometabolic health: an energy metabolism perspective. Nutrients 14, 489 (2022).
Ezpeleta, M. et al. Time-restricted eating: watching the clock to treat obesity. Cell Metab. 36, 301–314 (2024).
de Cabo, R. & Mattson, M. P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 381, 2541–2551 (2019).
Liu, L., Chen, W., Wu, D. & Hu, F. Metabolic efficacy of time-restricted eating in adults: a systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 107, 3428–3441 (2022).
Liu, D. et al. Calorie restriction with or without time-restricted eating in weight loss. N. Engl. J. Med. 386, 1495–1504 (2022).
Wei, X. et al. Effects of time-restricted eating on nonalcoholic fatty liver disease: the TREATY-FLD Randomized Clinical Trial. JAMA Netw. Open 6, e233513 (2023).
Deng, Y. et al. Effects of time-restricted eating on intrahepatic fat and metabolic health among patients with nonalcoholic fatty liver disease. Obesity 32, 494–505 (2024).
Neeland, I. J. et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 7, 715–725 (2019).
Sutton, E. F. et al. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 27, 1212–1221.e1213 (2018).
Liu, J., Yi, P. & Liu, F. The effect of early time-restricted eating vs. later time-restricted eating on weight loss and metabolic health: a network meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 108, 1824–1834 (2023).
Xie, Z. et al. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat. Commun. 13, 1003 (2022).
Zhang, L. M. et al. Randomized controlled trial for time-restricted eating in overweight and obese young adults. iScience 25, 104870 (2022).
Queiroz, J. D. N. et al. Cardiometabolic effects of early v. delayed time-restricted eating plus energetic restriction in adults with overweight and obesity: an exploratory randomised clinical trial. Br. J. Nutr. 129, 637–649 (2023).
He, M. et al. Time-restricted eating with or without low-carbohydrate diet reduces visceral fat and improves metabolic syndrome: a randomized trial. Cell Rep. Med. 3, 100777 (2022).
Pavlou, V. et al. Effect of time-restricted eating on weight loss in adults with type 2 diabetes: a randomized clinical trial. JAMA Netw. Open 6, e2339337 (2023).
Lin, S. et al. Time-restricted eating without calorie counting for weight loss in a racially diverse population: a randomized controlled trial. Ann. Intern. Med. 176, 885–895 (2023).
Jamshed, H. et al. Effectiveness of early time-restricted eating for weight loss, fat loss, and cardiometabolic health in adults with obesity: a randomized clinical trial. JAMA Intern. Med. 182, 953–962 (2022).
Lewis, P., Oster, H., Korf, H. W., Foster, R. G. & Erren, T. C. Food as a circadian time cue—evidence from human studies. Nat. Rev. Endocrinol. 16, 213–223 (2020).
Lopez-Minguez, J., Gómez-Abellán, P. & Garaulet, M. Timing of breakfast, lunch, and dinner. Effects on obesity and metabolic risk. Nutrients 11, 2624 (2019).
Dote-Montero, M. et al. Efficacy of different 8 h time-restricted eating schedules on visceral adipose tissue and cardiometabolic health: a study protocol. Nutr., Metab. Cardiovasc. Dis. 34, 177–187 (2024).
Silva, V. S. D. & Vieira, M. F. S. International Society for the Advancement of Kinanthropometry (ISAK) Global: international accreditation scheme of the competent anthropometrist. Rev. Bras. Cineantropom. Desempenho Hum. 22, e70517 (2020).
Stergiou, G. S. et al. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J. Hypertens. 39, 1293–1302 (2021).
Battelino, T. et al. Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol. 11, 42–57 (2023).
Martínez-González, M. A. et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS ONE 7, e43134 (2012).
Estruch, R. et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 378, e34 (2018).
Bull, F. C. et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 54, 1451–1462 (2020).
Verheggen, R. J. et al. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes. Rev. 17, 664–690 (2016).
Ross, R. et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann. Intern. Med. 133, 92–103 (2000).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Acknowledgements
This study (project reference PID2022.141506OB.I00) is funded by the MCIU/AEI /10.13039/501100011033 and by ERDF EU A Way of Making Europe to J.R.R.; Junta de Andalucía, Consejería de Transformación económica, Industria, Conocimiento y Universidades (A-CTS-516-UGR20) to J.R.R.; the University of Granada Plan Propio de Investigación-Excellence actions: Unit of Excellence on Exercise Nutrition and Health (UCEENS) to J.R.R.; the Consejo Superior de Deportes, Ministerio de Cultura y Deporte (project reference EXP_75091), Plan de Recuperación, Transformación y Resiliencia -Funded by the European Union – NextGenerationEU to I.L.; the Government of Navarra, Departamento de Desarrollo Economico y Empresarial (0011-1365-2021-00070), Plan de Promoción de Grupos de Investigación de la Universidad Pública de Navarra to I.L.; Juan de la Cierva Formación (FJC2020-044453-I to J.M.A.A. and FJC2020-043385-I to A.C.-C.) funded by the Ministerio de Ciencia e Innovación and by the European Union NextGenerationEU/PRTR; Recualification of the Spanish University System 2021–2023 from the Spanish Ministry of Universities (RD 289/2021 to R. Cupeiro), funded by the European Union NextGenerationEU; Spanish Ministry of Universities (FPU 18/03357 to M.D.-M. and FPU21/01161 to A.C.J.); Spanish Ministry of Science, Innovation and Universities under Beatriz Galindo’s 2022 fellowship program (ref: BG22/00075 to J.H.M.). In addition, funding was provided from the EXERNET Research Network on Exercise and Health (DEP2005-00046/ACTI; 09/UPB/19; 45/UPB/20; 27/UPB/21 to I.L. and J.R.R.). R.d.C. is funded through the Intramural Research Program of the National Institute on Aging. This work is part of a doctorate thesis conducted in the Official Doctoral Program in Biomedicine of the University of Granada, Spain.
