Abstract
The hippocampus has a specialized microarchitecture, is situated at the nexus of multiple macroscale functional networks, contributes to numerous cognitive as well as affective processes and is highly susceptible to brain pathology across common disorders. These features make the hippocampus a model to understand how brain structure covaries with function, in both health and disease. Here we introduce HippoMaps, an open access toolbox and online data warehouse for the mapping and contextualization of subregional hippocampal data in the human brain (http://hippomaps.readthedocs.io). HippoMaps capitalizes on a unified hippocampal unfolding approach as well as shape intrinsic registration capabilities to allow for cross-participant and cross-modal data aggregation. We initialize this repository with a combination of hippocampal data spanning three-dimensional ex vivo histology, ex vivo 9.4-Tesla magnetic resonance imaging (MRI), as well as in vivo structural MRI and resting-state functional MRI obtained at 3 Tesla and 7 Tesla, together with intracranial encephalography recordings in patients with epilepsy. All code, data and tools are openly available online, with the aim of fostering further community contributions.
Similar content being viewed by others
Main
The hippocampus has long been regarded as a model to understand how brain structure spatially covaries with function. On the one hand, hippocampal anatomy is organized in both anterior–posterior and proximal–distal dimensions1,2. Anterior–posterior organization is emphasized in foundational descriptions of its anatomical segments (that is, head, body and tail) as well as gradual functional differentiation along the hippocampal long axis3,4,5. Perpendicular to this, there is a preserved arrangement of hippocampal subfields along the proximal–distal (also referred to as medio–lateral) axis2,5,6,7,8. These macroanatomical and microstructural features have been suggested to directly relate to hippocampal circuit organization and its embedding within macroscale functional networks, contributing to specific hippocampal functions and its role as a nexus connecting paralimbic, sensory and heteromodal association systems, notably the default mode network9,10,11,12. Its broad involvement in multiple networks is compatible with the key role the hippocampus plays in numerous cognitive and affective processes, including memory and language function, together with affective reactivity and stress as well as spatial navigation. The hippocampus is also recognized as one of the proximate evolutionary origins of the neocortex13,14, making it a candidate structure to investigate principles of evolutionary conservation and innovation in the primate lineage15. Collectively, these insights contribute to the notion that the hippocampus is a microcosm of the brain, and that an assessment of its subregional organization provides key insights into human neural architecture.
The fine-grained subregional organization of the hippocampus contrasts the coarse assessment of this structure by most contemporary neuroimaging investigations, which often still treat this complex archicortical structure as a single entity, or even erroneously label it as ‘subcortical’. This is, in part, due to technical limitations: since the hippocampus is thinner and more tightly convoluted than the neocortex, it is difficult to appreciate its cortical architecture in magnetic resonance imaging (MRI) or the extent of its three-dimensional (3D) convolutions in sparse histology slices. Relatively few studies have compared its microstructural to mesoscale structural and functional features directly, with most studies opting instead to apply subfield parcellation as a proxy2,16,17,18. At the level of the neocortex, there has, on the other hand, been an increasing repertoire of comprehensive open tools for contextualization of findings. With HippoMaps, we now expand anatomy-driven neuroinformatics and multiscale contextualization methods to the human hippocampus.
HippoMaps benefits from technical innovations in hippocampal image processing and analysis. First, it leverages a unified hippocampal segmentation and surface-mapping approach using deep learning-based image processing19, imposing a previously known topology20 and shape-inherent interparticipant alignment21. Similar to neocortical surface extraction and registration procedures22,23, this allows for topology-informed interparticipant registration to a standardized unfolded space21. This has begun a new wave of high-sensitivity hippocampally focused studies in topics including the mapping of histology features7,24, blood perfusion25,26, biophysically constrained diffusion27, hippocampal sclerosis28, neurodevelopmental trajectories29, functional connectivity30, visual receptive field mapping31 and cross-species comparison15. With the increasing aggregation of hippocampal features in a common reference space, it is now possible to devise repositories that allow for a broad contextualization of hippocampal findings. Such work may aid in the interpretation of findings from new studies and experiments, for example by allowing for the cross-referencing of results against established features of hippocampal functional and structural organization.
HippoMaps is conceptualized as an open access toolbox and online data warehouse for hippocampal analysis and multiscale contextualization. HippoMaps aggregates normative hippocampal data obtained from 3D ex vivo histology, high-field imaging and ultrahigh-field in vivo MRI at 3 Tesla and 7 Tesla, as well as intracranial electroencephalography (iEEG) data into a common unfolded coordinate system. Moreover, HippoMaps implements a range of nonparametric statistical tests to evaluate the similarity of standardized surface maps, while controlling for spatial autocorrelation within the hippocampal sheet-like topology. This will provide a statistical foundation for accurate enrichment analysis in the hippocampus32.
