Abstract
Coronary artery disease (CAD) is a major cause of ill health and death worldwide. Coronary computed tomographic angiography (CCTA) is the first-line investigation to detect CAD in symptomatic patients. This diagnostic approach risks greater second-line heart tests and treatments at a cost to the patient and health system. The National Health Service funded use of an artificial intelligence (AI) diagnostic tool, computed tomography (CT)-derived fractional flow reserve (FFR-CT), in patients with chest pain to improve physician decision-making and reduce downstream tests. This observational cohort study assessed the impact of FFR-CT on cardiovascular outcomes by including all patients investigated with CCTA during the national AI implementation program at 27 hospitals (CCTA n = 90,553 and FFR-CT n = 7,863). FFR-CT was safe, with no difference in all-cause (n = 1,134 (3.2%) versus 1,612 (2.9%), adjusted-hazard ratio (aHR) 1.00 (0.93–1.08), P = 0.97) or cardiovascular mortality (n = 465 (1.3%) versus 617 (1.1%), aHR 0.96 (0.85–1.08), P = 0.48), while reducing invasive coronary angiograms (n = 5,720 (16%) versus 8,183 (14.9%), aHR 0.93 (0.90–0.97), P < 0.001) and noninvasive cardiac tests (189/1,000 patients versus 167/1,000), P < 0.001). Implementation of an AI-diagnostic tool as part of a health intervention program was safe and beneficial to the patient pathway and health system with fewer cardiac tests at 2 years.
Similar content being viewed by others
Main
Coronary artery disease (CAD) remains a major cause of symptoms, morbidity and death worldwide. International guidelines endorse coronary computed tomography angiography (CCTA) as a first-line investigation for patients with suspected stable symptomatic CAD, principally to diagnose or exclude CAD1,2. The National Institute of Health and Care Excellence (NICE) in the UK was the first to recommend this diagnostic strategy for patients with possible anginal symptoms and no known CAD3. This decision was controversial, as CCTA has limited ability to link the presence of obstructive CAD to symptoms such as angina, and evidence from health systems where CCTA was adopted early showed that increased inappropriate invasive coronary angiograms (ICAs) and percutaneous coronary interventions (PCIs) could be a consequence of this diagnostic pathway4.
To further improve the CCTA diagnostic pathway and reduce the risk of unnecessary future tests or revascularizations, NICE subsequently recommended a new artificial intelligence (AI)-augmented technology, computed tomography (CT)-derived fractional flow reserve (FFR-CT) (HeartFlow), to be used on CCTA images when there was the presence of possibly flow-limiting CAD5. FFR-CT utilizes CCTA raw images to generate an AI deep-learned coronary segmentation that is combined with a three-dimensional computational fluid dynamics model of coronary arterial blood flow6,7. This technology is dependent on high-quality imaging and close adherence to the recommended guidelines on performing CCTA to produce an accurate three-dimensional model. FFR-CT models the combined anatomy and physiology of coronary arteries to inform the interpreting physician whether any CAD seen is probably flow limiting and, thus, causing patient symptoms. Several randomized controlled trials have demonstrated the diagnostic accuracy of FFR-CT and its potential to transform the patient chest pain pathway by reducing unnecessary ICA at a modeled neutral cost8,9.
NICE considered the evidence for the use of the AI technology based on its clinical effectiveness, system impact on the National Health Service (NHS) and likely cost–benefit, stating that FFR-CT had high accuracy, would reduce unnecessary invasive tests and save the NHS £9.1 million per year5. Despite this, the uptake of FFR-CT in clinical practice was limited due to the cost of the technology, hospital funding constraints and a ‘black box’ component to the external AI analysis10. The challenges to implementing AI decision support systems have been recognized globally, including at the 2024 Responsible AI for Social and Ethical Healthcare conference11.
In an attempt to address these obstacles, NHS England launched an innovation and technology program to centrally fund the use of new AI technologies within the national healthcare system. FFR-CT was chosen with the aim of improving patient care by avoiding unnecessary downstream tests, while benefiting an overburdened healthcare system. This retrospective quasi-experimental observational cohort study was designed to examine whether this specific health intervention of implementing an AI tool (FFR-CT) in a national health system was clinically useful and safe by improving clinical and health system outcomes12,13.
Results
Patient characteristics
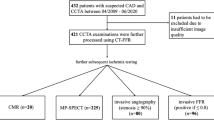
Between April 2017 and December 2020, 102,616 CCTAs were performed at 27 sites across a widespread geographic distribution, at secondary and tertiary hospitals in England that are representative of NHS clinical practice (Extended Data Figs. 1 and 2). There were 289 (0.28%) patients without an NHS number, 5,674 (5.5%) patients withdrew their consent, 6,100 (5.9%) CCTA were repeat studies on the same patient during the study period and 20 (0.0001%) patients had a post-mortem CCTA. The final study population of 90,553 patients consisted of 35,688 who had undergone CCTA before the introduction of FFR-CT, and 54,865 CCTA after FFR-CT was available at their hospital (Fig. 1). The mean age was 58 ± 13 years, 48.1% female, with varied ethnicity (78.7% white British or Irish, 2.2% black, 1.4% mixed race, 8.2% Asian, 2.4% other, 7.1% unstated).
The median follow-up for the total population was 1,211 (interquartile range (IQR) 535) days, with 98.1% (n = 88,842) completing 2 years follow-up. The cohorts were well matched for demographic and cardiovascular disease risk factors (Table 1) with clinically small but statistically significant differences in patients’ age, hypertension, heart failure, valve disease, chronic obstructive pulmonary disease (COPD) and chronic kidney disease. Among 54,865 CCTA patients who had FFR-CT testing available to their hospital, 7,863 (14.1%) went on to undergo FFR-CT analysis. This cohort were older (63 (IQR 55–71) years), with greater cardiovascular risk factors compared with the total population as they were selected for further testing on the basis of the presence of CAD (Supplementary Table 1).
