Abstract
Chronic infection with Helicobacter pylori is a modifiable cause of gastric cancer. To assist policymakers in advocating for and planning prevention strategies, we projected the future burden of gastric cancer, including that attributable to H. pylori, among a cohort of young people born in 2008–2017. Expected gastric cancer cases, in the absence of intervention, were quantified in 185 countries by combining national age-specific incidence rates from GLOBOCAN 2022 and cohort-specific mortality rates from the United Nations’ demographic projections. Globally, 15.6 million (95% uncertainty interval 14.0–17.3 million) lifetime gastric cancer cases are expected within these birth cohorts, 76% of which are attributable to H. pylori. Two-thirds of cases will be concentrated in Asia, followed by the Americas and Africa. Whereas 58% of cases are expected in traditionally high-incidence areas for gastric cancer, 42% of cases are expected to occur in lower-incidence areas owing to demographic changes, particularly in sub-Saharan Africa, where the future burden could be six times greater than estimated in 2022. A shift in focus toward the life course of today’s young people and their prospects of developing gastric cancer, with or without effective interventions, underscores the need for greater investment in gastric cancer prevention, including the implementation of population-based H. pylori screen-and-treat strategies.
Similar content being viewed by others
Main
Although some higher-resource countries, notably in East Asia, have put in place organized early detection programs1,2, gastric cancer remains the fifth most common cause of cancer death worldwide3, and there is evidence that the disease will remain an important public health problem for the foreseeable future unless effective measures are implemented4. Long considered an ‘unplanned triumph’ of primary prevention5, gastric cancer has been prone to underinvestment relative to other infection-related cancers, such as cervical or liver cancer3,6,7. Of note, gastric cancer incidence rates at younger ages (<50 years) are increasing in both low- and high-incidence populations8,9,10,11, which may, in turn, result in a deceleration or reversal of the long-term declines in incidence rates. Further, the numbers of gastric cancer cases and deaths are projected to increase in the next decades through population aging and growth irrespective of overall trends4.
Most gastric cancers, especially noncardia gastric cancer (NCGC), are caused by chronic infection with Helicobacter pylori12,13 and can be prevented by treatment of the infection with a combination of antibiotics and proton pump inhibitors. A systematic review of the available randomized controlled trials and observational studies showed that studies of different designs consistently found that H. pylori treatment prevents gastric cancer in H. pylori-positive individuals14. The working group convened by the International Agency for Research on Cancer/World Health Organization (IARC/WHO) in 2013 recommended that countries explore the possibility of introducing population-based H. pylori screen-and-treat programs based on the local disease burden, competing health priorities and cost-effectiveness analyses15. Nevertheless, there have been few attempts to implement such programs at the population level even in high-risk areas, although several European countries have recently initiated pilot studies as part of Europe’s Beating Cancer Plan16. While a vaccine against H. pylori would be a powerful tool for gastric cancer prevention and would serve to overcome concerns surrounding antibiotic treatment, there appears little momentum at present to advance its development17.
To assist policymakers and local stakeholders in advocating for and implementing prevention strategies, this study first quantifies the future burden of gastric cancer among individuals born between 2008 and 2017, assuming no changes in the current control measures for gastric cancer. We then estimate the number of cancers attributable to H. pylori infection as potentially preventable cancers within the same birth cohorts, taking into account the gastric cancer subsite. We present the results for the global population and for 185 countries by world region and Human Development Index (HDI).
Results
Expected gastric cancer cases by region, HDI and incidence
Assuming no change in future incidence rates, we would expect a lifetime estimate of 15.6 million new gastric cancer cases (95% uncertainty interval (UI) 14.0–17.3 million) among all men and women born between 2008 and 2017 globally (Table 1). The Asian continent is the major contributor to the expected burden, with more than 10.6 million cases (95% UI 9.8–11.4 million; 68% of all cases), followed by the Americas (2.0 million (95% UI 1.8–2.1 million); 13%), Africa (1.7 million (95% UI 1.3–2.4 million); 11%), Europe (1.2 million (95% UI 1.1–1.3 million); 8%) and Oceania (0.07 million (95% UI 0.05–0.20 million); 0.4%) (Table 1). Among the expected gastric cancer cases, approximately 76% are attributable to H. pylori infection globally, with 8.0 million gastric cancer cases in Asia attributable to the bacteria (accounting for 67% of the global burden of gastric cancer cases attributable to H. pylori), followed by 1.5 million (13%) and 1.4 million (12%) cases in the Americas and Africa, respectively.
Examining the burden by world subregion, we estimated that 5.9 million (95% UI 5.6–6.1 million) gastric cancer cases in eastern Asia and 2.9 million (95% UI 2.6–3.2 million) cases in southern Asia are expected to occur. These numbers are followed by 1.6 million (95% UI 1.5–1.8 million) in Latin America and the Caribbean and 1.4 million (95% UI 1.0–2.1 million) expected cases in sub-Saharan Africa (Table 1). More than 70% of the estimated burden of gastric cancer is concentrated in countries with very high (3.1 million, 95% UI 2.9–3.3 million) or high (7.9 million, 95% UI 7.3–8.3 million) HDI (Table 1). Although more than half of all gastric cancer cases are expected to occur in areas where the age-standardized incidence rates of the disease are greater than 10 in 100,000, a further 42% of cases are expected in areas where the rates are lower than this threshold (Table 1). The stratified analyses by sex and world region are shown in Extended Data Tables 1 and 2. The expected number of gastric cancer cases attributable to H. pylori infection is higher in women (16%) than in men (9%) in Africa, whereas the opposite is true for Asia (71% in men versus 61% in women).