Results
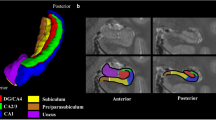
We present hippocampal maps in a standardized folded and unfolded space for multimodal and multiscale datasets consisting of histology, structural MRI, resting-state functional MRI (rsfMRI) and iEEG (Fig. 1). This includes 30 distinct group-averaged maps that have been preprocessed and curated. Despite surface meshes having differing tessellations (Fig. 1b), they can be interpolated in unfolded space to match microscale features (for example, 3D reconstructed histological stains) to MRI or vice versa, spanning a scale of micrometers to millimeters. Using a similar approach, even sparsely sampled data can be spatially mapped across the hippocampus. In this case, we map the centroids of iEEG channels to their nearest corresponding hippocampal vertices. However, in principle, this could also apply to other sparse (or scattered) data such as tissue punches, other invasive recording devices, small resections or other irregularly spaced sampling methods. Similarities between maps are quantified using spatial correlation using permutations to account for spatial autocorrelation.
a, At-a-glance sections of online documentation. b, Surface folding and density are matched to a given sample shape and resolution. Mapping to a standardized unfolded space enables registration and interpolation across scales and data formats, which can then be followed by averaging within a modality, comparison between modalities by spatial correlation, or comparison to anterior–posterior and subfield-related axes of hippocampal organization. c, Initial HippoMaps data includes 30 highly quality and lightweight surface maps, with extensibility as other experiments are uploaded. Blue maps are derived from histology, red from MRI, green from rsfMRI, violet from iEEG and yellow from morphology. d, Example of how maps are compared via spin test-corrected spatial correlation. ADC, apparent diffusivity coefficient; FA, fractional anisotropy; FC, functional connectivity; Hist., histology; IntTS, intrinsic timescale; ReHo, regional homogeneity.
Histology
Histology is considered a neuroanatomical gold standard, and is the basis for most parcellations and descriptions of brain regions33. Here we examined cytoarchitectonic data collected from BigBrain Merker staining for cell bodies34, 3D polarized light imaging (PLI) of neural processes35 and the AHEAD dataset with different stains serving as markers of neurons, myelin and subtypes of interneurons36 (Fig. 2a). Most features showed banding in the proximal–distal direction, in alignment with the subfield atlas.
a, Sample slices and average 3D maps of histological features. Maps are averaged across depths and, where possible, samples. Numbered lines indicate the approximate locations shown on the corresponding boxes in b. b, Example of microstructural profile shapes from five evenly spaced bins across the proximal–distal axis of the BigBrain Merker stain map. Gray indicates points outside the gray matter mask. c, Correlation between microstructural profiles, concatenated across modalities, at each vertex (left). Dimensionality reduction into primary diffusion embedding components 1–3 (right). Scale bars are arbitrary unless indicated otherwise. max., maximum; min., minimum; mMPC, multimodal microstructural profile covariance.
Microstructural (or laminar) profiles are shown for five regions of interest across the proximal–distal axis of the BigBrain Merker stain (Fig. 2b). This is a common method for characterizing laminar structure37. They show a tight unimodal distribution in the distal cornu ammonis (CA) fields, and a more bimodal distribution in the subiculum as expected based on their known laminar architectures1. Profiles for all vertices were concatenated across all stains to make multimodal profiles. That is, for a given vertex, vectors of laminar profiles were concatenated across all modalities, and an affinity matrix was calculated as the correlation between all these extended, or multimodal, profiles, which we call a multimodal microstructural profile covariance matrix (Fig. 2c). Diffusion map embedding, a nonlinear dimensionality reduction technique38,39,40, decomposed the multimodal microstructural profile covariance matrix into primary components that highlighted the differences between vertices with respect to all modalities and depths. In the first component, a sharp boundary was seen between the subicular complex and proximal CA1 and the rest of the hippocampus. The second and third components in turn highlighted the CA2–3 regions and CA1 with parts of the subiculum, respectively. This is data-driven evidence that subfields across the proximal–distal extent of the hippocampus, rather than anterior–posterior or other patterns, account for structural variance in the hippocampus with respect to these stains. These data-driven decompositions thereby echo classical and recent neuroanatomy descriptions of hippocampal microstructure1,2,41.
Structural MRI
MRI is a key tool for studying human neuroanatomy and structure–function relations due to its noninvasive nature and potential for biomarker discovery. The aggregated in vivo 7-Tesla (7-T) and ex vivo 9.4-T scanning are especially powerful, achieving greater resolution and contrast than typical 3-T or 1.5-T clinical scans. Here we provide healthy normative maps for such scans (Fig. 3a) including popular acquisitions: quantitative T1 relaxometry (qT1) and its nonquantitative ex vivo inverse: R1, T2* and its inverse R2*, proton density, diffusion-weighted imaging (DWI) estimates of fractional anisotropy and apparent diffusivity coefficient, and magnetic transfer ratio (MTR). Note that DWI and MTR images were prone to image artifacts including ringing, leading to ripple-like patterns on individual participant maps and lower interparticipant consistency. Fortunately, since these artifacts are not in-phase between participants, they are not present in the group-averaged maps.
a, Sample slices and averaged 3D maps of 9.4-T ex vivo and 7-T in vivo structural MRI features. Maps are averaged across depths and, where possible, samples. b, Consistency, as measured by the correlation between all pairs of individual sample maps (mean ± s.d., n = 6 and n = 190 pairwise comparisons for ex vivo and in vivo data, respectively, all P < 0.001, one-sample, two-sided t-tests). c, Correlation between microstructural profiles, concatenated across modalities, at each vertex (left). Dimensionality reduction into primary diffusion embedding gradients 1–3 (right). Scale bars are arbitrary unless indicated otherwise. MPC, microstructural profile covariance.