All-cause mortality and cardiovascular outcomes
There were 2,746 deaths, of which 1,082 were cardiovascular, 1,129 myocardial infarction (MI) and 13,903 ICA performed at 2 years. The 90-day, 1-year and 2-year numbers (%) for each group are reported in Table 2.
There was lower all-cause (n = 1,134 (3.2%) versus 1,612 (2.9%), hazard ratio (HR) 0.92 (0.856–0.996), P = 0.04) and cardiovascular mortality (n = 465 (1.3%) versus 617 (1.1%), HR 0.86 (0.765–0.973), P = 0.02) rates observed in the FFR-CT available group at 2 years compared with the unavailable group (Fig. 2). There was no significant difference in MI events (n = 425 (1.2%) versus 704 (1.3%), HR 1.08 (0.96–1.22), P = 0.24). Rates of all ICA inclusive of those progressing to revascularization (n = 5,720 (16.0%) versus 8,183 (14.9%), HR 0.92 (0.89–0.96), P < 0.001) and ICA with no subsequent revascularization (n = 3,117 (8.7%) versus 4,002 (7.3%), HR 0.83 (0.79–0.87), P < 0.001) were significantly lower in the FFR-CT available group.
Nonbalanced prognostic factors at baseline were entered into a multivariable Cox-regression model (age, hypertension, heart failure, COPD, valve disease and chronic kidney disease). Once adjusted for baseline differences in co-morbidities, there was no significant difference in all-cause (adjusted HR (aHR) 1.00 (0.93–1.08), P = 0.97) or cardiovascular mortality (aHR 0.96 (0.85–1.08), P = 0.48) risk between the FFR-CT available and FFR-CT unavailable groups at 2 years. Adjusted risk of MI was higher in the FFR-CT available group (aHR 1.18 (1.05–1.34), P = 0.006) (Table 2 and Extended Data Fig. 3). The risk reduction in all ICA (aHR 0.93 (0.90–0.97), P < 0.001) and ICA without revascularization (aHR 0.84 (0.80–0.88), P < 0.001) remained after covariate adjustment (Table 2) and sensitivity analysis (Supplementary Table 2). Assessment of the proportional hazards assumption by testing for a zero slope in the scaled Schoenfeld residuals for each Cox model showed proportionality for all outcomes. Propensity score matching (PSM) resulted in two cohorts of 30,665 patients (FFR-CT unavailable and FFR-CT available) (Extended Data Fig. 4 and Supplementary Table 3). The PSM analysis showed that all-cause, cardiovascular death and MI at 2 years did not significantly differ (P = 0.95, P = 0.85 and P = 0.17) between groups. Lower all ICA and ICA without revascularization was still observed in the FFR-CT available cohort compared with the unavailable cohort (P < 0.005 and P < 0.001) (Extended Data Fig. 5).
Downstream secondary cardiovascular tests (excluding ICA) were selected from 92 different diagnostic codes (Supplementary Table 5). These cardiac imaging modalities were subcategorized into cardiovascular magnetic resonance imaging (MRI), cardiac CT, nuclear medicine, stress echocardiography and invasive intracoronary imaging (optical coherence tomography, intravascular ultrasound and invasive FFR). A total of 15,942 subsequent non-ICA cardiovascular tests were performed within 2 years of the index CCTA (178.5/1,000 patients). Noninvasive cardiovascular tests performed were lower at 2 years after FFR-CT (n = 6,777 (189/1,000 patients) versus 9,169 (167/1,000), P < 0.001) with a 12% relative risk reduction in the FFR-CT available cohort (HR 0.88 (0.85–0.92), P < 0.001) of having a downstream test. There was reduced likelihood of having a repeat cardiac CT (HR 0.87 (0.80–0.93), P < 0.001), second-line stress echocardiogram (HR 0.52 (0.44–0.62), P < 0.001) or nuclear stress testing (HR 0.61 (0.56–0.67), P < 0.001). The number of cardiovascular magnetic resonance scans performed was higher in the FFR-CT available cohort (HR 1.06 (1.00–1.13), P = 0.042). Invasive intracoronary imaging represented a small number of second-line tests (4.6/1,000 patients), but these increased significantly (HR 1.70 (1.36–2.12), P < 0.001) after FFR-CT availability (Fig. 3).
Subcategorized into noninvasive tests (cardiovascular MRI, cardiac CT, nuclear medicine (positron emission tomography and radionuclide imaging) and echocardiography (excluding transthoracic echocardiography)) and invasive tests (total ICA and intracoronary imaging (optical coherence tomography (OCT), intravascular ultrasound (IVUS) and invasive FFR (FFR))). The error bars indicate the 95% CIs.
Coronary revascularization
There was an early increase in PCI that was sustained at 2 years in the FFR-CT available group (n = 1,912 (5.4%) versus 3,161 (5.8%), aHR 1.09 (1.03–1.15, P = 0.002) (Table 2 and Supplementary Fig. 3). The likelihood of receiving coronary artery bypass grafting (CABG) did not change (n = 691 (1.9%) versus 1,020 (1.9%), aHR 1.01 (0.91–1.11), P = 0.89). Total revascularization rates (PCI and CABG) were higher in the FFR-CT available group (n = 2,603 (7.3%) versus 4,181 (7.6%), aHR 1.06 (1.01–1.11), P = 0.02). The proportion of patients going to ICA who received revascularization (revascularization ratio) was higher in the FFR-CT available cohort (48.3% versus 52.9%, P < 0.001) (Table 2). There was no significant difference in the treatment time from CCTA to revascularization between the FFR-CT unavailable and FFR-CT available cohorts nor the group who had adjunct FFR-CT analysis (Extended Data Fig. 6).