Expected gastric cancer cases and the cases attributable to H. pylori infection
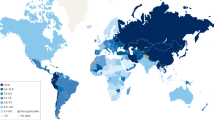
About two-fifths (42%) or 6.5 million (95% UI 6.1–6.9 million) new cases among these cohorts will occur in China and India alone (Fig. 1a and Extended Data Table 3). A further 5.6 million (95% UI 4.8–6.5 million, 36%) cases will occur among cohorts born in 25 countries where the expected burden is between 100,000 and 1 million cases (Japan, Iran, Russia, Brazil, Vietnam, Turkey, the USA, Pakistan, Bangladesh, Mexico, South Korea, Colombia, Peru, Philippines, Afghanistan, Myanmar, Democratic Republic of the Congo, Ethiopia, Kenya, Egypt, Nigeria, Tanzania, Mali, Indonesia and Uzbekistan; Fig. 1b and Extended Data Table 3). An additional 2.3 million (95% UI 1.8–3.2 million, 15%) cases are expected in countries where the expected burden is between 35,000 and 100,000 cases (Fig. 1c), with the remaining 8% (1.2 million (95% UI 0.9–1.9 million) cases) occurring in the remaining 158 countries (Extended Data Fig. 1 and Extended Data Table 3).
Expected numbers of country-specific gastric cancer cases in individuals born between 2008 and 2017 in the absence of changes in the current control measures for gastric cancer and the cases attributable to H. pylori infection. a–c, Countries are grouped according to their contribution to the overall gastric cancer burden, with countries sorted according to the expected number of gastric cancer cases and subsequently grouped into the following five categories: >1,000,000 (group A; a), 100,000–1,000,000 (group B; b) and 35,000–100,000 (group C; c). Countries from groups D and E are included in Extended Data Fig. 1, with the corresponding number of gastric cancer cases provided in Extended Data Table 3.
Ratio of change
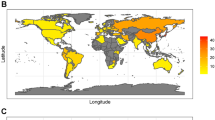
The effect of future demographic changes on the national and regional gastric cancer burden is illustrated in Figs. 2 and 3 by comparing the average number of expected gastric cancer cases across a lifetime in an average single birth cohort relative to the national GLOBOCAN estimates for the year 2022. Demographic changes are expected to markedly increase the gastric cancer burden. In most of sub-Saharan Africa, where the current burden is low, the future burden in an average single birth cohort could be six times higher than at present (Figs. 2 and 3), with the burden in Asia and Latin America expected to be two to six times higher. Conversely, fewer gastric cancer cases relative to the 2022 GLOBOCAN estimates are expected in cohorts from the South Korea, Japan and some European countries with high or very high HDI (Fig. 2).
Ratio of the average number of expected lifetime gastric cancer cases in an average single birth cohort across birth cohorts born between 2008 and 2017 versus the total number of cases estimated cross-sectionally in 2022 (indicated here as the ratio of change). The designations used and the presentation of the material in this article do not imply the expression of any opinion whatsoever on the part of WHO and the IARC about the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries.
Age-specific distribution of the average number of expected gastric cancer cases in an average single birth cohort across birth cohorts of individuals born between 2008 and 2017 in the absence of changes in the current control measures, compared with those estimated cross-sectionally in 2022. ‘Ratio’ indicates the ratio of change.
The correlation between the ratio of the future-to-current number of gastric cancer cases (ratio of change) and country-specific HDI was −0.71 (95% confidence interval −0.78, −0.64; Fig. 4). Twenty-three of forty-eight sub-Saharan African countries and territories with low or middle HDI are expected to have a future burden that is more than six times higher than estimated in 2022. By contrast, some East Asian countries with very high HDI and the highest incidence rates worldwide at present, such as the South Korea and Japan, are expected to have a decreased future burden, with a ratio of change of <1.
Effect of various assumptions on the expected number of gastric cancer cases
In sensitivity analyses, we assessed the impact of various scenarios on the expected number of gastric cancer cases (Fig. 5 and Extended Data Table 4). Under the scenarios of year-on-year decreases or increases of −3% or 3% in the NCGC incidence rates over 20 years, a 38.2% decrease or 66.5% increase in the expected cases is estimated. This contrasts with the more modest impact of the same range of trends in cardia gastric cancer (CGC) rates of only a 12% change in the estimates. Still, under this assumption of −3% year-on-year decreases in the NCGC incidence rates over 20 years, the estimated number of gastric cancer cases in our cohort will be higher than the cross-sectional estimates from GLOBOCAN 2022 (ratio of change = 1.1).
This figure shows the variation in the overall lifetime expected number of gastric cancer cases in individuals born between 2008 and 2017 according to changes in the model parameters. The variation could be read in terms of the absolute number of cases (top x axis) or the ratio of the lifetime expected number of gastric cancer cases (bottom x axis). The reference model parameters are the same as the ones assumed in the main text (that is, no competitive risk between CGC and NCGC, median UN death rate scenario, no annual percentage change (APC) for CGC and NCGC, no changes in the current public health intervention (PHI) for gastric cancer control). Each line on the y axis represents the specific parameter changed compared to the reference while all the other parameters remain unchanged. The tested parameters are as follows: (1) competing rate: including the CGC competing rate in the NCGC burden calculation and vice versa 2 (yes). (2) Death rate: changing the overall cause of death competing risk for the UN lower (lwr) or upper (upr) scenario. (3) APC CGC (10 years): applying APCs of −3%, −2%, −1%, 1%, 2% and 3% for 10 years to the CGC incidence rate. (4) APC CGC (20 years): applying APCs of −3%, −2%, −1%, 1%, 2% and 3% for 20 years to the CGC incidence rate. (5) APC NCGC (10 years): applying APCs of −3%, −2%, −1%, 1%, 2% and 3% for 10 years to the NCGC incidence rate. (6) APC NCGC (20 years): applying APCs of −3%, −2%, −1%, 1%, 2% and 3% for 20 years to the NCGC incidence rate. (7) PHI impact: applying the population-level impact of H. pylori screen-and-treat strategies by 80–100%.