Multiple scans were available for averaging (n = 4 left + right hippocampi at 9.4 T and n = 20 left + right hippocampi at 7 T), enabling a calculation of consistency across samples via Pearson’s R (Fig. 3b) consistency. DWI and qT1 maps were also calculated in a second validation dataset, consisting of 82 locally scanned healthy participants (including the subset from the MICA-MICs dataset) with a 3 T scanner, which showed similar patterns (Extended Data Fig. 1). Multimodal microstructural profile covariance matrixes were generated as above and were reduced using diffusion map embedding into primary components, which again highlighted differences across subfields. Only the third component showed anterior–posterior differences, largely within the CA1 subfield.
rsfMRI
fMRI during the resting state (rsfMRI) allows interrogation of intrinsic brain function via the analysis of spontaneous activity and its statistical dependencies, and has become a key technique in the mapping of functional–anatomical systems10,42,43. Here we examined several features of rsfMRI in 88 healthy participants scanned at 3 T. Intrinsic timescale is a measure of the time it takes for the temporal autocorrelation to drop below a threshold44 (Fig. 4a). Regional homogeneity considers the similarity between adjacent vertices’ time series, which is thought to indicate the extent of horizontal (that is, between cortical columns) excitatory connectivity45 (Fig. 4b). Finally, macroscale functional connectivity is by far the most popular rsfMRI feature, with many rich properties that have been explored with respect to white matter connections46, network properties47, organizational gradients39,48 and many other summary metrics. For simplicity, we examined connectivity between all hippocampal vertices and neocortical parcels from the Schaeffer400 parcellation49 (Fig. 4c). Repetition of these analyses in a smaller sample of 7-T rsfMRI data showed consistent results (Extended Data Fig. 2).
a, Intrinsic timescale (recurrence). b, Regional homogeneity (short range connectivity). c, Functional connectivity (long range; to the neocortex). d, Cross-sample (that is, participants and hemispheres) consistency (mean ± s.d., n = 19,503 pairwise comparisons, all P < 0.001, one-sample, two-sided t-tests). e, Decomposition of functional connectivity patterns across hippocampal vertices into primary diffusion map embedding gradients. Scale bars are arbitrary unless indicated otherwise.
As mentioned above, functional connectivity is a rich measure that can be summarized in many ways. Here we identified gradual components of intrinsic hippocampal connectivity variations (Fig. 4e) using the aforementioned nonlinear decomposition techniques. Consistent with previous work3,4,5,12,50,51, we found anterior–posterior differentiation in the first hippocampal component, together with proximal–distal banding with CA1 in particular differing from the other subfields. Neocortical counterparts of this component show that anterior and CA1 regions shared more connectivity with temporal pole, insula and frontal regions whereas more posterior and non-CA1 subfields shared connectivity with more posterior parietal and visual areas, again consistent with previous findings12. The second component also showed differentiation of CA1 from subiculum and CA2–3 in the more middle and posterior regions, with neocortical correspondences to medial prefrontal and posterior cingulate regions for CA1 and more visual areas for CA2–3 and posterior subiculum.
Intracranial EEG
Invasive recording methods such as iEEG provide a direct measure of neural activity at high temporal resolution but typically have lower spatial coverage and are limited to neurological patient populations. In that sense, they can be considered as scattered spatial data, which can be interpolated or extrapolated for hippocampal mapping as described in Fig. 1b, following previous approaches52. We use common measures of the periodic component of iEEG data, as shown by power-spectrum density and also further simplified to delta, theta, alpha, beta and gamma band powers from low to high frequencies, respectively. Power-spectrum densities and band powers derived from hippocampal channels resembled those derived from all channels (Fig. 5a). Extrapolating channel information across neighboring vertices from a given hippocampus, a spatial pattern emerged in which both proximal–distal and anterior–posterior differences were seen (Fig. 5b). Band power is a limited measure of the full power-spectrum density however, and so in Fig. 5c we performed diffusion map embedding of the full power-spectrum density. This showed a graded primary anterior–posterior component driven by higher theta and alpha power in the posterior and higher delta power in the anterior hippocampus. The second component showed increased delta power in the anterior and posterior hippocampus, while the third component showed a slight increase in delta and decrease in theta in the subiculum. Results were consistent when using an open iEEG atlas52 or locally collected data from patients53, showing largely conserved patterns in Extended Data Fig. 3.
a, Left: power-spectrum density plots of all channels (n = 4279) and hippocampal channels (<5 mm from any hippocampal midthickness vertex) (n = 81), lines show mean ± s.d. shaded. Right: log normal power within each band for each hippocampal channel, with vertical lines indicating the median and with corresponding bands from all channels in gray. b, Spatial extrapolation weighted by geodesic distance shows largely anterior–posterior differences in band powers. c, Power-spectrum densities reduced into primary diffusion map embedding components. Scale bars are arbitrary unless indicated otherwise.
Feature combinations
The biggest advantage of a common hippocampal mapping space is that it allows for direct spatial correlation between features from different scales and methods. In Fig. 6a, we examined relationships between all maps shown above using Pearson’s R with an adapted spin test significance testing to control for spatial autocorrelation in the data27. We also compared morphological measures of thickness, gyrification and curvature that are generated within the HippUnfold workflow (Extended Data Fig. 4). Previous work24 showed that these features differed between MRI and histology, with the latter showing greater detail including more gyrification and lower thickness. Overall, this revealed many greater-than-chance correlations (Fig. 6a). This was especially strong within methodologies, but significant correlations between methods using different spatial scales were also observed.
a, Correlation matrix of all features, after resampling to a common 0.5-mm vertex-spacing surface. b, Diffusion map embeddings 1–3 across all features. c, Alignment of gradients 1 and 2 to hippocampal subfields, proximal–distal and anterior–posterior axes. d, Absolute correlation between each feature map and the anterior–posterior (AP) axis (Pearson’s R) and the maximum permuted subfield labels (Spearman’s R). Scale bars are arbitrary unless indicated otherwise. A, anterior; L, lateral; M, medial; P, posterior.