FFR-CT subgroup analysis
Among the 7,863 patients who received FFR-CT analysis, 7,091 (90.1%) had a stenosis-specific result and 7,844 (99.7%) had a distal vessel result, leaving 19 (0.2%) with no FFR-CT value. A stenosis-specific positive FFR-CT (≤0.80) was observed in 4,390 (55.8%) patients. A positive FFR-CT predicted cardiovascular mortality (HR 3.00 (1.33–6.76), P = 0.008), MI (HR 4.76 (2.91–7.77), P < 0.001) and revascularization (HR 13.47 (10.74–16.89), P < 0.001) at 2 years. ICA rates after a positive FFR-CT were higher than for those with a negative FFR-CT, or no stenosis-specific FFR-CT value (FFR-CT ≤0.80, n = 2,406 (54.9%), FFR-CT >0.80, n = 299 (11.1%), no FFR-CT value, n = 36 (4.8%), P < 0.001) (Table 3).
AI implementation and learning
From a baseline in which no NHS site was commissioned in March 2018, within 12 months, 27 different hospitals implemented the AI technology. At the end of the program, 54 sites were commissioned and utilizing the AI technology in routine healthcare settings. National implementation was geographically equitable with balanced representation from across England, urban and rural, academic and nonacademic centers (Extended Data Fig. 1). The median time from funding to starting an FFR-CT program was 4.7 months (IQR 2.4–7.6) with some variability between centers and regions (North 4.6 (2.3–8.3), Midlands 0.8 (0.0–8.0), Southeast 5.4 (3.5–11.0), Southwest 5.1 (2.0–8.6)) (Extended Data Fig. 2b). The indices of multiple deprivation (IMD) score, which ranks populations from the least deprived to the most deprived areas, varied between hospitals according to geographic region and population served. There was no difference in the population IMD score before or after FFR-CT introduction (mean 20.58 (±15.9), pre-FFR-CT 20.61, post-FFR-CT 20.52), nor in the FFR-CT tested cohort (20.1), indicating that the availability and use of FFR-CT was nondiscriminatory according to the level of deprivation or social class (Supplementary Table 6).
The learning curve was determined on an institutional basis by assessing the patient outcomes and resource utilization from the FFR-CT tested cohort (n = 7,863) by the center experience (first 75 FFR-CT cases versus >75 cases). This demonstrated a change in practice and learning over time. The frequency of positive FFR-CT results increased (55% versus 60%), with no change in ICA (24.2% versus 23.8%) or revascularization (19.4% versus 19.4%) rates, resulting in an increased revascularization ratio (53.4% versus 56.2%). There was an associated reduction in the number of second-line downstream tests performed over time (220.5 versus 183.2/1,000 patients) (Supplementary Table 7).
Discussion
This study has shown that a national technology implementation program enabled the rapid, equitable uptake of a new AI health technology in the health system. The introduction of FFR-CT was beneficial to the patient care pathway by reducing ICA and noninvasive functional cardiovascular tests performed 2 years after CCTA. The AI decision support tool appears safe with no significant difference in all-cause or CV mortality. The potential for increased risk of nonfatal MI during the technology availability will require longer-term assessment and consideration. AI implementation and clinical utility was balanced across populations, geographic location and institutional settings, with evidence of physician learning as use of the technology increased.
AI clinical decision support systems such as FFR-CT are being welcomed by overburdened healthcare systems with the aim of improving efficiencies and costs. Patients and clinicians often have greater apprehension about the use of AI in healthcare14. The introduction of new technologies and AI tools such as this constitute a complex health intervention in clinical practice and public health, aimed at improving patient outcomes. Assessing the real-world impact and clinical performance is difficult due to multiple interactions that may occur after a single health intervention15. These include differing groups or organizations being targeted, implementation of the AI technology outside of guidelines, and human factors such as the effects of time and learning16. Effectiveness, as assessed in randomized trials against usual treatment, frequently does not apply to real-world practice, limiting the impact and interpretability of trial results. Previous experience with computer-aided detection has highlighted the challenges in translation of trial results with use of technology versus real-world usage. In breast screening, for instance, the improved diagnostic accuracy in randomized controlled trials actually had reduced specificity with no benefit in the real world17,18. This study was designed as a real-world study to evaluate the national implementation of FFR-CT on patients and the wider health system by including every patient that underwent a CCTA at 27 different hospitals over a 3-year period, during which the technology was made available19.
This study provides insights into contemporary clinical practice and the type of patient undergoing investigation for suspected CAD20. A primary reason for NHS England recommending the adoption of FFR-CT was to improve the patient pathway by reducing unnecessary future downstream tests, especially invasive angiography. The clinical effectiveness of FFR-CT has previously been demonstrated by randomized trials showing lower rates of ICA compared with the usual treatment of noninvasive stress imaging tests at 9 and 12 months (refs. 9,8). Our real-world study population is 45 times larger than the comparator studies, has a longer follow-up and is more representative of contemporary clinical practice, as both cohorts were investigated with CCTA as the primary test under the same national guidance. After FFR-CT implementation, a reduction in all ICA and ICA without revascularization occurred early (90 days) and was sustained at 2 years after CCTA. National ICA rates were dropping before the introduction of FFR-CT related to the increased use of CCTA alone21, yet we have demonstrated that adding FFR-CT to this pathway further reduces rates of all coronary angiography by 7% and fewer inappropriate ICA procedures that did not result in treatment by 16%. The rate of ICA after a positive FFR-CT was a modest 55%. This points to the appropriate use of FFR-CT as an AI-diagnostic aid tool, where the AI-reported value gives a likelihood of functional significance (Extended Data Fig. 8), which must be integrated with the other imaging and clinical factors such as vessel size, location of the disease, or the patient being rendered asymptomatic on medications. The AI tool intends to assist clinicians and does not remove the need for clinical judgment.
