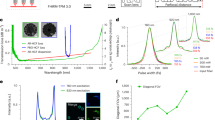
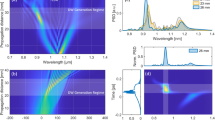
Abstract
Optical approaches to monitor neural activity are transforming neuroscience, owing to a fast-evolving palette of genetically encoded molecular reporters. However, the field still requires robust and label-free technologies to monitor the multifaceted biomolecular changes accompanying brain development, aging or disease. Here, we have developed vibrational fiber photometry as a low-invasive method for label-free monitoring of the biomolecular content of arbitrarily deep regions of the mouse brain in vivo through spontaneous Raman spectroscopy. Using a tapered fiber probe as thin as 1 µm at its tip, we elucidate the cytoarchitecture of the mouse brain, monitor molecular alterations caused by traumatic brain injury, as well as detect markers of brain metastasis with high accuracy. We view our approach, which introduces a deep learning algorithm to suppress probe background, as a promising complement to the existing palette of tools for the optical interrogation of neural function, with application to fundamental and preclinical investigations of the brain and other organs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data underlying the conclusions of this manuscript are available from the corresponding authors and the following link: https://doi.org/10.5281/zenodo.14016545 (ref. 70), under CC BY-NC-SA 4.0.
Code availability
The code underlying the conclusions of this manuscript is available from the corresponding authors and at the following link: https://doi.org/10.5281/zenodo.14016545 (ref. 70), under CC BY-NC-SA 4.0.
References
Abdelfattah, A. et al. Neurophotonic tools for microscopic measurements and manipulation: Status report. Neurophotonics 9, 1–86 (2022).
Patriarchi, T. et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360, eaat4422 (2018).
Sabatini, B. L. & Tian, L. Imaging neurotransmitter and neuromodulator dynamics in vivo with genetically encoded indicators. Neuron 108, 17–32 (2020).
Day‐Cooney, J., Dalangin, R., Zhong, H. & Mao, T. Genetically encoded fluorescent sensors for imaging neuronal dynamics in vivo. J. Neurochem. 164, 284–308 (2023).
Pal, A. & Tian, L. Imaging voltage and brain chemistry with genetically encoded sensors and modulators. Curr. Opin. Chem. Biol. 57, 166–176 (2020).
Moreaux, L. C. et al. Integrated neurophotonics: toward dense volumetric interrogation of brain circuit activity – at depth and in real time. Neuron 108, 66–92 (2020).
Jones, R. R., Hooper, D. C., Zhang, L., Wolverson, D. & Valev, V. K. Raman techniques: fundamentals and frontiers. Nanoscale Res. Lett. 14, 1–34 (2019).
Lee, H. J. et al. Label-free vibrational spectroscopic imaging of neuronal membrane potential. J. Phys. Chem. Lett. 8, 1932–1936 (2017).
Zhang, L. et al. Spectral tracing of deuterium for imaging glucose metabolism. Nat. Biomed. Eng. 3, 402–413 (2019).
DePaoli, D. et al. Rise of Raman spectroscopy in neurosurgery: a review. J. Biomed. Opt. 25, 1–36 (2020).
Jermyn, M. et al. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci. Transl. Med. 7, 274ra19 (2015).
Ji, M. et al. Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy. Sci. Transl. Med. 5, 201ra119-201ra119 (2013).
Orringer, D. A. et al. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nat. Biomed. Eng. 1, 0027 (2017).
Trägårdh, J. et al. Label-free CARS microscopy through a multimode fiber endoscope. Opt. Express 27, 30055–30066 (2019).
Lombardini, A. et al. High-resolution multimodal flexible coherent Raman endoscope. Light Sci. Appl. 7, 10 (2018).
Desroches, J. et al. A new method using Raman spectroscopy for in vivo targeted brain cancer tissue biopsy. Sci. Rep. 8, 1792 (2018).
Liu, Z. et al. Raman fiber photometry for understanding mitochondrial superoxide burst and extracellular calcium ion influx upon acute hypoxia in the brain of freely moving animals. Angew. Chem. Int. Ed. Engl. 61, e202111630 (2022).
Gebrekidan, M. T., Knipfer, C. & Braeuer, A. S. Refinement of spectra using a deep neural network: fully automated removal of noise and background. J. Raman Spectrosc. 52, 723–736 (2021).
Wahl, J., Sjödahl, M. & Ramser, K. Single-step preprocessing of Raman spectra using convolutional neural networks. Appl. Spectrosc. 74, 427–438 (2020).
Huang, J. et al. Remote SERS detection at a 10-m scale using silica fiber SERS probes coupled with a convolutional neural network. Opt. Lett. 48, 896–899 (2023).
Kazemzadeh, M. et al. Cascaded deep convolutional neural networks as improved methods of preprocessing Raman spectroscopy data. Anal. Chem. 94, 12907–12918 (2022).