When we assessed the population-level impact of H. pylori screen-and-treat strategies, the expected number of gastric cancer cases would be reduced by up to 75% with 100% effectiveness of the intervention, compared with 67.5% and 60% when 90% and 80% of impact were assumed, respectively (Fig. 5). These analyses are also presented by sex and region (Extended Data Fig. 2). While little differences were observed between men and women in terms of the expected number of gastric cancer cases under these scenarios, the impact of using the lower or higher 95% projections of the United Nations (UN) death rates was relatively larger in Africa and Oceania compared to other regions, indicating that life expectancy in these regions has a larger effect on the estimated number of gastric cancer cases.
Discussion
In the absence of changes in the current practices of gastric cancer prevention, we expect that 15.6 million gastric cancer cases worldwide will occur among individuals born between 2008 and 2017, with around three-quarters of these preventable if H. pylori infection is eradicated. Our results highlight Asia as the main contributor to the estimated burden of gastric cancer (10.6 million cases), followed by the Americas (2.0 million cases) and Africa (1.7 million cases). The impact of demographic changes will be striking in these latter continents, with the lifetime gastric cancer burden among these birth cohorts in Africa, for example, estimated to be nearly six times greater than the currently estimated burden in 2022.
In Asia, the major contributor to the current gastric cancer burden worldwide, few countries have introduced gastric cancer prevention programs. While the South Korea2, Japan1 and China18 continue focusing efforts on organized programs through national or regional endoscopic screening for gastric cancer, population-based H. pylori screen-and-treat programs are also being progressively implemented, for example, in the Matsu Islands19, Japan (through national health insurance coverage of H. pylori treatment for patients with endoscopically confirmed gastritis)20, and Bhutan21. In 2023, Bhutan completed time-bound national programs for gastric cancer prevention as part of the Health Flagship Project, a population-based H. pylori screen-and-treat program for persons aged 18–75 years, and population-based endoscopic screening for precancerous lesions for those aged 40–75 years21.
The lack of public health action on gastric cancer prevention in the Americas has been highlighted22. This is despite the substantial H. pylori-attributable gastric cancer burden in the region and evidence from a long-term chemoprevention trial showing that H. pylori eradication had a long-term beneficial effect, including substantial reductions in histological progression in a high-risk Hispanic population23. In the USA, there are currently no national guidelines or formal recommendations for gastric cancer prevention, although gastric cancer disproportionately affects Asian Americans, Hispanic Americans, African Americans and American Indian–Alaska Native individuals6, and an increasing trend in young individuals (age <50 years) has been observed between 2016 and 2022, most notably in women24.
Similarly, in Africa, no known population-based gastric cancer prevention programs are available despite H. pylori infection being very common on the continent. Efforts are ongoing to improve data collection and formulate evidence-based and locally relevant practice guidelines on H. pylori management in Africa25. Our results endorse the importance of making changes to the current practice and urge regional health systems to be prepared to manage the growing burden of this largely preventable disease by planning pilot and feasibility projects, including H. pylori screen-and-treat programs.
The observation of the contrasting low risk of gastric cancer despite ubiquitous H. pylori infection, for example, in Africa26, may in part reflect the lack of robust cancer registries to capture the actual number of cases, emphasizing the importance of establishing high-quality population-based registries. This may also partly be an effect of competing mortality from H. pylori-related gastrointestinal conditions27. Gastric cancer is already a common cancer in some western African countries, such as Senegal, Guinea and Mali. Given the anticipated increasing burden of gastric cancer shown here, local cancer control planning must be reconfigured to embrace prevention policies. More than one-quarter of the future global burden is expected to occur in countries that can be categorized as having very low incidence rates. These findings further highlight the fact that gastric cancer will remain a major public health problem globally over the next decades, with the substantial demographic-driven increase in burden in these traditionally low-risk areas compounding the continuing high burden in high-risk areas.
In our study, we observed that much of the global burden of gastric cancer continues to occur in very high- or high-HDI countries in East Asia, mostly in China, due to their large and aging populations. In these birth cohorts, we used the most recent attributable fraction estimates of H. pylori infection for CGC (62%) and NCGC (79%) in China as measured by a sensitive immunoblot assay28,29. The findings suggest that the beneficial effects of H. pylori treatment extend beyond NCGC in this high-burden setting, leading to larger estimates of impact than previously understood. A large-scale trial of community-based H. pylori eradication in China reported no severe intolerable adverse events while highlighting its potential as a public health policy for gastric cancer prevention30.
Given that gastric cancer is largely preventable, more active intervention and control programs should be implemented in these high-resource East Asian countries. This can be done by prioritizing gastric cancer prevention and allocating resources to the primary prevention of the disease, considering the continued high H. pylori-attributable burden that is foreseen. Our finding of a negative correlation between the ratio of future-to-current number of gastric cancer cases and HDI suggests that prevention strategies need to be adapted to each country’s resource level. Treatment of H. pylori at the population level is currently the best evidence-based and most affordable approach that could be adapted in low- to medium-HDI settings, along with early diagnosis focusing on detecting symptomatic patients as early as possible for better treatment and survival, compared to endoscopy-based screening programs or cancer treatment. The potential of the population-level impact of H. pylori screen-and-treat strategies in reducing the future number of gastric cancer cases is clearly shown in our results. Previous cost-effectiveness analyses consistently reported that H. pylori screen-and-treat strategies are a cost-effective intervention for gastric cancer prevention, even in low-risk settings31.