We performed a dimensionality reduction as in previous figures, but this time across all features from all methods overviewed here (Fig. 6b). As seen in previous results, both proximal–distal or subfield-related and anterior–posterior differences were seen. For additional visualization, we plotted the two most dominant components with color coding according to subfield and continuous anterior–posterior and proximal–distal gradients (Fig. 6c). The proximal–distal and anterior–posterior axes of the hippocampus are closely aligned to components 1 and 2, respectively, with component 1 explaining approximately twice the variance (Fig. 6b). This suggests that while these two axes emerge as natural summaries of many hippocampal feature maps, the proximal–distal direction is stronger.
A summary of which measures are most correlated with the anterior–posterior and subfield axes of the hippocampus is provided in Fig. 6d. As expected, the strongest subfield relationships were observed in histological features such as Calbindin and Calretinin staining, or thickness measures at a histological level of precision. Many structural 9.4-T and 7-T features also showed strong subfield correlations, especially qT1 and qR1. This is encouraging given the increasing availability and adoption of quantitative T1 sequences. The rsfMRI and iEEG features used were still moderately correlated with subfield division, but iEEG and rsfMRI maps showed strong correlations with the anterior–posterior hippocampal axis. Some caution should be exercised here: iEEG data were sparsely sampled and so after extrapolation each band power map was very smooth, which could amplify correlation values (but not significance, since spin test permutations were used to control for spatial autocorrelation). Note also that laminar profiles were not used in this analysis, and histological measures can benefit from the information added by such methods due to their high precision.
Usability experiment and documentation
HippoMaps as an open toolbox and online data warehouse paves the way for multiple future research avenues. We anticipate that as hippocampal mapping studies are performed in other research areas, authors can use the initial maps provided here as comparisons and will upload their own maps in the spirit of open and reproducible science, and to boost the visibility of their work. To this end, we provide a set of Python tools, well documented example code to reproduce the maps shown here (labeled as tutorials), and guidelines for how other experimenters should upload their maps to this repository. We have and will continue to answer questions and create community resources via GitHub (https://github.com/MICA-MNI/hippomaps), and all current maps are available on the Open Science Framework (https://osf.io/92p34/).
An example experiment with task fMRI using the mnemonic similarity task is illustrated in Extended Data Fig. 5. This task is designed to probe pattern separation, a process thought to preferentially involve hippocampal subregions54. This can be seen most strongly in subiculum for the successful pattern separation trials, whereas trials with new stimuli showed anterior–posterior differentiation, similar to previous work11. Comparing these maps directly to microcircuit features provides context for the demands of these two task conditions: pattern separation was strongly correlated to detailed maps of functional connectivity with the neocortex and histologically derived thickness, whereas novelty was moderately correlated to iEEG-derived alpha band power and intrinsic timescale (Extended Data Fig. 5, right). Further task fMRI results from an object-pairing memory task, as well as replication data of the mnemonic similarity task at 7 T, are shown in Extended Data Fig. 6.
An example experiment comparing 33 patients with temporal lobe epilepsy (TLE) to 42 healthy, age-matched and sex-matched controls scanned at 3-T MRI is illustrated Extended Data Fig. 7. Reductions in hippocampal thickness and gyrification are seen, with the greatest changes in CA1 and CA4 subfields, which have previously been identified as vulnerable areas1,55,56,57. These patient-control differences were found to be consistent between participants. Comparing thickness reduction patterns to other maps shows moderate correlations with healthy levels of MRI-derived thickness and parvalbumin staining. Gyrification loss was moderately correlated with MRI-derived MTR and healthy gyrification in histology.
Discussion
Despite its critical role in human brain organization in both health and disease, the field lacks a standardized framework to aggregate, represent and compare structural and functional features of the hippocampus. The current work presented HippoMaps as a centralized toolbox and online data warehouse for hippocampal subregional analysis and contextualization. HippoMaps will provide key guidance to: (1) compare hippocampal features derived from different methods, in particular to cross-reference in vivo imaging measures with ex vivo, (2) interrogate structure–function relationships, for example by contextualizing task-based fMRI findings or intracranial neural recording against spatial patterns obtained from anatomical and microstructural measures, (3) contextualizing case-control deviations in clinical populations against established principles of subregional hippocampal organization and (4) refining our understanding of hippocampal circuitry, by mapping its functional connectivity and microstructure for a better understanding of its computational operations and transfer functions at the subregional level. HippoMaps is fully open access and designed according to community standards (http://hippomaps.readthedocs.io), to facilitate its dissemination and usability. As such, we anticipate that HippoMaps will represent a powerful analytical ally for fundamental and clinical neuroscientists alike. Considering the important role the hippocampus plays in human neuroanatomy and cognition1,58 and its important computational properties59,60, it may furthermore provide key insights and guidance into the design and validation of emerging bio-inspired AI architectures.