Noninvasive downstream cardiovascular tests also reduced by 12% after the introduction FFR-CT, in particular the likelihood of having a repeat CCTA or nuclear stress test. A small but significant increase in the rates of cardiac MRI and invasive coronary tests were also observed. The increase in cardiac MRI despite greater use of CCTA after NICE guidance has been previously reported and does not appear to have been dampened by the first-line use of CCTA nor the addition of FFR-CT. An increase in invasive physiological and intracoronary imaging has also been observed but in this instance may reflect greater awareness and need to test stenoses after a positive FFR-CT, which previously would have been deemed unlikely to be flow limiting. This could be interpreted as improved clinical practice.
If FFR-CT is considered as another diagnostic test, the total number of diagnostics performed at 2 years is higher in the FFR-CT available group. However, from the patients’ perspective, FFR-CT is not another test, as it is a post-test AI analysis that occurs on the already acquired CCTA, saving further diagnostics referral waiting time, additional days missed from work and transport costs for hospital visits. The healthcare system could potentially also benefit from a reduction in overall test demand, which requires infrastructure (echo, MRI and CT equipment) and has direct workforce implications5. These are key paybacks that healthcare funders want to realize from the introduction of AI diagnostic aids.
Revascularization rates were higher after the FFR-CT available time period, with a small increase in PCI and a modest nonsignificant reduction in CABG. This shift of patients from CABG to PCI is consistent with the randomized controlled PRECISE study that compared a precision FFR-CT strategy with a routine cardiac testing strategy. This could reflect a change from three-vessel CAD, which is normally treated by CABG, to two- or one-vessel CAD, where PCI is more appropriate and feasible22. The impact of reduced ICA and increased PCI resulted in an improved revascularization ratio (patients at ICA receiving revascularization). This conversion rate of diagnostic test to treatment in one procedure is an important measure of FFR-CT efficacy compared with alternative non-AI, functional stress tests, which are associated with lower revascularization rates, thus wasting limited, expensive healthcare resources and putting the patient through an unnecessary invasive procedure with a risk of costly complications23.
Reduced unnecessary tests and improved CAD pathway efficiency are important to patients and healthcare providers, but any health intervention or new AI technology must first and foremost be safe11,15. We observed lower rates of all-cause mortality and cardiovascular mortality after the introduction of FFR-CT. The cohorts were overall well matched, with the group investigated before FFR-CT availability having a clinically small but a statistically significant increased number of risk factors such as heart failure, hypertension and kidney disease, whereas the post-FFR-CT group was older. These small differences in population risk factors probably explain the unadjusted lower all-cause and cardiovascular mortality rates observed in the FFR-CT available group, as their CAD burden should be similar given the knowledge that this does not change at a population level24,25. Higher PCI rates are unlikely to have had a significant impact, given that increased PCI has not been shown from studies in patients with stable CAD to improve mortality26,27,28,29,30. Overall, adjusted mortality and CV mortality were not different between groups and time periods, which is notable given the context of an adverse change in CV mortality trends since 2020 (ref. 31). The increased adjusted risk of MI in the FFR-CT available group at 2 years is difficult to interpret, especially given the lack of difference in unadjusted rates and on propensity matching (Extended Data Fig. 5). Increased MI events were observed in the PRECISE study and attributed to increased periprocedural events8,32. Our data show that the MI events increased in the FFR-CT available period with the event lines crossing at 90 days, similar to the increase in PCI (Extended Data Fig. 3). The FFR-CT available group had longer exposure to the coronavirus disease 2019 (COVID-19) pandemic period. This time period was associated with increased MIs and mortality in the general population and may be another possible explanation for differences observed between the groups, as MI events were higher late in the follow-up period that crossed over into the pandemic (Extended Data Fig. 7)33,34. It is difficult to attribute the increased adjusted risk of MI to the use of FFR-CT in 8.5% of the population, as there are many factors at play, including patient baseline risks, human factors and the pandemic.
One argument for using traditional functional stress tests over FFR-CT is the wealth of prognostic data available from them that is lacking in the FFR-CT literature. Small observational studies have suggested that a positive FFR-CT was associated with increased risk of MI and cardiovascular death compared with a negative FFR-CT; however, this was based on low numbers of events35,36. Due to the population size and number of clinical events in our study, we were able to show that FFR-CT provides patient-specific prognostic information, as the risk of MI or cardiovascular death increased three- to fivefold with a positive FFR-CT.
Our study suggests the potential of a centralized healthcare intervention program introducing AI technologies to improve AI utilization and support the transition to AI healthcare15,37. AI system challenges still exist, as, despite the technology being free to use, it took many centers up to 36 months to set up the information technology infrastructure and information governance processes required to use this cloud-based technology. Although the growth was substantial, in 3 years only 54 of 124 (44%) acute NHS England trusts were using the AI solution, despite unlimited central funding. High costs of FFR-CT would undoubtedly impact the affordability and, thus, accessibility for future broader use of this AI tool. Once implemented, the utilization of this AI technology was beneficial with generalizability across sites and populations (geography, age, gender and social deprivation). Human factors such as continuous learning appear to occur, with greater case selection and a reduction in additional testing associated with higher FFR-CT practice, thus implying that, with greater user experience, use of the technology can be adapted and potentially improve.
These results establish that the national introduction of a new AI technology FFR-CT by NHS England into the health system was beneficial to the patient chest pain pathway, supporting NICE’s recommendation and NHS England policymakers’ decision to centrally fund a new AI health technology. Implications for clinical practice could be wider utilization of FFR-CT to reduce unnecessary downstream tests. Future research will need to focus on the impact of increased revascularization, MI risk, longer-term impact and overall costs. Cost-effectiveness was fundamental to NICE’s recommendation and will be as important to the healthcare system as the clinical effectiveness of this health intervention38. There should be further investigation into the ability of FFR-CT to predict adverse cardiac events and whether it can be used to improve patient outcomes by more personalized patient-specific decision-making and revascularization.