Based on data from randomized controlled trials, Ford et al.32 estimated that more than 8.7 million disability-adjusted life-years would be gained if population-based H. pylori screening and treatment are implemented globally. Their review of four randomized controlled trials with long-term follow-up reconfirmed the long-term efficacy of H. pylori eradication in preventing gastric cancer cases and deaths among healthy individuals with H. pylori infection and also showed that the benefits were not limited to very old adults33. Despite the accumulating evidence, very few countries have piloted H. pylori screen-and-treat programs at the population level. Our projections of the potentially modifiable gastric cancer burden provide policymakers with critical information relevant to cancer control planning. In addition, effective strategies will have additional benefits in reducing other important clinical conditions, including peptic ulcer disease, dyspepsia, iron deficiency and idiopathic thrombocytopenic purpura15.
In Europe, with the adoption of Europe’s Beating Cancer Plan in 2021 and subsequent recommendations on gastric cancer prevention made by the European Council, population-based H. pylori screen-and-treat programs are emphasized as an important tool for gastric cancer prevention, especially for those with an intermediate to high gastric cancer burden34.
The implementation outcomes of such programs in Europe, including the collection of cost data, are currently being analyzed in two European Union projects focusing on gastric cancer prevention: EUROHELICAN (‘Accelerating gastric cancer reduction in Europe through H. pylori eradication’) and TOGAS (‘Towards gastric cancer screening implementation in the European Union’)35. The IARC/WHO has developed global guidance on the implementation of population-based H. pylori screen-and-treat strategies for gastric cancer prevention by convening an international expert working group36. The group sought to examine various aspects, including concerns surrounding a potential increase in antibiotic resistance, to tailor future gastric cancer prevention efforts accordingly36.
While our study findings highlight the potential public health impact of H. pylori screen-and-treat approaches that are evidence-based, relatively simple and effective, and safe and inexpensive to implement, relative to cancer treatment, in mitigating the increasing gastric cancer burden, the importance of continued efforts to develop an H. pylori vaccine needs to be stressed. As shown in the sensitivity analyses, the population-level impact of H. pylori treatment has substantial potential in determining the future burden of gastric cancers. This indicates that, as in human papillomavirus vaccine programs for cervical cancer prevention or hepatitis B vaccine programs to reduce the risk of liver cancer, an H. pylori vaccine would greatly advance the fight against gastric cancer, given that vaccination is one of the most context-responsive prevention strategies and is highly adaptable, especially in low- and middle-income settings where we expect to see a high number of H. pylori-attributable gastric cancer cases. Currently, only one H. pylori vaccine has passed phase 3 of a clinical trial (NCT02302170)37. More investment in future vaccine trials focusing on pediatric populations should be made, clarifying the mechanisms of vaccine-associated immunoprotection38. The accelerated development of vaccines against SARS-CoV-2 may facilitate new approaches for developing H. pylori vaccines39. An H. pylori vaccine could then take advantage of well-established existing financing and logistical support mechanisms40, which would help reduce barriers and challenges in reaching target populations and therefore bridge the inequity gap and ensure sustainability.
Some important limitations of the study need to be highlighted. Our analyses were based on data from GLOBOCAN, Cancer Incidence in Five Continents (CI5) and UN World Population Prospects, whose quality and accuracy depend on the availability and reliable and timely collection of data. There is likely an underestimation of H. pylori-attributable gastric cancers that are not captured in local and national registries, especially in lower-resource settings due to the relative paucity of high-quality population-based cancer registries. The information on gastric cancer and its subsite as extracted from population-based registries available from the IARC’s CI5 Volume XII (CI5-XII) database, a compilation of high-quality cancer incidence data at the national or subnational level, is particularly sparse in Africa41. Similarly, a marked proportion of all gastric cancers was categorized as having an overlapping (4%) or unspecified (26%) location, demonstrating the difficulty in estimating the site-specific proportions of gastric cancer. In an attempt to mitigate potential misclassification, we included only registries with at least 25% of topographically specified cases (C16.0 to C16.8) in this study for subsite-specific analyses, although the extended inclusion of cancer registries not meeting this criterion did not materially affect the results. Likewise, the HDI also relies on available national data and may mask subnational inequalities. Nevertheless, these reference data represent the best of what is currently available worldwide, and the results thus reflect the current knowledge on the expected and preventable burden.
In conclusion, we have projected the regional and country-specific burden of gastric cancer and estimated the burden attributable to H. pylori infection, assuming no change in the current age-specific rates of gastric cancer and no change in the current gastric cancer control measures. Our future estimates highlight an increasing burden of gastric cancer in areas traditionally considered to have low incidence, including Africa, and are of immediate relevance to public health decision-makers seeking to recalibrate prevention strategies based on local cancer profiles. Shifting the focus of gastric cancer burden projections from the traditional cross-sectional viewpoint toward the expected gastric cancer burden in young cohorts across their life course can aid policymakers in implementing effective interventions as part of a gastric cancer prevention program.
Methods
Country classification
We present the contribution to the expected absolute burden of gastric cancer in the year 2022 of the 185 countries included in IARC’s GLOBOCAN database hosted at the Global Cancer Observatory42, grouped by continent, UN world region and level of HDI. The HDI is reported by the UN Development Programme (https://hdr.undp.org/data-center) as a measure of the social and economic development of countries; details on the data sources and methods used in developing the GLOBOCAN estimates at the national level are available elsewhere43. Countries were sorted by the expected number of cases and subsequently grouped into the following five categories of incidence burden: >1,000,000, 100,000–1,000,000, 35,000–100,000, 10,000–35,000 and <10,000. We also grouped countries based on their age-standardized incidence rates to illustrate the gastric cancer burden: 0–5 in 100,000, >5–10 in 100,000 and >10 in 100,000. The HDI was further stratified by the predefined UN Development Programme four-tier categories into the low (<0.55), middle (0.55–0.70), high (0.70–0.79) and very high (≥0.80) levels.