We anticipate that surface-based registration will become the standard for subregional hippocampal mapping, as it has in the neocortex22,61,62,63. HippoMaps is a major step in advancing the usability of this methodology, generating utilities, scientific context and an open community for examining the hippocampus in detail. Moreover, our repository is designed to employ the same data standards that have already been extensively developed for neocortical brain imaging data including Brain Imaging Data Standards (BIDS)64, Neuroimaging Informatics Technology Initiative (NIfTI) or Geometry Informatics Technology Initiative (GIfTI) file formatting65 and Findability, Accessibility, Interoperability and Reusability principles66. Our online tutorials also showcase the straightforward interplay between HippUnfold, HippoMaps and other community tools for surface analysis including Connectome Workbench, BrainStat and NiLearn67. Despite its demonstrated benefits, surface-based alignment is not yet universal for the neocortex and certainly still in its infancy for the hippocampus. Thus, while we encourage the use of surface-based methods, we also provide code and examples of how to map volumetrically aligned hippocampal data (for example, in a standard volumetric space such as MNI152 or others) to hippocampal surfaces for comparison and contribution to HippoMaps. In the field, work progresses at the level of hippocampal subfield parcellation at the level of histology, for example to derive additional subregional divisions68,69,70. Moreover, there have been ongoing efforts by the neuroimaging community to harmonize boundary heuristics2,71. Under the HippoMaps framework, descriptions go beyond typical unitary descriptions of the hippocampus and beyond its parcellation into subfields to the level of mapping vertex-wise or columnar structure of hippocampal archicortex. The columnar level represents an important structural and functional modularization of the brain72, and has the potential to unlock new facets of hippocampal computation. As such, different subfield parcellations can also be converted to surface format and integrated seamlessly within the HippoMaps warehouse. Thus, we apply considerable futureproofing, and we encourage the broader hippocampal research community to upload their own maps to this repository under our support, curation and online guidelines and tutorials.
HippoMaps critically depends on the quality of repository data. Some maps varied between individuals, as reflected in lower consistency metrics. This was strongest in diffusion-derived MRI, magnetization transfer and fMRI maps. This can reflect idiosyncratic imaging artifacts that average out over large samples, or systematic imaging artifacts such as dropout near major tissue interfaces. In fMRI this could also reflect true interindividual variability that is not accounted for by structural alignment alone. It is notable that some features showed extreme intensity values at the anterior and posterior edges: these are relatively small in native space and so have limited constituent data and are prone to interpolation artifacts. Thus, the anterior and posterior edges of each map should be interpreted with some caution. Consistency was not evaluated in histology due to small sample sizes, and histology is generally less scalable than MRI making averages across many samples costly. However, as new high throughput methods become more widespread, invaluable datasets such as BigBrain, AHEAD and others, can provide a high level of anatomical precision and a breadth of extracted features. Future data uploaded to HippoMaps should, thus, aim to include state-of-the-art acquisition methods, averages over many samples where possible, and apply robust preprocessing and quality control to minimize artifacts that limit the quality of comparisons and conclusions about hippocampal organization that can be drawn.
Multi-feature aggregation as in the HippoMaps repository provides extensive opportunities to assess relationships between hippocampal structure and function, to cross-validate in vivo measures with ex vivo imaging as well as histological data. Structural and microstructural data derived from 3D histology and MRI currently aggregated support a close alignment of many feature maps with the classic subfields account of the hippocampal circuitry. Moreover, several measures, particularly those derived from functional modalities such as rsfMRI or iEEG, lend additional evidence for anterior–posterior differentiation of the hippocampal formation. Specifically, diffusion map embedding of hippocampal rsfMRI connectivity and iEEG power-spectrum densities showed that anterior–posterior differentiation captured most inter-regional variance, whereas histological and structural MRI measures showed primarily proximal–distal or subfield-related differentiation. The consistently repeated structural motifs across the anterior–posterior axis of the hippocampus are suggestive of parallel repeated computations being performed on different input and output information across the anterior–posterior hippocampal axis, in line with previous accounts3,4. These two dimensions have also been suggested to topographically represent the functional embedding of the broader mesiotemporal region in large-scale functional networks, in particular default mode and multiple demand networks9,10,73,74, which provides a potential substrate for the parametric mixing of both functional systems in macroscale brain function7. It is, therefore, not surprising that two axes explain the greatest proportion of the variance across all maps in the current repository as well, consolidating the notion that a two-dimensional organization may serve as a powerful summary descriptor for a broad array of hippocampal structural and functional features5.
We provide adapted methods to control for autocorrelation when comparing spatial maps to one another in the hippocampus. We specifically adapted Moran’s spectral randomization ‘spin test’ permutation and Eigenstrapping permutation testing that have previously been introduced to study neocortical data27,32,40,75,76. These methods reveal robust correlations between many of the maps included here. Many of these relationships support the validity of the methods being applied, for example between in vivo qT1 and ex vivo R1 that are inverses of one another. Another example is that functional connectivity of the hippocampus was strong to default mode neocortical areas, as shown in previous work9,12,77,78, with connectivity being strongest in the subiculum. This recapitulates the role of the subiculum as the primary output structure of the hippocampus, and contributions of the hippocampus to functions typically ascribed to the default mode network such as mind-wandering, episodic recall or future-thinking that are frequent during rest74,79,80,81,82,83,84. Some relationships reveal new information about the methods themselves: PLI transmittance is thought to reflect many microscopic structures under the broad heading of ‘neural processes’ or ‘nerve fibers’85,86. Across the extent of the hippocampus, this feature correlated with Bielschowsky and Thionin staining, R2*, average neocortical functional connectivity, and, most substantially, rsfMRI intrinsic timescale. Intrinsic timescale is hypothesized to relate to recurrent connections87, which could indeed be supported by dense neural processes. Finally, we illustrate contextualization via nonlinear diffusion map embedding across maps. When applied to all maps, we show data-driven separation of subfields, in line with previous work. We also note that in this latent space, CA4 closely resembles CA1, even though they are not adjacent topologically. This fits descriptions of CA4 as having a wide pyramidal layer with large and dispersed neurons, similar to CA1 (ref. 1), and indeed in some cases these two areas have similar disease vulnerabilities, for example in drug-resistant TLE55. Future work may determine more selectively what features make these two regions similarly vulnerable or explore conditions with differential vulnerability.