Strengths of this study are its quasi-experimental observational design that resulted in total population inclusion, thus reducing any selection bias. The use of hospital-linked outcomes data reduced recollection bias that can be a limitation of observational studies, and it is reassuring that many of the key findings of this study are consistent with the randomized trial evidence9. There remains the risk of information bias utilizing HRs to adjust for imbalances in baseline potential prognostic confounders; thus, we supplemented our analysis with a PSM model.
The study has several weaknesses. As an observational study, causation cannot be attributed but should be considered in the context of wider multifactorial confounders. The use of routinely collected healthcare data enables large-scale studies such as this and limits misclassification, observer and recall bias but may be imperfect in the lack of adjudication of every event. The data are based on clinical coding and International Classification of Diseases (ICD) subgroups and so, on occasion, lack granularity, such as the inability to differentiate the type of MI. Patients were followed up for 2 years at different time points, rather than a single longitudinal cohort design. Cohort assignment depended upon when the patient had their scan and whether the AI technology was available at their hospital. Thus, referral bias cannot be totally excluded, as changes in practice can occur over time regardless of guidelines. The benefit of utilizing FFR-CT in the English NHS cannot be generalized to all healthcare systems, as each health intervention is unique to the environment in which it is implemented. The COVID-19 pandemic occurred during the study, meaning that the post-FFR-CT group had a greater follow-up time period during COVID. Our analysis suggests this had no significant impact on downstream test differences.
In conclusion, this study shows that a widescale national implementation of an AI diagnostic aid was feasible and resulted in widespread use of the technology. The use of FFR-CT in the stable CAD pathway was beneficial by reducing subsequent invasive and noninvasive cardiac tests and safe with no difference in MIs, all-cause or CV mortality. Implications for clinical practice could be the wider utilization of FFR-CT to reduce unnecessary downstream tests, and health policymakers may advocate more directed adoption programs to increase the uptake of AI tools within the health system.
Methods
Study design and population
FISH&CHIPS was a multicenter, retrospective, quasi-experimental, observational cohort study. The study aimed to determine the safety and benefit of FFR-CT introduction into NHS England by comparing a clinical population undergoing CCTA for investigation of possible stable CAD after the introduction of FFR-CT with a previous ‘standard of care’ population undergoing CCTA before FFR-CT availability with the use of existing second-line functional cardiac investigations (such as myocardial perfusion scintigraphy, stress echocardiography and perfusion cardiac MRI).
Hospitals participating in the NHS Innovation Technology Payment (ITP) program were invited. Sites were required to have performed >50 FFR-CT studies in the first year of their program to reduce learning curve bias. Participants were required to be age ≥18 years and have undergone a CCTA from April 2017 to December 2020. Nondedicated CCTA scans including CT calcium score alone, CT aorta and CT for transcatheter aortic valve implantation planning were excluded.
The study population was divided into two groups based on whether their CCTA was performed before or after FFR-CT became available at their hospital. All patients were investigated under the same NICE chest pain guidelines, with total inclusion; thus, both groups should be comparable and representative of a typical chest pain population, without the preselection bias often found in randomized controlled trials.
Data source
Clinical events were determined from routinely collected healthcare data from April 2016 to April 2022, using a previously validated methodology39. This provided 6 years of event data with a median 1 year prior and 3.3 years follow-up after CCTA. Data were requested before CCTA to ensure accurate baseline patient characterization. Data from all hospital episodes, including diagnostic coding, were obtained from NHS Digital’s Data Access Reporting System. This comprised the hospital episode statistics (HES) admitted patient care (APC), critical care (CC), emergency care (ECDS) and outpatient care (OPC) datasets. Diagnostic tests performed were captured in the diagnostic imaging dataset (DIDS). Mortality data and cause of death were obtained from the Office for National Statistics (ONS)-linked HES dataset. FFR-CT analysis was performed by a commercial company (HeartFlow) in accordance with the ITP funding. The decision to send a CCTA for FFR-CT analysis was at the discretion of the clinical team. FFR-CT was performed on a per-patient and per-vessel basis, then reported to the physician for clinical interpretation. Pseudonymized FFR-CT analysis was provided by HeartFlow to the study team for data linkage. Analysis of the FFR-CT data is based on the lowest stenosis specific value (2 cm distal to a stenosis) reported per patient.
Study outcomes
The study was designed to evaluate the early-stage use of an AI decision support system in accordance with reporting guidelines, by focusing on the clinical utility, safety and human factors associated with AI implementation40. Differences in health-related events between the populations were used to determine the primary objectives of safety and impact of implementing FFR-CT on the health system. Primary safety outcomes included all-cause mortality, cardiovascular mortality and MI event rates. Primary impact outcomes included the rates of downstream tests performed after the index CCTA; including ICA with and ICA without revascularization as well as noninvasive functional tests or repeat CCTA. Secondary analysis categorized FFR-CT patients’ outcomes according to their FFR-CT result. Human factors were assessed by describing the uptake and utilization across different sites and population demographics, as well as any impact of learning over time.
Clinical outcomes were reported using the hospital admission diagnostic codes from the OPCS Classification of Interventions and Procedures (OPCS-4) and equivalent International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10)-World Health Organization version definitions. Cardiovascular deaths included I00-I99, excluding I26-28 (pulmonary heart disease), I60-69 (cerebrovascular disease) and I80-89 (diseases of the veins and lymphatics). MI was defined as a new admitted patient episode with a diagnostic code of MI (I21/I22) (SupplementaryTable 4).