Site-specific proportions of gastric cancer
To take into account the current evidence on potentially differential relationships between H. pylori infection and gastric cancer subsite by region28, we obtained the site-specific proportions of gastric cancer from the CI5-XII database44 for 52 countries where subsite classification is available, including a ‘cardia’ category (C16.0). Previous versions of the CI5 were used if no information was available in CI5-XII at the country level (ten countries), and other cancer registry data were used for eight other countries (see below for details).
Source of gastric cardia and noncardia cancers
Country-specific (when available) and subregional proportions of CGC versus NCGC were based on the following:
-
CI5-XII for Algeria, Australia, Austria, Belarus, Belgium, Benin, Brazil, Canada, Chile, China, Colombia, Costa Rica, Croatia, Cyprus, Czechia, Denmark, Ecuador, Estonia, Finland, France, Germany, Guadeloupe, Iceland, Iran, Ireland, Israel, Italy, Japan, Latvia, Lithuania, Malta, Martinique, Netherlands, New Caledonia, New Zealand, Norway, Peru, Poland, Portugal, Puerto Rico, Russia, Réunion, Singapore, Slovenia, South Korea, Spain, Sweden, Switzerland, Turkey, Ukraine, the UK and the USA
-
CI5-XI for Bulgaria, French Guiana, Jamaica, Jordan, Saudi Arabia, Slovakia and Vietnam
-
CI5-X for Egypt, Qatar and Tunisia
-
Other cancer registry data with at least 25% of topographically specified cases for French Polynesia, Kazakhstan, Nigeria, South Africa, Pakistan, Romania, Serbia and Vanuatu
When country-specific estimates from the proportions of cardia and noncardia cancers were not available, we attributed the mean values of the UN subregional proportions estimated from a hierarchical logistic random-effects model.
The ‘noncardia’ category includes six different topographic locations (C16.1 to C16.6), plus an overlapping category (C16.8) and a ‘not otherwise specified’ category (C16.9). The estimated numbers of noncardia cancer cases were obtained by applying the proportion of NCGC cases in existing local cancer registries (that is, C16.1 to C16.9) to the total number of gastric cancer cases by sex, age groups (<65 and 65+ years) and world area as estimated in the GLOBOCAN database, given that subsite data are not available. Using this method, we made the strong assumption that all cases in C16.8 and C16.9 are noncardia. To compute the proportions, we considered only the registries with at least 25% of topographically specified cases (C16.0 to C16.8). The proportions of CGC and NCGC were estimated using country-specific data for those with more than four cases of gastric cancer per age group and sex. Country-specific data were pooled at the UN subregional level to estimate the proportion of cardia and noncardia cases for countries with insufficient data. More details are available elsewhere45. To assess the robustness of our estimates to the cancer registry selection, we reconducted the analysis including the following 30 additional countries where registries had <25% of topographically specified cases: Argentina, Bahrain, Bosnia and Herzegovina, Botswana, Brunei, Congo (Brazzaville), Cuba, Côte d’Ivoire, Ghana, Guinea, India, Kenya, Kuwait, Lebanon, Libya, Malawi, Malaysia, Mali, Mauritius, Morocco, Namibia, Oman, Philippines, Seychelles, Thailand, Trinidad and Tobago, Uganda, Uruguay, Zambia and Zimbabwe. The results did not materially change.
Number of gastric cancers attributable to H. pylori infection
Accurate quantification of the fraction of gastric cancer cases attributable to H. pylori is highly dependent on obtaining accurate estimates of relative risk. In this study, we used data from improved studies that assessed the relative risk using prediagnostic samples with long-term follow-up, as well as studies using more sensitive immunoblotting than ELISA, to be consistent with our previous work on the global burden of cancer attributable to infections12,46. The estimated number of gastric cancer cases attributable to chronic infection with H. pylori was obtained using the attributable fractions previously calculated from prospective studies that applied immunoblotting to detect H. pylori at least 10 years before cancer diagnosis46, separately for NCGC (89% worldwide) and CGC (29% in eastern Asia, 0% in the rest of the world)12, except for China for which we used the latest estimates from a more recently published large-scale prospective study (78.5% for NCGC and 62.1% for CGC)29. These H. pylori-attributable cases are assumed to be potentially preventable through implementing population-based H. pylori screen-and-treat programs. Of note, H. pylori infection rates were not incorporated into our analyses, as we assumed no change in the current practices of gastric cancer prevention and no change in age-specific gastric cancer incidence rates in future years.
Expected lifetime number of gastric cancers
We estimated the expected lifetime number of gastric cancers among ten birth cohorts aged 5–14 years in 2022 (that is, those born between 2008 and 2017), up to the time point when the individuals would reach 84 years old, by adapting ATLAS, a previously published cancer progression model47. The statistical package for the ATLAS model is implemented in the methis.atlas R package. More information about the model is available on the IARC’s METHIS website (https://iarc-miarc.gitlab.io/methis/methis.website/docs/models/atlas.html). ATLAS combines the age-specific incidence estimates from the Global Cancer Observatory GLOBOCAN (https://gco.iarc.fr//today/en) project and the cohort-specific mortality rate projections by age from the UN Department of Economic and Social Affairs, Population Division, allowing for the competing risk of dying from any cause before being diagnosed with gastric cancer. Accounting for cohort-specific mortality rates by age enabled us to incorporate future demographic changes in our projections.