At the level of the neocortex, several packages already exist to facilitate the contextualization of results88,89. With HippoMaps, such an approach is now also possible for the hippocampal region, and we demonstrate the contextualization of task fMRI maps during an episodic memory paradigm as well morphological alterations in patients with TLE relative to healthy individuals. Such approaches can help to clarify the hypothetical role of microstructural features in specific hippocampal computations, such as pattern separation60,90, pattern completion91,92,93 and novelty detection93,94. These previously assumed relations of function to microstructure have generally relied on parcellations of the hippocampus into stereotyped subfields; with HippoMaps, it is instead possible to compare functional and microstructural maps directly without any predefined subfield labeling. In addition to offering potential increases in anatomical specificity, this representation may also lend itself more naturally to sensitive spatial correlation with autocorrelation control through permutation testing. One area for future work will lie in consolidating mesoscale connectivity with detailed descriptions of the internal hippocampal circuitry, which will not only help to further understand the computations of specific hippocampal subregions but may also clarify the different substrates of computation27,95. Indeed, hippocampal circuitry has inspired the basic ways in which we think about biological computation, spurring principles such as long-term potentiation96 and carrying important computational models such as the Boltzmann machine97 and Tolman–Eichenbaum machine98. Even more recent computational models and associated theory still center around hippocampal structure as told through a stereotyped subfield architecture98,99. Formal mapping, rather than stereotyped descriptions, can extend this work, building up biological plausibility of such models and scaffolding our understanding of these systems. For this reason, HippoMaps may also provide precise macroscale, mesoscale and microscale hippocampal features in a common same space to further identify and harness computational properties of its circuitry.
Methods
Datasets
To provide broad coverage of many areas of hippocampal research, we initialize HippoMaps with 30 minimally processed but spatially aligned data spanning 3D ex vivo histology, high-field in vivo structural imaging as well as rsfMRI and iEEG. These data originate from open-source resources including BigBrain34, AHEAD36, MICs100, PNI101 and the MNI open iEEG atlas52, and are further supplemented with locally collected data including further healthy structural and fMRI obtained at 3 T and 7 T, as well as iEEG data obtained from patients with epilepsy who also underwent pre-implantation multimodal MRI. These are summarized in Extended Data Table 1, and the following sections provide details of each dataset and preprocessing.
Histology
BigBrain, both AHEAD brain samples and one 3D-PLI hemisphere were manually segmented and processed with HippUnfold in our previous work19,21. Quantitative data were then sampled on hippocampal surfaces using the Connectome Workbench tool ‘volume-to-surface-mapping’ with trilinear interpolation (https://www.humanconnectome.org/software/connectome-workbench). In the current work, we sampled 100 μm3 images to save computational resources (summarized for all data in Extended Data Table 1). Masks of local outliers were defined as being more than four standard deviations from the average of a ten vertex radius and were then dilated by two vertices to discard data at an interface with missing areas where excess stain or other artifacts can accumulate. Surface-based interpolation (linear) and extrapolation (nearest neighbor) were used to fill in missing data. Finally, microstructural profiles over 25 surface depths were postprocessed for outliers and alignment. Microstructural profiles are highly sensitive to errors in tissue segmentation that can cause a profile to shift in the vertical direction (that is, toward the superficial or deep parts of the cortex). We aimed to account for this sensitivity by including an additional postprocessing step to vertically translate profiles until they are maximally aligned. This was accomplished by first sampling laminar depths both within and outside (above and below) the hippocampal cortical mantle to collect data from any tissue that may have been under segmented. We then translate these profiles vertically by shifting them up or down by a given number of depth-wise surfaces. The amount of translation is that which maximizes the alignment of a given profile to the global average of all profiles from a given map (that is, across all vertices). This is overviewed in Extended Data Fig. 8. This was especially important since some of the AHEAD brain data showed misalignment between modalities, leading to some vertices being outside the hippocampus in some cases, depending on the image. By extrapolating these surfaces beyond the original inner and outer hippocampal bounds, we aimed to capture the actual hippocampal tissue intensities rather than those of the surrounding white matter or background. These were then aligned by translation in the laminar direction that maximized correlation to the global average microstructural profile. Translations were limited to 25% of the surface thickness at each column or vertex.