Downstream cardiovascular diagnostic tests were collected from the DIDS, categorized as per the National Interim Clinical Imaging Procedure code set used for the coding of clinical imaging procedures in electronic systems in the NHS.
Statistical analysis
Descriptive data continuous variables are reported as mean (± standard deviation) or median (IQR) and categorical as number (%). Comparisons were performed using Student’s t-test, Mann–Whitney test or chi-square test where appropriate. Time to first event was calculated using Kaplan–Meier methodology from the time of the CCTA until 2 years. Cox-proportional regression was used to determine the univariable HR of clinical outcomes at 2 years after CCTA41,42,43. Prognostic covariates that were different between the groups at entry into the study were entered into an adjusted multivariable Cox regression model41,44. Additional multivariable sensitivity analysis were performed using the differences in baseline characteristics with the inclusion of FFR-CT availability as an interaction term for predicting ICA. Age was recalculated as mean-centered age for the interaction term to reduce multicollinearity. PSM was performed for the primary outcomes to help determine causal effect. The PSM model used a 1:1 nearest-neighbor method, with assessment of post-match covariate by standardized mean difference. A standardized mean difference of <5% was used for the matching. Matched-pairs analysis was performed using Cox-proportional hazard models stratified on the matched pairs. The 95% confidence intervals (CIs) are reported with P values, and a two-sided P value <0.05 was considered statistically significant. All statistical analysis was performed in the R stats package (R documentation, version 3.6.2).
Ethics and consent
The Confidentiality Advisory Group (CAG) approved the use of confidential patient information without consent on the basis of health and social care research in the public interest (National Health Service Act 2006, s251, ‘Control of patient information’; CAG reference 20CAG0101). Ethical approval was obtained from the Health Regulatory Authority (Integrated Research Application System project ID 285996; Research Ethics Committee reference 20/NW/0430). The NHS ‘Opt out of research’ database was queried with participants excluded if consent was withdrawn. The study was performed in accordance with the Declaration of Helsinki and principles of good clinical practice.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Supporting data are available via the UK Data service repository at https://reshare.ukdataservice.ac.uk and via figshare at https://doi.org/10.6084/m9.figshare.28398374 (ref. 45), including the study protocol and the algorithms for defining clinical outcomes from HES data. Dataset availability is subject to controlled access due to the CAG approvals and NHS England’s data sharing contract (CON-317153-H1H4Z (version 2.03)). Individual deidentified, aggregated participant data that underlie the study reported outcomes will be made available in accordance with the ethical approvals, NHSE data sharing framework contract and the Medical Research Council Industrial Collaboration Agreement (MICA). Any data sharing is subject to a data sharing agreement (DSA) between parties. Each DSA will detail: (1) the data to be provided; (2) the legal basis for sharing data; (3) the purpose of the sharing and use of the data; (4) the expected benefits to health and/or social care by sharing the data; (5) the data transfer method; (6) any associated DSAs; (7) any special terms and conditions for the use or reuse of the data; and (8) any charges payable for the provision of the data. Requests for data sharing should be communicated in writing to the research governance team at Liverpool Heart and Chest Hospital (Research.Governance@lhch.nhs.uk) specifying the nature of the request. All external requests will be responded to within 2 weeks by the sponsors director of research, with an estimated timeframe of 3 months from the date of request to DSA approval. The study ethics approvals allow data storage up to 15 years.
Code availability
The code used to determine clinical events from the HES datasets is available via the UK Data service repository at https://reshare.ukdataservice.ac.uk; search ‘Implementation of a national AI technology program on cardiovascular outcomes and the health system’ (https://doi.org/10.5255/UKDA-SN-857706). The underlying methodology for FFR-CT computational analysis has been published previously; however, the source code for FFR-CT computation is proprietary, so it cannot be made available publicly.
References
Gulati, M. et al. AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 144, e368–e454 (2021).
Knuuti, J. et al. ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 41, 407–477 (2020).
Chest Pain of Recent Onset: Assessment and Diagnosis. NICE Guidelines (NICE, 2010).
Inohara, T. et al. Appropriateness ratings of percutaneous coronary intervention in Japan and its association with the trend of noninvasive testing. JACC Cardiovasc. Inter. 7, 1000–1009 (2014).
HeartFlow FFRCT for Estimating Fractional Flow Reserve from Coronary CT Angiography (MTG32) (National Institute for Health and Care Excellence, 2017); https://www.nice.org.uk/guidance/mtg32/resources
Taylor, C. A., Fonte, T. A., Min, J. K., City, R. & Angeles, L. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve scientific basis. J. Am. Coll. Cardiol. 61, 2233–2241 (2013).
Taylor, C. A. et al. Patient-specific modeling of blood flow in the coronary arteries. Comput. Methods Appl. Mech. Eng. 417, 116414 (2023).
Douglas, P. S. et al. Comparison of an initial risk-based testing strategy vs usual testing in stable symptomatic patients with suspected coronary artery disease: the PRECISE randomized clinical trial. JAMA Cardiol. 8, 904–914 (2023).
Curzen, N. et al. Fractional flow reserve derived from computed tomography coronary angiography in the assessment and management of stable chest pain: the FORECAST randomized trial. Eur. Heart J. 42, 3844–3852 (2021).
Clarke, S. & Ray, S. Cardiology Getting it Right the First Time (GIRFT) Programme National Specialty Report (NHS England, 2021).
Goldberg, C. B. et al. To do no harm—and the most good—with AI in health care. Nat. Med. 30, 623–627 (2024).
Youssef, A. et al. Ethical considerations in the design and conduct of clinical trials of artificial intelligence. JAMA Netw. Open 7, e2432482 (2024).
Flanagin, A. et al. Reporting use of AI in research and scholarly publication—JAMA network guidance. JAMA 331, 1096–1098 (2024).