Briefly, for a given cohort, the expected cumulative number of gastric cancers between age 5 and 84 years was calculated using the 5-year age group-specific gastric cancer incidence rate projections from GLOBOCAN 2022, and 5-year age group-specific mortality rates were obtained from the UN Department of Economic and Social Affairs, Population Division48.
Let A5 be the age group 5 years and A84 the age group 84 years. Let us denote by λC and λM the gastric cancer incidence rate and mortality rate, respectively, both assumed to be piecewise constant functions in each 5-year age group. That is, for i = 5, …, 84, λC,i and λM,i denote the constant Ai-specific gastric cancer incidence and mortality rates, respectively. Let us also designate by \({A}_{i}^{\textrm{L}}\) and \({A}_{i}^{\textrm{U}}\) the lower and upper bounds of age interval Ai, with, for i > 0, \({A}_{i}^{\textrm{L}}={A}_{i-1}^{\textrm{U}}\). Then, the survival free from an event (that is, gastric cancer diagnosis or death) by age \({A}_{I}^{\textrm{U}}\) for a cohort of individuals aged 5 years at the start of follow-up can be calculated according to the following formula:
Now, the cumulative incidence of gastric cancer by age \({A}_{I}^{\textrm{U}}\) can be calculated as
We then have
Based on the assumption that the age group-specific incidence rates were stable across birth cohorts, the cumulative incidence between ages 5–14 years in 2022 and age 84 years was then obtained by summing the age group-specific contributions: \({\rm{Cum}}{{\rm{I}}}_{5-84}={\sum }_{i=5}^{84}{\rm{Cum}}{{\rm{I}}}_{i}\). Finally, the cumulative number of gastric cancer cases was calculated by multiplying this cumulative incidence by the size of the cohort at age 5–14 years in 2022, estimated from the UN database. UIs were estimated with Monte Carlo simulation combining the uncertainty about the overall gastric cancer incidence as reported in GLOBOCAN 2022 (central estimates and 95% UI), age-specific distribution of gastric cancer (GLOBOCAN 2022), proportions of CGC and NCGC, and mortality rate prediction uncertainties as reported by the UN Population Division (median estimates and 95% UI).
To illustrate the effect of future demographic changes on country-specific gastric cancer burden, we compared the average number of lifetime gastric cancer cases (up to age 84 years) that would be expected in an average single birth cohort born between 2008 and 2017—assuming no changes in the current practices of gastric cancer prevention—versus the total number of cases as estimated in the GLOBOCAN database, calculating a ratio between the two estimates (termed as the ‘ratio of change’). We assumed no change in the annual incidence of gastric cancer attributable to modifications in H. pylori prevalence or gastric cancer screening practices. The correlation between the above-mentioned ratio of change and country-specific HDI was calculated using Pearson’s coefficient (r). Country-specific 95% UIs for the average number of expected cases, obtained using simulation, combine the uncertainty in the gastric cancer incidence estimates from GLOBOCAN 2022 (central estimates and 95% UI) and all-cause mortality projections from the UN (median variant and 95% UI). These two sources of uncertainty are considered independent. No trends in age-specific gastric cancer incidence rates are considered.
Sensitivity and stratified analyses
We additionally conducted sensitivity analyses to quantify how much our estimates are affected by varying patterns of disease burden, life expectancy and cancer control practice. We also performed stratified analyses by sex and world region. The lifetime number of gastric cancers was estimated according to different model parameter disruptions in the 2008–2017 birth cohorts. Model outcomes were compared to the reference scenario, that is, (1) no competitive risk between CGC and NCGC, (2) median UN death rate scenario, (3) no APC in the incidence of CGC and NCGC, and (4) no change in the current public health intervention for gastric cancer control. The estimates were expressed in terms of the ratio of change and the percentage of change in the lifetime number of gastric cancer cases expected. The tested parameters included the following: (1) competing rate of the CGC versus NCGC burden; (2) death rate incorporating the lower 95% (optimistic) versus the higher 95% (pessimistic) estimates of the probabilistic projection of crude death rate by country or area in 2024–2100 from the UN model; (3) APC in the incidence of CGC and NCGC for 10 and 20 years, separately, by applying varying levels of APC (−3% to 3%); and (4) population-level impact of H. pylori screen-and-treat strategies by 80–100%.
All analyses were conducted with R (version 4.4.1) and the methis.atlas package (version 0.3.0).
Ethics and inclusion statement
This study was conducted with a commitment to providing policymakers with evidence that is both globally comparable and regionally meaningful. GLOBOCAN and CI5 estimates are based on data from population-based cancer registries and vital statistics registries worldwide, including low- and middle-income countries, and are relevant to all countries. Global estimates generated through this research are designed to be actionable and relevant to regional policymakers, providing them with timely, high-resolution data to inform decision-making and prioritize interventions tailored to local needs.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data used in this analysis are publicly available. GLOBOCAN estimates are available from the International Agency for Research on Cancer’s Global Cancer Observatory (https://gco.iarc.fr//today/en). The United Nations Development Programme’s Human Development Index data are available at https://hdr.undp.org/data-center/documentation-and-downloads. The United Nations World Population Prospects 2022 Probabilistic Projections data are available at https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf.
Code availability
All custom code used for the analysis of the data is available on GitLab (https://gitlab.com/iarc-ice/miarc/atlas-hpylori). No restrictions on the availability of the code have been set. The statistical package for the ATLAS model is implemented in the methis.atlas R package. More information about the model is available on the IARC’s METHIS website (https://iarc-miarc.gitlab.io/methis/methis.atlas/).