MRI
In the PNI101 dataset, 10 healthy participants (5 men and 5 women, age 26.8 ± 4.61 years) underwent 3 imaging sessions to include additional acquisition modalities and for rescanning to provide additional image contrast. T1 images were acquired with an MP2RAGE sequence with the following parameters: voxel size 0.5 mm3, repetition time 5,170 ms, echo time 2.44 ms, flip angle 4°, field of view 260 × 260 mm2, iPAT acceleration factor 3, partial Fourier 6/8, 320 sagittal slices, with a final matrix size 320 × 320 × 320. Superresolution sampling was performed to improve the contrast available in these images. That is, all qT1 images were upsampled from 0.5 mm3 to 0.25 mm3, and rigidly registered usings ANTs102 to the first qT1 image for each participant. These high-resolution, high-contrast images were then run through HippUnfold v.1.3.0. Diffusion-weighted images were acquired with the following parameters: voxel size 1.1 mm3; repetition time 7,383 ms; echo time 70.60 ms; flip angle 90°; refocusing angle 180°; field of view 211 × 211 mm2; multiband factor 2 and echo spacing 0.79 ms with three shells at B values of 300, 700 and 2,000 s mm−2. b0 images acquired in reverse phase encoding direction are also provided for distortion correction of DWI scans. Derivative apparent diffusivity coefficient and fractional anisotropy were generated within micapipe, which uses MRtrix103. MTR was acquired with the following parameters: voxel size 0.7 mm3, repetition time 95 ms, echo time 3.8 ms, flip angle 5°, field of view 230 × 230 mm2, 240 sagittal slices, pulse width 50 ms and interpulse delay 1 ms. T2* was acquired with the following parameters: voxel size 0.7 mm3; repetition time 43 ms; multi-echo times 6.46 ms, 11.89 ms, 17.33 ms, 22.76 ms, 28.19 ms and 33.62 ms; flip angle 13°; field of view 220 × 220 mm2 and 160 sagittal slices. Each of these structural images were rigidly registered to this superresolution qT1 image and sampled on hippocampal surfaces provided by HippUnfold, as above. Resting-state and task fMRI data were acquired using an echo planar imaging sequence with the following parameters: voxel size 1.9 mm3, repetition time 1,690 ms, multi-echo times 10.80 ms, 27.3 ms and 43.8 ms, flip angle 67°, field of view 224 × 224 mm2, 775 slices oriented to AC-PC-39°, multiband factor 3 and echo spacing 0.53 ms.
The MICs dataset release initially included 50 participants100, but here we include proceeding scans with the same protocol from 99 healthy participants (54 men and 45 women, age 29.6 ± 9.7 years) and 33 patients with TLE (12 men and 11 women, age 42.4 ± 11.0 years). Patients’ scans were compared to only a subset of the MICs data to maximize demographic similarity (21 men and 21 women, age 40.4 ± 10.4 years). qT1 scans were acquired with an MP2RAGE sequence with the following parameters: voxel size 0.8 mm3, repetition time 5,000 ms, echo time 2.9 ms, flip angle 5°, iPAT acceleration factor 3, bandwidth 270 Hz per px, partial Fourier 6/8, 240 sagittal slices, with a final matrix size of 320 × 320 × 240. Diffusion-weighted images were acquired with the following parameters: voxel size 1.6 mm3, repetition time 3,500 ms, echo time 64.40 ms, flip angle 90°, refocusing angle 180°, field of view 240 × 240 mm2, multiband factor 3, echo spacing 0.76 ms, B value 0, with three shells at B values 300, 700 and 2,000 s mm−2. b0 images acquired in reverse phase encoding direction are also provided for distortion correction of DWI scans. rsfMRI data were acquired using an echo planar imaging sequence with following parameters: voxel size 3.0 mm3, repetition time 600 ms, echo time 30 ms, flip angle 52°, field of view 240 × 240 mm2, multiband factor 6 and echo spacing 0.54 ms. All scans were preprocessed using micapipe v.0.2.0 (ref. 104) followed by HippUnfold v.1.3.0. Each of these structural images was registered within micapipe and sampled on hippocampal surfaces provided by HippUnfold, as above.
fMRI data were sampled onto hippocampal surfaces with an average vertex spacing of 2 mm, in correspondence with the lower resolution of this data, making 419 vertices × N timepoints. Custom code was used to compute intrinsic timescale, regional homogeneity, and functional connectivity analyses of resting-state data. For task data, NiLearn67 was used to construct general linear models. All trial types were modeled separately after convolution with a hemodynamic response function and its derivative and dispersion, alongside regressors of no interest for motion (six degrees of freedom) and drift (eight frequencies).
For comparison between patients with TLE and healthy control participants, a subset of 81 controls was selected to match age and sex of the TLE group. All patients with TLE had a clear lateralization of primary epileptogenic zones (15 left and 20 right). Only ipsilateral hemispheres were examined here.