Richardson, J. P. et al. Patient apprehensions about the use of artificial intelligence in healthcare. NPJ Digit. Med. 4, 140 (2021).
Vasey, B. et al. Reporting guideline for the early-stage clinical evaluation of decision support systems driven by artificial intelligence: DECIDE-AI. Nat. Med. https://doi.org/10.1038/s41591-022-01772-9 (2022).
Craig, P. et al. Medical Research Council Guidance. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ https://doi.org/10.1136/bmj.a1655 (2008).
Gilbert, F. J. et al. Single reading with computer-aided detection for screening mammography. N. Engl. J. Med. 359, 1675–1684 (2008).
Lehman, C. D. et al. Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Intern. Med. 175, 1828–1837 (2015).
Boyko, E. J. Observational research—opportunities and limitations. J. Diabetes Complications https://doi.org/10.1016/j.jdiacomp.2013.07.007 (2013).
SCOT-HEART Investigators et al. Coronary CT angiography and 5-year risk of myocardial infarction. N. Engl. J. Med. 379, 924–933 (2018).
Weir-McCall, J. R. et al. National trends in coronary artery disease imaging: associations with health care outcomes and costs. JACC Cardiovasc. Imaging 16, 659–671 (2023).
Collet, C. et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur. Heart J. 39, 3689–3698 (2018).
Curzen, N. P., Nolan, J., Zaman, A. G., Norgaard, B. L. & Rajani, R. Does the Routine Availability of CT-Derived FFR Influence Management of Patients with Stable Chest Pain Compared to CT Angiography Alone? The FFR CT RIPCORD Study (2016).
Shaw, L. J. et al. Coronary computed tomographic angiography as a gatekeeper to invasive diagnostic and surgical procedures: results from the multicenter confirm (coronary CT angiography evaluation for clinical outcomes: an international multicenter) registry. J. Am. Coll. Cardiol. 60, 2103–2114 (2012).
Williams, M. C., Moss, A., Nicol, E. & Newby, D. E. Cardiac CT improves outcomes in stable coronary heart disease: results of recent clinical trials. Curr. Cardiovasc Imaging Rep. 10, 14 (2017).
Sedlis, S. P. et al. Effect of PCI on long-term survival in patients with stable ischemic heart disease. N. Engl. J. Med. 373, 1937–1946 (2015).
Maron, D. J. et al. Initial invasive or conservative strategy for stable coronary disease. N. Engl. J. Med. 382, 1395–1407 (2020).
Mehta, S. R. et al. Complete revascularization with multivessel PCI for myocardial infarction. N. Engl. J. Med. 381, 1411–1421 (2019).
Al-Lamee, R. & Jacobs, A. K. ISCHEMIA trial: was it worth the wait? Circulation https://doi.org/10.1161/CIRCULATIONAHA.120.045007 (2020).
Ko, D. T. et al. Assessing the association of appropriateness of coronary revascularization and clinical outcomes for patients with stable coronary artery disease. J. Am. Coll. Cardiol. 60, 1876–1884 (2012).
Woodruff, R. C. et al. Trends in cardiovascular disease mortality rates and excess deaths, 2010–2022. Am. J. Prev. Med https://doi.org/10.1016/j.amepre.2023.11.009 (2023).
Lansky, A. J. & Stone, G. W. Periprocedural myocardial infarction prevalence, prognosis, and prevention. Circ. Cardiovasc. Interv. 3, 602–610 (2010).
Dale, C. E. et al. The impact of the COVID-19 pandemic on cardiovascular disease prevention and management. Nat. Med. 29, 219–225 (2023).
Eberhardt, N. et al. SARS-CoV-2 infection triggers pro-atherogenic inflammatory responses in human coronary vessels. Nat. Cardiovasc. Res. 2, 899–916 (2023).
Nørgaard, B. L. et al. Prognostic value of coronary computed tomography angiographic derived fractional flow reserve: a systematic review and meta-analysis. Heart 108, 194–202 (2022).
Madsen, K. T. et al. Prognostic value of coronary CT angiography–derived fractional flow reserve on 3-year outcomes in patients with stable angina. Radiology 308, e230524 (2023).
How to support the transition to AI-powered healthcare. Nat. Med. https://doi.org/10.1038/s41591-024-02897-9 (2024).
Hlatky, M. A. et al. Randomized comparison of chest pain evaluation with FFRCT or standard care: factors determining US costs. J. Cardiovasc. Comput. Tomogr. 17, 52–59 (2023).
Stables, R. H. et al. Routine pressure wire assessment versus conventional angiography in the management of patients with coronary artery disease: the RIPCORD 2 trial. Circulation 146, 687–698 (2022).
Reddy, S. et al. Evaluation framework to guide implementation of AI systems into healthcare settings. BMJ Health Care Informatics https://doi.org/10.1136/bmjhci-2021-100444 (2021).
Jachno, K. M. et al. Examining evidence of time-dependent treatment effects: an illustration using regression methods. Trials 23, 857 (2022).
Gassama, M., Bénichou, J., Dartois, L. & Thiébaut, A. C. M. Comparison of methods for estimating the attributable risk in the context of survival analysis. BMC Med Res Methodol. 17, 10 (2017).
Royston, P. & Parmar, M. K. An approach to trial design and analysis in the era of non-proportional hazards of the treatment effect. Methodology https://doi.org/10.1186/1745-6215-15-314 (2014).
Royston, P. & Parmar, M. K. B. The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat. Med. 30, 2409–2421 (2011).
Fairbairn, T. A. et al. Implementation of a national AI technology programme on cardiovascular outcomes and the health system; Nat. Med. 2025. figshare https://doi.org/10.6084/m9.figshare.28398374 (2025).