Author information
Authors and Affiliations
Contributions
M.D.-M.: conceptualization, methodology, project administration, investigation and writing—original draft. A.C.-J., E.M.-R., M.O., J.E. and A.C.-C.: project administration, investigation and writing—review and editing. M.C., J.M.A.A., A.L.-V., R. Cupeiro, A.D.l.O., P.V.G.P., V.C.-B., A.M.-G., A.Z., E.P., N.Á.d.E., E.G., C.A.B., M.T.G.C., J.L.M.-R., F.I. and R. Cabeza: investigation and writing—review and editing. F.J.A.-G.: methodology, investigation and writing—review and editing. J.H.M. and A.C.-B.: statistical analysis and writing—review and editing. R.d.C.: writing—review and editing. M.M.-T.: supervision, funding acquisition and writing—review and editing. I.L.: conceptualization, methodology, project administration and supervision, funding acquisition and writing—review and editing. J.R.R.: conceptualization, methodology, project administration and supervision, funding acquisition and writing—original draft.
Corresponding authors
Ethics declarations
Competing interests
J.R.R. has received lecture fees from Novo Nordisk and Abbott for research unrelated to this study. All other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Leonie Heilbronn, Alexandra Johnstone and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Sonia Muliyil, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Estimated changes in time (min/day) in MVPA, LPA, SB, and sleep, as measured by accelerometry among the usual care (A), early TRE (B), late TRE (C), and self–selected TRE (D) groups after the 12–week intervention.
Two-sided P value from multivariate analysis of variance with Lawley-Hotelling trace test. Changes were estimated from the isometric log-ratio coordinates between the group compositional means at the 12-week measurement relative the overall compositional mean at baseline. MVPA, moderate-to-vigorous physical activity; LPA, light physical activity; SB, sedentary behavior; TRE, time-restricted eating.
Extended Data Fig. 2 Median eating window time (hours; A), eating window length (hours; B), and percentage of adherent days (%; C) among the UC, early TRE, late TRE, and self–selected TRE groups over the 12–week intervention period.
Panel A represents the median eating windows for participants across the usual care (UC), early time–restricted eating (TRE), late TRE, and self–selected TRE groups, with the intensity of colors representing adherence to the protocol. Black dotted rectangles indicate group–median eating windows. Panel B shows the duration of the eating window for each intervention group (violin plot), with dots representing the distribution of individual data. Panel C illustrates the percentage of adherent days per group over the 12–week intervention period (adherence defined as an eating window ≥12 hours for usual care and ≤9 hours for TRE groups), with dots representing the distribution of individual data. Data in the Panel B are median, interquartile range, and 1.5x interquartile range. Data in panel C are means with 95% confidence intervals. Statistical analysis applied was analysis of variance with post–hoc Bonferroni analysis for significant differences between groups (P < 0.05). The P values reported are two-sided and Bonferroni-adjusted P values. Groups sharing similar letters denote significant differences. This figure includes only participants with eating window records for more than 60% of the intervention days.
Extended Data Fig. 3 Changes in VAT volume (cm3; A and F), VAT percentage (%; B and G), body weight (kg; C and H), fat–free mass (kg; D and I), and fat mass (kg; E and J) in men and women among the UC, early TRE, late TRE, and self–selected TRE groups after the 12-week intervention.
Data are raw means with 95% confidence interval. Similar letters represent significant differences between groups as determined by post-hoc Bonferroni correction for multiple comparisons (P < 0.05). The P values reported are two-sided and Bonferroni-adjusted P values. Secondary analysis (body weight changes in kilograms) between early time-restricted eating (TRE) and usual care (UC, men: P < 0.008; women: P = 0.003), late TRE and UC (women: P = 0.005), and self-selected TRE and UC (men: P < 0.001). Changes were calculated as the difference between postintervention and preintervention values. VAT percentage indicates changes relative to preintervention values. Intervention effects on primary and secondary outcomes at 3 months after the intervention were assessed based on repeated-measures linear mixed-effects multilevel models, which included random cluster (site) effects. Individual measures of change were therefore modelled as the function of randomly assigned group, site, assessment time, and their interaction terms, stratifying the analysis by sex (that is, separately for men and women). Model-based estimations were performed with an intention-to-treat approach using the restricted maximum-likelihood method, the model assuming that missing values were missing-at-random.
