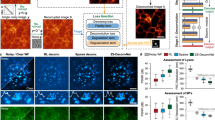
Abstract
In fluorescence microscopy, a persistent challenge is the defocused background that obscures cellular details and introduces artifacts. Here, we introduce Dark sectioning, a method inspired by natural image dehazing for removing backgrounds that leverages dark channel prior and dual frequency separation to provide single-frame optical sectioning. Unlike denoising or deconvolution, Dark sectioning specifically targets and removes out-of-focus backgrounds, stably improving the signal-to-background ratio by nearly 10 dB and structural similarity index measure of images by approximately tenfold. Dark sectioning was validated using wide-field, confocal, two/three-dimensional structured illumination and one/two-photon microscopy with high-fidelity reconstruction. We further demonstrate its potential to improve the segmentation accuracy in deep tissues, resulting in better recognition of neurons in the mouse brain and accurate assessment of nuclei in prostate lesions or mouse brain sections. Dark sectioning is compatible with many other microscopy modalities, including light-sheet and light-field microscopy, as well as processing algorithms, including deconvolution and super-resolution optical fluctuation imaging.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
General data are provided to reviewers and uploaded to figshare at https://figshare.com/articles/dataset/Dark-sectioning/24607614 (ref. 54). Some small size data are in the tags of GitHub at https://github.com/Cao-ruijie/Dark-sectioning.
Code availability
Software for Dark sectioning is uploaded to GitHub at https://github.com/Cao-ruijie/Dark-sectioning. It contains three versions: Fiji (based on Java), Windows.exe and MATLAB code.
References
Betzig, E., Trautman, J. K., Harris, T. D., Weiner, J. S. & Kostelak, R. L. Breaking the diffraction barrier: optical microscopy on a nanometric scale. Science 251, 1468–1470 (1991).
Gustafsson, M. G. L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198, 82–87 (2000).
Hell, S. W. & Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780–782 (1994).
Huang, X. et al. Fast, long-term, super-resolution imaging with Hessian structured illumination microscopy. Nat. Biotechnol. 36, 451–459 (2018).
Mo, Y. et al. Quantitative structured illumination microscopy via a physical model-based background filtering algorithm reveals actin dynamics. Nat. Commun. 14, 3089 (2023).
Demmerle, J. et al. Strategic and practical guidelines for successful structured illumination microscopy. Nat. Protoc. 12, 988–1010 (2017).
Li, Z. et al. Fast widefield imaging of neuronal structure and function with optical sectioning in vivo. Sci. Adv. 6, eaaz3870 (2020).
Bayguinov, P. O. et al. Modern laser scanning confocal microscopy. Curr. Protoc. Cytom. 85, e39 (2018).
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005).
Dan, D. et al. Super-resolution and optical sectioning integrated structured illumination microscopy. J. Phys. D Appl. Phys. 54, 074004 (2020).
Guo, X. et al. Rapid 3D isotropic imaging of whole organ with double-ring light-sheet microscopy and self-learning side-lobe elimination. Biomed. Opt. Express 14, 6206–6221 (2023).
Temma, K. et al. Selective-plane-activation structured illumination microscopy. Nat. Methods 21, 889–896 (2024).
Ou, Z. et al. Achieving optical transparency in live animals with absorbing molecules. Science 385, eadm6869.
Richardson, D. S. et al. Tissue clearing. Nat. Rev. Methods Prim. 1, 84 (2021).
Lim, D., Chu, K. K. & Mertz, J. Wide-field fluorescence sectioning with hybrid speckle and uniform-illumination microscopy. Opt. Lett. 33, 1819–1821 (2008).
Qiao, C. et al. Evaluation and development of deep neural networks for image super-resolution in optical microscopy. Nat. Methods 18, 194–202 (2021).
Qian, J. et al. Structured illumination microscopy based on principal component analysis. eLight 3, 4 (2023).
Zhao, W. et al. Enhanced detection of fluorescence fluctuations for high-throughput super-resolution imaging. Nat. Photonics 17, 806–813 (2023).
Zhao, W. et al. Sparse deconvolution improves the resolution of live-cell super-resolution fluorescence microscopy. Nat. Biotechnol. 40, 606–617 (2022).
Ingaramo, M. et al. Richardson–Lucy deconvolution as a general tool for combining images with complementary strengths. ChemPhysChem 15, 794–800 (2014).
Hou, Y. W. et al. Multi-resolution analysis enables fidelity-ensured deconvolution for fluorescence microscopy. eLight 4, 14 (2024).
Rodrigues, M. C. M. & Militzer, M. Application of the Rolling ball algorithm to measure phase volume fraction from backscattered electron images. Mater. Charact. 163, 110273 (2020).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Zhang, Y. et al. Rapid detection of neurons in widefield calcium imaging datasets after training with synthetic data. Nat. Methods 20, 747–754 (2023).