Acknowledgements
We are grateful to the trials and information governance teams at Liverpool Heart and Chest Hospital and NHS England’s database team. This study was funded by the UK Medical Research Council (MR/T024933/1), and Liverpool Heart and Chest Hospital was the study sponsor. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
FISH&CHIPS study steering committee: T.A.F., J.C., G.Y.H.L., L.M., E.N., M. Schmitt, M. Shaw and J.W.-M. FISH&CHIPS data analysis team, Liverpool Heart and Chest Hospital: I.K., L.T. and M. Shaw. Statistical analysis: G.B., Department of Health Data Science, University of Liverpool, and M. Shaw, Liverpool Heart and Chest Hospital. HeartFlow investigators: S.M. and C.R. Conceptualization: S.D., T.A.F., E.N., M. Schmitt and J.W.-M. Data curation: J.C., T.A.F., I.K., M. Shaw and L.T, Formal analysis: G.B., T.A.F., I.K., M. Shaw and L.T. Funding acquisition: T.A.F., G.Y.H.L., E.N., J.W.-M. and J.W.-M. Investigation: J.C., T.A.F., I.K., G.Y.H.L., L.M., E.N., M. Shaw, M. Schmitt and J.W.-M. Methodology: G.B., T.A.F. and M. Shaw. Project administration: J.C. and L.T. Writing—original draft: T.A.F. Writing—review and editing: T.A.F., G.Y.H.L., L.M., E.N., M. Schmitt, J.W.-M., S.S., A.C., C.L., S.W., S.I., A.B., I.S., J.P.G., M.M., A.R., A. Beattie, J.C., P.H., N.B., B.H., J.R., O.W., V.V., R.B., P.O'K., A.D., G.M., S.D., H.M., V.P. and J.S.
Corresponding author
Ethics declarations
Competing interests
The authors received no specific funding for this work. S.M. and C.R. are employees of HeartFlow. An MRC Industrial Collaboration Agreement was signed between collaborators and HeartFlow. HeartFlow provided the pseudo-anonymized FFR-CT data but had no role in the study design, data collection, analysis, interpretation of the data, writing or decision to submit the manuscript. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Konstantinos Rizas, Yan-Ran Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ming Yang, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 English hospital site map.
A map of NHS England with the 25 different NHS Trusts plotted, their corresponding Integrated Care Board (ICB) and number of patients (n) contributed to the study.
Extended Data Fig. 2 Patient recruitment and site onboarding for the AI technology.
a Patient recruitment and drop out for 6 months, 12 months and 24 month time points: Categorized as pre (blue line) or post (red line) FFR-CT availability. All 27 sites provided patient data from April 2017 – April 2020 with 2 sites (UHD and BRI) providing data from April 2017-December 2020. b Site FFR-CT availability: Introduction of FFR-CT at a site level was used to define whether the CCTA was performed before or after FFR-CT was made available at their site. The bubbles represent the time that FFR-CT was made available for clinical use and the number of CCTA scans performed at each site over the study recruitment time period (April 2017-December 2020).
Extended Data Fig. 3 Event curves for myocardial infarction and percutaneous intervention at different time points.
a Myocardial Infarction (MI) events and B. Percutaneous Intervention (PCI) rates at 90 days, 1 year and 2 years. Cox proportional hazards univariate analysis (unadjusted) p values. The shaded areas indicate the 95% CIs.
Extended Data Fig. 4 Propensity Score Matching balancing.
Propensity Score Matching covariate balancing pre and post matching using a standard mean difference of <0.05.
Extended Data Fig. 5 Event curves for the primary outcomes of the Propensity Matched Scoring population.
a. All-cause death, b. Cardiovascular death, c. Myocardial infarction, d. ICA with no revascularization. Kaplan-Meier (KM) charts of the cumulative incidence of the individual primary objectives over 2 years post index CCTA on the Propensity Matched population (n = 30,665 in each group). Blue represents pre FFR-CT availability. Red line represents post FFR-CT availability. The shaded areas indicate the 95% CIs.
Extended Data Fig. 6 Referral to treatment time.
Time (in years) from CCTA date (Time 0) to the date of revascularisation (PCI or CABG) for the 6,784 patients who had coronary revascularisation within the 2-year follow-up. The mean wait was 0.41 years for both FFR-CT unavailable and FFR-CT available groups. The group who received FFR-CT had a insignificant shorter wait (0.4 years). The box represents the interquartile range with the median as the middle segment, the mean as the black dot and the whiskers as minimum and maximum waits.
Extended Data Fig. 7 Autoregressive Integrated Moving Average curve for the incidence of myocardial infarction over teh study time period.
Autoregressive Integrated Moving Average (ARIMA) method were performed. Weekly observed rates of all primary outcomes were assessed before and after FFR-CT introduction, then modelled for observed versus expected changes in outcomes post-health intervention over time. The dashed black line represents the time that FFR-CT was made available to the hospital sites.
Extended Data Fig. 8 FFR-CT report example.
A positive FFR-CT (centre panel). The LAD shows a gradual reduction from proximal to distal vessel with a stenosis specific value of 0.72 and distal vessel value of 0.65. The circumflex was ‘negative’ at 0.89 and the RCA was occluded. The scale on the left panel shows the degree of flow limitation and likelihood of functional significance as a continuum with the margins of error on the right panel to help the physician in their decision process.
Supplementary information
Supplementary Information
Methods, Supplementary Tables 1–7, Stastical analysis plan and Protocol.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fairbairn, T.A., Mullen, L., Nicol, E. et al. Implementation of a national AI technology program on cardiovascular outcomes and the health system. Nat Med 31, 1903–1910 (2025). https://doi.org/10.1038/s41591-025-03620-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-025-03620-y
This article is cited by
-
Artificial intelligence in estimating instantaneous wave-free ratio: a systematic literature review of techniques
The International Journal of Cardiovascular Imaging (2026)