Extended Data Fig. 4 Glucose levels during a 24–hour period as measured by continuous glucose monitoring over 14 days in both the baseline and the last 2 weeks of the 12–week intervention for men and women in the usual care (A and E), early TRE (B and F), late TRE (C and G), and self-selected TRE (D and H) groups.
The solid lines represent mean glucose levels, and the shaded area shows the 95% confidence interval. The color intensity of the eating window for time-restricted eating (TRE) groups corresponds to the number of participants having their meals at that specific time of day.
Extended Data Fig. 5 Estimated changes in time (min/day) in MVPA, LPA, SB, and sleep, as measured by accelerometry in men and women among the usual care (A and E), early TRE (B and F), late TRE (C and G), and self–selected TRE (D and H) groups after the 12–week intervention.
Two-sided P value from multivariate analysis of variance with Lawley-Hotelling trace test, stratifying the analysis by sex (that is, separately for men and women). Changes were estimated from the isometric log-ratio coordinates between the group compositional means at the 12-week measurement relative the overall compositional mean at baseline. MVPA, moderate-to-vigorous physical activity; LPA, light physical activity; SB, sedentary behavior; TRE, time-restricted eating.
Extended Data Fig. 6 Median eating window time (hours; A), eating window length (hours; B), and percentage of adherent days (%; C) for men among the UC, early TRE, late TRE, and self–selected TRE groups over the 12–week intervention period.
Panel A represents the median eating windows for men across the usual care (UC), early time–restricted eating (TRE), late TRE, and self–selected TRE groups, with the intensity of colors representing adherence to the protocol. Black dotted rectangles indicate group–median eating windows. Panel B shows the duration of the eating window for each intervention group (violin plot), with dots representing the distribution of individual data. Panel C illustrates the percentage of adherent days per group over the 12–week intervention period (adherence defined as an eating window ≥12 hours for usual care and ≤9 hours for TRE groups), with dots representing the distribution of individual data. Data in the Panel B are median, interquartile range, and 1.5x interquartile range. Data in panel C are means with 95% confidence intervals. Statistical analysis applied was analysis of variance with post–hoc Bonferroni analysis for significant differences between groups (P < 0.05). The P values reported are two-sided and Bonferroni-adjusted P values. Groups sharing similar letters denote significant differences. This figure includes only participants with eating window records for more than 60% of the intervention days.
Extended Data Fig. 7 Median eating window time (hours; A), eating window length (hours; B), and percentage of adherent days (%; C) for women among the UC, early TRE, late TRE, and self–selected TRE groups over the 12–week intervention period.
Panel A represents the median eating windows for women across the usual care (UC), early time–restricted eating (TRE), late TRE, and self–selected TRE groups, with the intensity of colors representing adherence to the protocol. Black dotted rectangles indicate group–median eating windows. Panel B shows the duration of the eating window for each intervention group (violin plot), with dots representing the distribution of individual data. Panel C illustrates the percentage of adherent days per group over the 12–week intervention period (adherence defined as an eating window ≥12 hours for usual care and ≤9 hours for TRE groups), with dots representing the distribution of individual data. Data in the Panel B are median, interquartile range, and 1.5x interquartile range. Data in panel C are means with 95% confidence intervals. Statistical analysis applied was analysis of variance with post–hoc Bonferroni analysis for significant differences between groups (P < 0.05). The P values reported are two-sided and Bonferroni-adjusted P values. Groups sharing similar letters denote significant differences. This figure includes only participants with eating window records for more than 60% of the intervention days.
Supplementary information
Supplementary Information
Supplementary Tables 1–7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dote-Montero, M., Clavero-Jimeno, A., Merchán-Ramírez, E. et al. Effects of early, late and self-selected time-restricted eating on visceral adipose tissue and cardiometabolic health in participants with overweight or obesity: a randomized controlled trial. Nat Med 31, 524–533 (2025). https://doi.org/10.1038/s41591-024-03375-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41591-024-03375-y
This article is cited by
-
The impact of dietary interventions on cardiometabolic health
Cardiovascular Diabetology (2025)
-
Wellness advantages of religious fasting practices: a narrative review of the literature
Nutrire (2025)
-
Intermittent fasting is good for losing (some) weight
Nature Medicine (2025)
-
Sleep and circadian rhythms in cardiovascular resilience: mechanisms, implications, and a Roadmap for research and interventions
Nature Reviews Cardiology (2025)
-
Fasting mimicking diet cycles versus a Mediterranean diet and cardiometabolic risk in overweight and obese hypertensive subjects: a randomized clinical trial
npj Metabolic Health and Disease (2023)