References
Hamashima, C.; Systematic Review Group and Guideline Development Group for Gastric Cancer Screening Guidelines.Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn. J. Clin. Oncol. 48, 673–683 (2018).
Jun, J. K. et al. Effectiveness of the Korean National Cancer Screening Program in reducing gastric cancer mortality. Gastroenterology 152, 1319–1328 (2017).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Morgan, E. et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: a population-based modelling study. EClinicalMedicine 47, 101404 (2022).
Howson, C. P., Hiyama, T. & Wynder, E. L. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol. Rev. 8, 1–27 (1986).
Shah, S. C. Gastric cancer: a neglected threat to racial and ethnic minorities in the USA. Lancet Gastroenterol. Hepatol. 6, 266–267 (2021).
Herrero, R., Parsonnet, J. & Greenberg, E. R. Prevention of gastric cancer. JAMA 312, 1197–1198 (2014).
Arnold, M. et al. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 69, 823–829 (2020).
Anderson, W. F. et al. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA 303, 1723–1728 (2010).
Anderson, W. F. et al. The changing face of noncardia gastric cancer incidence among US non-Hispanic whites. J. Natl Cancer Inst. 110, 608–615 (2018).
Luo, G. et al. Global patterns and trends in stomach cancer incidence: age, period and birth cohort analysis. Int. J. Cancer 141, 1333–1344 (2017).
de Martel, C., Georges, D., Bray, F., Ferlay, J. & Clifford, G. M. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob. Health 8, e180–e190 (2020).
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. A Review of Human Carcinogens. Part B: Biological Agents Vol. 100B (IARC, 2012).
Ford, A. C., Yuan, Y., Park, J. Y., Forman, D. & Moayyedi, P. Eradication therapy to prevent gastric cancer in Helicobacterpylori-positive individuals: systematic review and meta-analysis of randomized controlled trials and observational studies. Gastroenterology https://doi.org/10.1053/j.gastro.2024.12.033 (2025).
Park, J. Y. et al. Summary of IARC Working Group Meeting on Helicobacter pylori eradication as a strategy for preventing gastric cancer. In IARC Helicobacter pylori Working Group. Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer (IARC Working Group Report No. 8) 1–4 (IARC, 2014).
Europe’s Beating Cancer Plan—Communication from the Commission to the European Parliament and the Council health.ec.europa.eu/system/files/2022-02/eu_cancer-plan_en_0.pdf (European Commission, 2021).
Sutton, P. & Boag, J. M. Status of vaccine research and development for Helicobacter pylori. Vaccine 37, 7295–7299 (2019).
Xu, H. & Li, W. Early detection of gastric cancer in China: progress and opportunities. Cancer Biol. Med. 19, 1622–1628 (2022).
Chiang, T.-H. et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut 70, 243–250 (2021).
Tsuda, M. et al. Effect on Helicobacter pylori eradication therapy against gastric cancer in Japan. Helicobacter 22, e12415 (2017).
Dorji, T. et al. Population-level cancer screening and cancer care in Bhutan, 2020–2023: a review. Lancet Reg. Health Southeast Asia 24, 100370 (2024).
Riquelme, A. et al. Recommendations for gastric cancer prevention and control in the Americas. Lancet Reg. Health Am. 27, 100608 (2023).
Piazuelo, M. B. et al. The Colombian chemoprevention trial: 20-year follow-up of a cohort of patients with gastric precancerous lesions. Gastroenterology 160, 1106–1117 (2021).
SEER*Explorer: An Interactive Website for SEER Cancer Statistics (Surveillance Research Program, National Cancer Institute, accessed 17 March 2025); seer.cancer.gov/statistics-network/explorer/
Mashiko, S. et al. Helicobacter pylori management in Africa: a survey of diagnostic, treatment, and related resources. Helicobacter 29, e13153 (2024).
Holcombe, C. Helicobacter pylori: the African enigma. Gut 33, 429–431 (1992).
Coates, M. M. et al. Burden of non-communicable diseases from infectious causes in 2017: a modelling study. Lancet Glob. Health 8, e1489–e1498 (2020).
Gu, J. et al. A systematic review and meta-analysis on the relative and attributable risk of Helicobacter pylori infection and cardia and non-cardia gastric cancer. Expert Rev. Mol. Diagn. 23, 1251–1261 (2023).
Yang, L. et al. The relative and attributable risks of cardia and non-cardia gastric cancer associated with Helicobacter pylori infection in China: a case–cohort study. Lancet Public Health 6, e888–e896 (2021).
Pan, K.-F. et al. Gastric cancer prevention by community eradication of Helicobacter pylori: a cluster-randomized controlled trial. Nat. Med. 30, 3250–3260 (2024).
Lansdorp-Vogelaar, I. et al. Cost-effectiveness of prevention and early detection of gastric cancer in Western countries. Best Pract. Res. Clin. Gastroenterol. 50–51, 101735 (2021).
Ford, A. C., Yuan, Y. & Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut 69, 2113–2121 (2020).
Ford, A. C., Yuan, Y. & Moayyedi, P. Long-term impact of Helicobacter pylori eradication therapy on gastric cancer incidence and mortality in healthy infected individuals: a meta-analysis beyond 10 years of follow-up. Gastroenterology 163, 754–756 (2022).
European Commission, Directorate-General for Research and Innovation, Group of Chief Scientific Advisors. Cancer Screening in the European Union (Publications Office of the European Union, 2022).
Leja, M. Where are we with gastric cancer screening in Europe in 2024? Gut 73, 2074–2082 (2024).