Zhang, Y. et al. Computational optical sectioning with an incoherent multiscale scattering model for light-field microscopy. Nat. Commun. 12, 6391 (2021).
He, K., Sun, J. & Tang, X. Single image haze removal using dark channel prior. IEEE Trans. Pattern Anal. Mach. Intell. 33, 2341–2353 (2010).
Golts, A., Freedman, D. & Elad, M. Unsupervised single image dehazing using dark channel prior loss. IEEE Trans. Image Process. 29, 2692–2701 (2019).
Gustafsson, M. G. L. et al. Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination. Biophys. J. 94, 4957–4970 (2008).
Cao, R. et al. Fast reconstruction and optical-sectioning three-dimensional structured illumination microscopy. Innovation 6, 100757 (2025).
Zhao, C. et al. Miniature three-photon microscopy maximized for scattered fluorescence collection. Nat. Methods 20, 617–622 (2023).
Wilson, T. & Masters, B. M. Confocal microscopy. Appl. Opt. 33, 565–566 (1994).
Culley, S. et al. Quantitative mapping and minimization of super-resolution optical imaging artifacts. Nat. Methods 15, 263–266 (2018).
Li, Y. et al. High-speed autopolarization synchronization modulation three-dimensional structured illumination microscopy. Adv. Photonics Nexus 3, 016001 (2024).
Zhanghao, K. et al. Super-resolution imaging of fluorescent dipoles via polarized structured illumination microscopy. Nat. Commun. 10, 4694 (2019).
Cao, R. et al. Open-3DSIM: an open-source three-dimensional structured illumination microscopy reconstruction platform. Nat. Methods 20, 1183–1186 (2023).
Qu, L. et al. Self-inspired learning for denoising live-cell super-resolution microscopy. Nat. Methods 21, 1895–1908 (2024).
Li, X. et al. Spatial redundancy transformer for self-supervised fluorescence image denoising. Nat. Comput. Sci. 3, 1067–1080 (2023).
Müller, M., Mönkemöller, V., Hennig, S., Hübner, W. & Huser, T. Open-source image reconstruction of super-resolution structured illumination microscopy data in ImageJ. Nat. Commun. 7, 10980 (2016).
Hobson, C. M. et al. Practical considerations for quantitative light sheet fluorescence microscopy. Nat. Methods 19, 1538–1549 (2022).
Zhao, Z. et al. Two-photon synthetic aperture microscopy for minimally invasive fast 3D imaging of native subcellular behaviors in deep tissue. Cell 186, 2475–2491.e222 (2023).
Wen, G. et al. High-fidelity structured illumination microscopy by point-spread-function engineering. Light. Sci. Appl. 10, 70 (2021).
Zhao, W. et al. Quantitatively mapping local quality of super-resolution microscopy by rolling Fourier ring correlation. Light. Sci. Appl. 12, 298 (2023).
Lu, Z. et al. Long-term intravital subcellular imaging with confocal scanning light-field microscopy. Nat. Biotechnol. 43, 569–580 (2024).
Zhang, W. et al. Optimized approach for optical sectioning enhancement in multifocal structured illumination microscopy. Opt. Express 28, 10919–10927 (2020).
Ren, W. et al. Expanding super-resolution imaging versatility in organisms with multi-confocal image scanning microscopy. Natl Sci. Rev. 11, nwae303 (2024).
Hou, Y. et al. Multi-resolution analysis enables fidelity-ensured deconvolution for fluorescence microscopy. eLight 4, 14 (2024).
Li, Y. et al. Real-time 3D single-molecule localization using experimental point spread functions. Nat. Methods 15, 367–369 (2018).
Sun, J. et al. Super-resolution imaging of mitochondrial cristae using a more hydrophobic far-red Si-rhodamine probe. Chem. Commun. 59, 13038–13041 (2023).
Sun, Y., Wang, Y., Li, W. & Li, C. Real-time dual-modal photoacoustic and fluorescence small animal imaging. Photoacoustics 36, 100593 (2024).
Shi, W. et al. Aberration correction for deformable-mirror-based remote focusing enables high-accuracy whole-cell super-resolution imaging. Photon. Res. 12, 821–832 (2024).
Stringer, C., Wang, T., Michaelos, M. & Pachitariu, M. Cellpose: a generalist algorithm for cellular segmentation. Nat. Methods 18, 100–106 (2021).
Stringer, C. & Pachitariu, M. J. B. Cellpose3: one-click image restoration for improved cellular segmentation. 2024.2002. 2010.579780 (2024).
Sekh, A. A. et al. Physics-based machine learning for subcellular segmentation in living cells. Nat. Mach. Intell. 3, 1071–1080 (2021).