Castellani, G., Croese, T., Peralta Ramos, J. M. & Schwartz, M. Transforming the understanding of brain immunity. Science 380, eabo7649 (2023).
Mancusi, R. & Monje, M. The neuroscience of cancer. Nature 618, 467–479 (2023).
Heng, H. P. S., Shu, C., Zheng, W., Lin, K. & Huang, Z. Advances in real‐time fiber‐optic Raman spectroscopy for early cancer diagnosis: pushing the frontier into clinical endoscopic applications. Transl. Biophotonics 3, e202000018 (2021).
Pisanello, M. et al. Tailoring light delivery for optogenetics by modal demultiplexing in tapered optical fibers. Sci. Rep. 8, 4467 (2018).
Stibůrek, M. et al. 110 μm thin endo-microscope for deep-brain in vivo observations of neuronal connectivity, activity and blood flow dynamics. Nat. Commun. 14(1), 1897 (2023).
Pisanello, F. et al. Dynamic illumination of spatially restricted or large brain volumes via a single tapered optical fiber. Nat. Neurosci. 20, 1180–1188 (2017).
Pisano, F. et al. Depth-resolved fiber photometry with a single tapered optical fiber implant. Nat. Methods 16, 1185–1192 (2019).
Lee, J., Wang, W. & Sabatini, B. L. Anatomically segregated basal ganglia pathways allow parallel behavioral modulation. Nat. Neurosci. 23, 1388–1398 (2020).
Koljenović, S. et al. Tissue characterization using high wave number Raman spectroscopy. J. Biomed. Opt. 10, 031116 (2005).
Koljenović, S. et al. Raman spectroscopic characterization of porcine brain tissue using a single fiber-optic probe. Anal. Chem. 79, 557–564 (2007).
Kirsch, M., Schackert, G., Salzer, R. & Krafft, C. Raman spectroscopic imaging for in vivo detection of cerebral brain metastases. Anal. Bioanal. Chem. 398, 1707–1713 (2010).
Priego, N. et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat. Med. 24, 1024–1035 (2018).
Krafft, C., Kirsch, M., Beleites, C., Schackert, G. & Salzer, R. Methodology for fiber-optic Raman mapping and FTIR imaging of metastases in mouse brains. Anal. Bioanal. Chem. 389, 1133–1142 (2007).
Short, K. W., Carpenter, S., Freyer, J. P. & Mourant, J. R. Raman spectroscopy detects biochemical changes due to proliferation in mammalian cell cultures. Biophys. J. 88, 4274–4288 (2005).
Siam, L. et al. The metastatic infiltration at the metastasis/brain parenchyma-interface is very heterogeneous and has a significant impact on survival in a prospective study. Oncotarget 6, 29254–29267 (2015).
Dankner, M. et al. Invasive growth associated with cold-inducible RNA-binding protein expression drives recurrence of surgically resected brain metastases. Neuro Oncol. 23, 1470–1480 (2021).
Berghoff, A. S. et al. Invasion patterns in brain metastases of solid cancers. Neuro Oncol 15, 1664–1672 (2013).
Surmacki, J. M. et al. Label-free monitoring of tissue biochemistry following traumatic brain injury using Raman spectroscopy. Analyst 142, 132–139 (2016).
Jamieson, L. E., Li, A., Faulds, K. & Graham, D. Ratiometric analysis using Raman spectroscopy as a powerful predictor of structural properties of fatty acids. R. Soc. Open Sci. 5, 181483 (2018).
van Doorn, H. C. et al. Raman spectroscopy for guidance of vulvar cancer surgery: a pilot study. Biomed. Opt. Express 12, 3008–3020 (2021).
Marro, M. et al. Dynamic molecular monitoring of retina inflammation by in vivo Raman spectroscopy coupled with multivariate analysis. J. Biophotonics 7, 724–734 (2014).
Ow, Y.-L. P., Green, D. R., Hao, Z. & Mak, T. W. Cytochrome c: functions beyond respiration. Nat. Rev. Mol. Cell Biol. 9, 532–542 (2008).
Amorini, A. M. et al. Severity of experimental traumatic brain injury modulates changes in concentrations of cerebral free amino acids. J. Cell. Mol. Med. 21, 530–542 (2017).
Bianco, M. et al. Orthogonalization of far-field detection in tapered optical fibers for depth-selective fiber photometry in brain tissue. APL Photonics 7, 026106 (2022).
Huang, Z. et al. Tapered optical fiber probe assembled with plasmonic nanostructures for surface-enhanced Raman scattering application. ACS Appl. Mater. Interfaces 7, 17247–17254 (2015).
Li, T., Yu, Z., Wang, Z., Zhu, Y. & Zhang, J. Optimized tapered fiber decorated by Ag nanoparticles for Raman measurement with high sensitivity. Sensors 21, 2300 (2021).