Park, J. Y. (ed.) Population-Based Helicobacter pylori Screen-and-Treat Strategies for Gastric Cancer Prevention: Guidance on Implementation (IARC Working Group Report No. 12) (IARC, 2025).
Zeng, M. et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386, 1457–1464 (2015).
Yunle, K., Tong, W., Jiyang, L. & Guojun, W. Advances in Helicobacter pylori vaccine research: from candidate antigens to adjuvants—a review. Helicobacter 29, e13034 (2024).
Park, J. Y. & Herrero, R. Recent progress in gastric cancer prevention. Best Pract. Res. Clin. Gastroenterol. 50–51, 101733 (2021).
Gavi, the Vaccine Alliance. Annual Progress Report 2022 (Gavi, 2022).
Li, M. et al. Population-based investigation of common and deviating patterns of gastric cancer and oesophageal cancer incidence across populations and time. Gut 72, 846–854 (2023).
Ferlay, J. et al. Global Cancer Observatory: Cancer Today (Version 1.1) (International Agency for Research on Cancer, accessed 2 February 2025); gco.iarc.who.int/today
Filho, A. M. et al. The GLOBOCAN 2022 cancer estimates: data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 156, 1336–1346 (2025).
Bray, F. et al. (eds) Cancer Incidence in Five Continents, Vol. XII (IARC CancerBase No. 19) (IARC, 2023).
Arnold, M., Ferlay, J., van Berge Henegouwen, M. I. & Soerjomataram, I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut 69, 1564–1571 (2020).
Plummer, M., Franceschi, S., Vignat, J., Forman, D. & de Martel, C. Global burden of gastric cancer attributable to Helicobacter pylori. Int. J. Cancer 136, 487–490 (2015).
Bonjour, M. et al. Global estimates of expected and preventable cervical cancers among girls born between 2005 and 2014: a birth cohort analysis. Lancet Public Health 6, e510–e521 (2021).
United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects 2022 population.un.org/wpp/ (UN, 2022).
Acknowledgements
This research was supported by an intramural grant from the International Agency for Research on Cancer/World Health Organization (IARC/WHO). Where authors are identified as personnel of the IARC/WHO, the authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policies or views of the IARC/WHO. We would like to acknowledge M. Colombet and H. Rumgay (IARC/WHO) for their assistance with data extraction from population-based cancer registries, and we thank all population-based cancer registries and their staff who contributed to sharing the cancer incidence data used to build the estimates applied in this study. We would also like to thank M. Bonjour (IARC/WHO) for the constructive discussions on how to adapt the ATLAS model to gastric cancer prevention and N. Akel (IARC/WHO) for English-language editing support.
Author information
Authors and Affiliations
Contributions
The study was conceptualized by J.Y.P., G.C. and I.B. Analyses were conducted by D.G. under the supervision of I.B. and with input from J.Y.P., C.J.A., F.B. and G.C. The first manuscript draft was prepared by J.Y.P. and D.G. All authors contributed to the editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Wanqing Chen, Shilpa Murthy and Kai-Feng Pan for their contribution to the peer review of this work. Primary Handling Editor: Ming Yang, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Country-specific number of gastric cancer cases expected in persons born between 2008 and 2017 in the absence of changes in the current control measures for gastric cancer (dark grey bar) and the cases attributable to H. pylori infection (light grey bar).
Countries are grouped according to their contribution to the overall gastric cancer burden, with countries sorted according to the expected number of gastric cancer cases and subsequently grouped into the following categories: 10,000 to 35,000 (group D) and less than 10,000 (group E).
Extended Data Fig. 2 Global analysis outcomes by various tested parameters by region and sex*.
*This figure shows variation of the overall lifetime expected number of gastric cancers born between 2008 and 2017 according to changes of the model parameters. The variation could be read in terms of absolute number of cases (upper x-axis) or ratio of the lifetime expected number of gastric cancers (lower x-axis). The reference model parameters are the same as the ones assumed in the main text (that is no competitive risk between cardia (CGC) and non-cardia (NCGC) gastric cancer, median UN death rate scenario, no annual percentage change (APC) for CGC and NCGC, no changes in the current public health intervention (PHI) for gastric cancer control). Each line on the y-axis represents the specific parameter changed compared to the reference while all the other parameters remain unchanged. The tested parameters are: 1. Competing Rate: Including CGC competing rate in the NCGC burden calculation and vice and versa 2 (yes). 2. Death Rate: Changing the overall cause of death competing risk for UN lower (lwr) or upper (upr) scenario. 3. APC CGC (10 years): Applying −3%, −2%, −1%, 1%, 2% and 3% APC for 10 years to the CGC incidence rate. 4. APC CGC (20 years): Applying −3%, −2%, −1%, 1%, 2% and 3% APC for 20 years to the CGC incidence rate. 5. APC NCGC (10 years): Applying −3%, −2%, −1%, 1%, 2% and 3% APC for 10 years to the NCGC incidence rate. 6. APC NCGC (20 years): Applying −3%, −2%, −1%, 1%, 2% and 3% APC for 20 years to the NCGC incidence rate. 7. PHI Impact: Applying population-level impact of H. pylori screen-and-treat strategies by 80–100%.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, J.Y., Georges, D., Alberts, C.J. et al. Global lifetime estimates of expected and preventable gastric cancers across 185 countries. Nat Med 31, 3020–3027 (2025). https://doi.org/10.1038/s41591-025-03793-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-025-03793-6
This article is cited by
-
Why internists should care about Helicobacter pylori: recapitulating gastric cancer prevention
Internal and Emergency Medicine (2025)
-
Association between Candida esophagitis and esophagojejunal leakage following total gastrectomy: a retrospective cohort study
Langenbeck's Archives of Surgery (2025)