Cao, R. & Xi, Peng. Dark-sectioning. figshare. Dataset. figshare https://doi.org/10.6084/m9.figshare.24607614.v1 (2024).
Acknowledgements
This work was supported by the funding support from the National Key R&D Program of China (2022YFC3401100 to P.X.), and the National Natural Science Foundation of China (62025501 and 92150301 to P.X., T2421003 to J.Q., 62335008 and 62405010 to M.L., 624B2009 to R.C. and 62305004 to B.J.). We thank the National Center for Protein Sciences and the Core Facilities of Life Sciences at Peking University. We thank Z. Chen at Shandong University for the culture of mouse spermatocytes and the label of the synaptonemal complex.
Author information
Authors and Affiliations
Contributions
P.X. and J.Q. supervised the project. R.C. and P.X. initiated and conceived the research. R.C. developed the algorithm and implemented the software. Yaning Li constructed the multi-mode SIM system. Yao Zhou conducted the light-sheet experiment. M.L. helped with the biological experiment design. F.L. and J.Q. conducted the brain tissue imaging. W.W. conducted the live-cell imaging experiment. G.Z. and J.W. conducted the 1p/2p joint experiment. G.W. conducted the THUNDER experiment. B.J. conducted the light-field experiment. W.R. conducted the SOFI experiment. Y.S. and C.L. conducted the small-animal imaging experiment. Z.Z. and J.W. provided 2pSAM data. W.Z. provided the MSIM data. J.S. conducted the STED imaging experiment. Y.H. helps the reconstruction of SecMRA. X.X. gave the analysis and discussion of data. J.H. and Yanye Lu conducted the DFCAN reconstruction. W.S., S.F. and Yiming Li conducted the STOMR experiment. Q.L. conducted the quantitate analysis. D.K. provided the prostate sample. Yuxuan Zhao and P.F. provided the whole-brain data and light-sheet support. R.C. analyzed the data and prepared the figures and videos. R.C., Y.L., M.L., J.Q. and P.X. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
P.X., R.C., Yaning Li and W. W. are inventors on a filed patent application (CN202311708895) related to this work. The other authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Jing Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Rita Strack, in collaboration with the Nature Methods team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
Summary of application scenarios of Dark sectioning.
Extended Data Fig. 2 The algorithm flow.
(a) conventional Dehaze method and (b) Dark sectioning, using actin filament in mouse kidney section as an example. (c) The Intermediate steps of Dark sectioning. Scale bar: 4 μm.
Extended Data Fig. 3 Schematic illustration of the (a) DMD-based multi-mode joint SIM microscope and (b) the comparison of TIRF, HiLo, WF, 2DSIM and 3DSIM illumination modalities for SIM.
Laser: MGL-FN-561-200mW, Changchun New Industries Optoelectronics Technology; Glan-Taylor polarizing prism (GTTP): GTP10-A, LBTek; Electro-optic modulator (EOM): M370, Conoptics; Quarter wave plate (QWP): wpa4420-450-650, Union Optic; Mirror (M): PF10-03-P01, Thorlabs; Beam expander (BE): GBE10-A, Thorlabs; Digital micro-mirror device (DMD): DLP6500 0.65 1080p MVSP Type A, Texas Instruments; Lens (L): AC254-300-A-ML, Thorlabs; Spatial mask (SM): custom-built; polarization-preserving dichroic mirror (PDM): ZT405-415/488/561/640-phaseR-UF, Chroma; Objective lens (OBJ): CFI Apochromat TIRF 100XC 1.49 NA Oil, Nikon; Tube lens (TL): TTL200-A, Thorlabs; Emission filter (EF): ET620/60 m, Chroma; sCMOS, ORCA-Flash4.0 V2, Hamamatsu.
Extended Data Fig. 4 Distinguishment of different image optimization methods including denoise, deconvolution and de-background.
(a) Denoise method using Hessian denoise as an example when imaging mitochondria. Deconvolution method using Sparse deconvolution as an example when imaging actin filaments. And de-background method using Dark sectioning as an example when imaging the mouse tail section. The red arrows mean the dense distribution of background which cannot be eliminated by denoise method. (b) Denoise, deconvolution, and de-background methods process actin filament in mouse kidney section, including Dark sectioning, Hessian denoise, and Sparse deconvolution with/without its de-background step. (c) Quantitively validation of different methods using 3DSIM images as GT in xoy and xoz plane with RSP and RSE listed. Scale bar: 4 μm. Axial scale: right part in (a) 209 layers, 125 nm per layer, (c) 31 layers, 125 nm per layer.
Extended Data Fig. 5 Simulation of Dark sectioning under different levels of defocus background.