Zheng, D. et al. Toward plasmonic neural probes: SERS detection of neurotransmitters through gold‐nanoislands‐decorated tapered optical fibers with sub‐10 nm gaps. Adv. Mater. 35, 2200902 (2023).
Gusachenko, I., Chen, M. & Dholakia, K. Raman imaging through a single multimode fibre. Opt. Express 25, 13782–13798 (2017).
Collard, L. et al. Holographic manipulation of nanostructured fiber optics enables spatially‐resolved, reconfigurable optical control of plasmonic local field enhancement and SERS. Small 18, e2200975 (2022).
Sharma, K. et al. Multifunctional optrode for opsin delivery, optical stimulation, and electrophysiological recordings in freely moving rats. J. Neural Eng. 18, 1741–2552 (2021).
Qazi, R. et al. Wireless optofluidic brain probes for chronic neuropharmacology and photostimulation. Nat. Biomed. Eng. 3, 655–669 (2019).
Spagnolo, B. et al. Tapered fibertrodes for optoelectrical neural interfacing in small brain volumes with reduced artefacts. Nat. Mater. 21, 826–835 (2022).
Zhang, S., Liu, Z., Zhang, Z., Wang, W. & Tian, Y. High‐density optophysiology platform for recording intracellular and extracellular signals across the brain of free‐moving animals. Angew. Chem. Int. Ed. Engl. 62, e202301382 (2023).
Krishna, S. et al. Glioblastoma remodelling of human neural circuits decreases survival. Nature 617, 599–607 (2023).
Sanchez-Aguilera, A. et al. Machine learning identifies experimental brain metastasis subtypes based on their influence on neural circuits. Cancer Cell 41, 1637–1649 (2023).
Eichmüller, O. L. & Knoblich, J. A. Human cerebral organoids: a new tool for clinical neurology research. Nat. Rev. Neurol. 18, 661–680 (2022).
Trujillo, C. A. et al. Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 25, 558–569 (2019).
Le Duigou, C. et al. Imaging pathological activities of human brain tissue in organotypic culture. J. Neurosci. Methods 298, 33–44 (2018).
Sileo, L., Pisanello, M., De Vittorio, M. & Pisanello, F. Fabrication of multipoint light emitting optical fibers for optogenetics. In Proceedings of SPIE: Optical Techniques in Neurosurgery, Neurophotonics, and Optogenetics II 9305, 93052O (2015).
Maglie, E. et al. Ray tracing models for estimating light collection properties of microstructured tapered optical fibers for optical neural interfaces. Opt. Lett. 45, 3856–3859 (2020).
Shi, L., Sordillo, L. A., Rodríguez-Contreras, A. & Alfano, R. Transmission in near-infrared optical windows for deep brain imaging. J. Biophotonics 9, 38–43 (2016).
Pisanello, M. et al. The three-dimensional signal collection field for fiber photometry in brain tissue. Front. Neurosci. 13, 82 (2019).
Tessier, M. et al. Bumetanide induces post-traumatic microglia–interneuron contact to promote neurogenesis and recovery. Brain 146, 4247–4261 (2023).
Le Losq, C. Rampy: a Python library for processing spectroscopic (IR, Raman, XAS…) data. Zenodo https://doi.org/10.5281/ZENODO.4715040 (2018).
Shalabaeva, V. et al. Time resolved and label free monitoring of extracellular metabolites by surface enhanced Raman spectroscopy. PLoS One 12, e0175581 (2017).
Di Mascolo, D., Coclite, A., Gentile, F. & Francardi, M. Quantitative micro-Raman analysis of micro-particles in drug delivery. Nanoscale Adv. 1, 1541–1552 (2019).
Das, G. et al. Principal component analysis based methodology to distinguish protein SERS spectra. J. Mol. Struct. 993, 500–505 (2011).
Rodriguez, A. & Laio, A. Clustering by fast search and find of density peaks. Science 344, 1492–1496 (2014).
Pisano, F. et al. Data and code for paper: vibrational fiber photometry: label-free and reporter-free minimally invasive Raman spectroscopy deep in the mouse brain. Zenodo https://doi.org/10.5281/zenodo.14016545 (2024).