(a) Simulated OTF of different layers in the z-axis. (b) Simulated profiles of OTF in different layers in the z-axis, with the different weights of OTF. (c) The lower weight means less in-focus information and more out-of-focus information, where w is the weight factor to control the ratio between in-focus and out-of-focus information. Simulated comparisons between GT, WF with 3DOTF, WF with 2DOTF, and Dark sectioning processed WF images using w = 0, 0.8, 0.9, and 0.98 of (d) circular and (e) linear structures. Lateral scale bar: 4 μm. Axial scale: 41 layers, 125 nm per layer.
Extended Data Fig. 6 Experiment results of samples in different depths.
Samples of (a) actin filament(~3 μm), (b) sunflower stem(~40 μm), and (c) mouse neurons(~200 μm). With the corresponding GT images and the comparison of different de-background methods. The standardized set of full metrics of RSP, RSE, and SSIM of (d) actin filament, (e) sunflower stem, and (f) mouse neurons using Confocal, OS-SIM, and 2p as GT. Scale bar: (a, b) 4 μm, (c) 20 μm.
Extended Data Fig. 7 Dark sectioning outperforms Thunder system of Leica.
MIP images of WF, Thunder’s ICC algorithm, and Dark sectioning of (a) aspergillus conidiophores and (b) actin filament. Lateral scale bar: 16 μm. Axial scale: 201 layers, 0.3 μm per layer.
Extended Data Fig. 8 Cross-validation of WF-Confocal joint system to validate the segmentation.
(a) Comparison between Confocal and WF images processed by different de-background methods, with (b) its corresponding 3D segmentation results. White, blue, and red zones mean correct zones, wrong zones, and missed zones, respectively. Quantitate metrics of the segmentation using Confocal as GT, containing (c) F1 score, (d) pixel accuracy, and (e) IoU. (f) Images are separated into 16 sub-ROIs to calculate the mean intensity of nuclei in every plane. There are 27 planes in total, and 432 ROIs in total. (g) The mean intensity of nuclei in each ROI of different methods with the (h) coefficients of mean intensity curves compared with the GT. Scale bar: 80 μm. Axial scale: 27 layers, 1 μm per layer.
Extended Data Fig. 9 Dark sectioning assists in long-term observation of live cells.
(a) Comparisons between WF and DarkWF of ER tubes in live COS-7 cells with a plasmid expressing GFP-tagged KDEL protein. (b) Comparisons between WF and DarkWF of tubulin (labeled by SiR Tubulin Kit, Cytoskeleton, CY-SC002) and lysosomal (labeled by LysoView™ 488, Biotium, 70067) in live COS-7 cells. (c) Comparisons between WF, DarkWF, and SIM, DarkSIM of mitochondria labeled by PKmito RED (Cytoskeleton, CY-SC052). Scale bar: 4 μm. Lateral scale bar: 4 μm.
Extended Data Fig. 10 Dark sectioning can be a complementary method for optical/dye-based background removal methods.
(a) Dark sectioning can be a complementary method for selective-plane structure illumination microscopy. Comparison of beads between WF, 3DSIM, WFDark, 3DSIMDark, and selective-plane 3DSIM, with the NanoJ analysis using selective 3DSIM as GT. (b) Dark sectioning can be a complementary method for confocal scanning light-field microscopy. The comparison between sLFM, sLEMDark, csLFM, and csFLMDarkof thick brain slice. (c) Dark sectioning can be a complementary method for multi-point structure illumination microscopy. Comparisons between WF, DarkWF, and SD, SDDark of mouse kidney section. The left bottom part is the RSP and RSE using (a) SPA-3DSIM, (b) csLFM, and (c) SD as GT, respectively. Scale bar: 4 μm. Axial scale: (c) 256 layers, 53.52 nm per layer.
Supplementary information
Supplementary Information
Supplementary Notes 1–8, Figs. 1–15, Software, Table 1 and Videos 1–5.
Supplementary Software
This contains the software (Matlab version) and example data of Dark sectioning.
Supplementary Video 1
Three-dimensional display of WF, DarkWF and confocal images of Aspergillus conidiophores.
Supplementary Video 2
Comparison between 1p, 1pDark and 2p microscopy to record the activity of mouse neurons.
Supplementary Video 3
Video of small-animal imaging for observing the vascular system in a live mouse with a comparison between SI and DarkSI.
Supplementary Video 4
Comparison between WF, DarkWF and SIM, DarkSIM of live mitochondria.
Supplementary Video 5
Screenshot video to guide the use of the software and the robustness in handling different types of data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, R., Li, Y., Zhou, Y. et al. Dark-based optical sectioning assists background removal in fluorescence microscopy. Nat Methods 22, 1299–1310 (2025). https://doi.org/10.1038/s41592-025-02667-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41592-025-02667-6
This article is cited by
-
High-fidelity tissue super-resolution imaging achieved with confocal2 spinning-disk image scanning microscopy
Light: Science & Applications (2025)
-
Time-deterministic cryo-optical microscopy
Light: Science & Applications (2025)