Acknowledgements
This work is dedicated to the memory of Prof. Marco Grande and to his legacy of scientific excellence and human kindness. M.M.-M, E.C., T.J.P., A.B., F.T., L.C., M.D.V., F.D.A., L.M.P., M.V. and F. Pisanello acknowledge funding from the European Union’s Horizon 2020 FET-Open Programme project NANOBRIGHT under grant agreement 828972. F. Pisano, M.B., M.P., L.S., M.D.V. and F. Pisanello acknowledge funding from the European Union’s Horizon 2020 Research and Innovation Programme project DEEPER under grant agreement 101016787. This research received support from the European Community under the program Horizon 2020 project ProID under grant agreement 964363. F. Pisano acknowledges funding from PARD 2024 from the University of Padua. M.M.-M. acknowledges funding from a postdoctoral grant (2023) from the Scientific Foundation of the Spanish Association Against Cancer. F.G. acknowledges funding from Associazione Italiana per la Ricerca sul Cancro – AIRC IG 2021 ID 25656. L.C., M.D.V. and F. Pisanello acknowledge funding from the Project 'RAISE (Robotics and AI for Socio-economic Empowerment)' (code ECS00000035) funded by European Union – NextGenerationEU PNRR MUR – M4C2 – Investimento 1.5 – Avviso 'Ecosistemi dell’Innovazione' CUP J33C22001220001. A.B. acknowledges funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement (101106602).
Author information
Authors and Affiliations
Contributions
F. Pisano and M.M.M. are co-first authors. M.S.A., E.C. M.K. and M.P. contributed equally and are listed in alphabetical order. F.G., F.D.A., M.D.V., L.M.P., M.V. and F. Pisanello jointly supervised the work and are co-last authors on this work. F. Pisano, M.P., M.G., A.B., F.T., F.D.A., M.D.V. and F. Pisanello developed the bench-top Raman system. M.P., M.S.A., F.T., F. Pisano and F. Pisanello developed the portable Raman system. M.M.-M., M.V., T.J.P. and L.M.P. tested the portable system. L.S. developed the tapered optical fiber for the Raman application. M.S.A., M.P. and F. Pisano performed the ex vivo experiments. M.M.-M., P.B. and M.V. developed the melanoma brain metastasis model and performed the in vivo experiments. E.C., T.J.P. and L.M.P. developed the traumatic brain injury model and carried out the immunohistochemistry. T.J.P., E.C., M.S.A. and L.M.P. performed the in vivo traumatic brain injury experiments and collected Raman data. F. Pisano, M.S.A., M.P., M.B., A.B., L.C., F.G. and F. Pisanello developed and performed the data analysis. M.K. developed and performed the deep learning network and analysis. M.B., M.G. and F. Pisano developed and performed the numerical simulations. F. Pisano and A.B. fabricated the micro-structured probes and performed the experiments. F.G. developed and performed the principal component analysis. F. Pisano, F.D.A., M.D.V., L.M.P., M.V. and F. Pisanello conceived the application of vibration fiber photometry in deep brain regions. F. Pisano and F. Pisanello conceptualized the work with contributions from all authors. F. Pisano drafted the manuscript and prepared the figures. All of the authors edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
L.S., M.D.V. and F. Pisanello are founders and hold private equity in Optogenix, a company that develops, produces and sells technologies to deliver light into the brain. L.S. and M.P. are employed by Optogenix. F. Pisano has been employed by Optogenix. Products of Optogenix have been used in this research. M.V. receives research funds from AstraZeneca. All other authors have no competing interests.
Peer review
Peer review information
Nature Methods thanks Hyeon Jeong Lee, Wei Min, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Nina Vogt, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Histology and reconstruction of fiber tracks.
a, Comparison of histology and PCA prediction for melanoma brain metastasis in ex vivo brain. Histological classification is overlaid with histological images for healthy tissue (green) and tissue with metastatic cells (red) within the collection area of the probe. Similarly, PCA classification in cluster 1, associated with healthy tissue (cyan), and cluster 2, associated with cancer (magenta),in the fingerprint (FP) (filled circles) and high-wavenumber (HW) (empty circles) ranges. b, Images of the fiber track position after in vivo experiments generating the spectra shown as orange dots in the PCA clusters in Fig. 2n. The images are acquired in fixed brain slices. The fiber track, pointed by red arrows, narrowly misses the brain metastasis. The image on the left was acquired on a brightfield fluorescence microscope; the image on the right was obtained by processing the left image with a high-pass filter to highlight the fiber track.
Extended Data Fig. 2 Supplementary data for ratiometric analysis.
a, Example of the raw Raman spectra used for ratiometric analysis acquired in metastatic tissue (magenta) and in a contralateral position in healthy tissue (cyan). b Derivative of Raman spectra for cancer free (cyan) and cancer invade (magenta) regions in the high-wavenumber (HW) range. For display reasons, the plot shows 1 measurement out of 5 for every implant position.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pisano, F., Masmudi-Martín, M., Andriani, M.S. et al. Vibrational fiber photometry: label-free and reporter-free minimally invasive Raman spectroscopy deep in the mouse brain. Nat Methods 22, 371–379 (2025). https://doi.org/10.1038/s41592-024-02557-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41592-024-02557-3
This article is cited by
-
In vivo multimodal neurochemical interfaces for real-time decoding of brain circuit
Nature Reviews Neuroscience (2025)