iEEG
Locally collected data came from 20 patients with epilepsy (7 men and 13 women; age 33.90 ± 9.02 years) implanted with deep intracranial electrodes as part of their standard of care. Only channels within the brain, free of artifacts, no ictal or interictal events, and during resting wakefulness with eyes closed were examined, making up 2,507 channels across the whole brain. When no continuous and artifact-free 60-s segments were found for a given channel, multiple discontinuous segments from the channel were concatenated with a 2-s zero-padded buffer between segments. Each patient also had an intraoperative computed tomography or MRI scan, from which all channels were located using the Intraoperative Brain Imaging System (Ibis)105. These scans were then rigidly registered to preoperative T1w images that were run through HippUnfold. From all channels, 44 (7 left and 37 right hemisphere) were within 5 mm of any hippocampal vertex. These were concatenated with similarly preprocessed data from ref. 52 consisting of 1,772 channels in standardized MNI152 (2009c, nonlinear, symmetric) volumetric space. HippUnfold was run on the MNI152 T1w template (https://github.com/khanlab/hippunfold-templateflow), allowing us to localize hippocampal electrodes (albeit, with less participant-specific precision). From all channels 37 (19 left and 18 right hemisphere) were within 5 mm of any hippocampal vertex. Power-spectrum densities were then calculated for the combined datasets using Welch’s method with a Hann window. Power-spectrum densities were then linearly resampled to a range of 0.5–80 Hz. Band powers for delta, theta, alpha, beta and gamma bands were calculated in the range of 0.5–4 Hz, 4–8 Hz, 8–13 Hz, 13–30 Hz and 30–80 Hz, respectively. Extrapolation of these sparsely sampled hippocampal vertex data was then performed using the method shown in Fig. 1c. That is, typical nearest-neighbor or linear interpolations were inappropriate since they would only consider one or two closest channels. Thus, we used a method that averages channels proportionally to their geodesic distance to a given vertex to maintain robustness while still providing some level of spatial differentiation based on where a given channel originated from.
Full details and code for each method described above are available within their respective online tutorials, while have been made simple and well commented to serve as examples for future hippocampal mapping projects via GitHub (https://github.com/MICA-MNI/hippomaps).
Surface mapping
All data were mapped to a standardized folded and unfolded surface space using HippUnfold v.1.3.0 (ref. 19). This entails tissue type segmentation using a deep UNet neural network, fitting of inner, outer and midthickness surfaces to hippocampal gray matter, mapping to a standardized unfolded rectangular space, and then registration in unfolded space to a standard, histology-derived generated atlas21. This standardized space is, thus, made equivalent across all participants. We mapped iEEG channel data to all vertices within <5 mm of the channel centroid, and average data across all channels from all patients with a weighting proportional to geodesic distance from those vertices. This extrapolation method is more robust than a linear or nearest-neighbor extrapolation, which would be strongly driven by only one or a few nearby vertices with data mapped to them, while also still preserving some spatial preference for data from nearby channels. In all cases, left and right hemispheres were averaged to increase signal and since no clear differences were seen between them.
In addition to inner, midthickness and outer surfaces, any number of intermediate surfaces can be generated at different depths or linearly extrapolated around the outer bounds of the hippocampus106 (Fig. 2). This is especially useful for submillimetric data, where laminar or microstructural profile information can be extracted.
Spatial comparisons
All surfaces have vertex-wise correspondence between participants and hemispheres, meaning that they can readily be averaged or used in other statistical operations. Similarities between maps can be quantified using spatial correlation, but spatial autocorrelation can compromise significance testing. HippoMaps provides several permutation test to ensure robustness against this issue, including Moran spectral randomization75, ‘spin’ tests32 and ‘Eigenstrapping’76. Figure 1d provides a brief overview of such a correlation using ‘spin’ test permutations. The HippoMaps tools include several methods for permutation testing of spatial correlations, most notable spin test correction27, Moran spectral randomization75 and Eigenstrapping76. These methods are overviewed in Extended Data Fig. 9, including an example of what a random permutation can look like. The spin test was observed to be most conservative, while Moran permutation was least conservative (that is, most likely to show a false positive as in the example shown).
Dimensionality reduction
Within each method (that is, histology, structural MRI, rsfMRI, iEEG), we performed dimensionality reduction to summarize the information contained across all group-average maps within that methodology. This was also repeated across all maps from all methods (Fig. 6). Dimensionality reduction consisted of nonlinear diffusion map embedding using BrainSpace40. Default parameters were used in all cases with a maximum of five components (although only the top three are shown), with the following exception: Pearson’s R was used in calculating affinity matrices in all cases, matching the methods used for spatial comparisons of maps as above, and sparsity was set to 0.1 instead of the default 0.9 in the final reduction across all modalities from all methods to better leverage the richness and reliability of the group-averaged maps. In the case of rsfMRI functional connectivity, components were further contextualized by showing their neocortical counterparts by averaging the connectivity of the top versus bottom 25% hippocampal vertices from each component. The same operation was used to show differences in power-spectrum density of the top versus bottom 10% of iEEG components.
Ethics and inclusion
The data used in HippoMaps was sourced from multiple open datasets. The MICs dataset was obtained with the approval of the Ethics Committee of the Montreal Neurological Institute and Hospital (2018–3469). The PNI dataset was obtained with approval by the Research Ethics Board of McGill University. The iEEG dataset was obtained with approval from the MNI as lead ethics organization (REB vote no. MUHC-15-950). All participants from the MICs, PNI and iEEG datasets provided written informed consent, which included a provision for openly sharing all data in anonymized form. The AHEAD dataset samples were collected from the body donation program of the University of Maastricht following a whole-body perfusion, for which written consent was obtained during life, and in accordance with the Dutch Burial and Cremation Act. The 3D-PLI dataset was collected through the body donor program of the University of Rostock, Germany, and in accordance with the local ethics committee. The BigBrain dataset was collected through the body donor program of the University of Düsseldorf in accordance with legal requirements. No further ethics approval was needed for the present study.
All listed authors contributed to either conception and design of the project, data acquisition, curation, analysis, interpretation, software development or paper preparation in accordance with Nature Methods’ authorship criteria. Author roles and responsibilities were additionally defined during regular meetings of the Helmholtz International BigBrain Analytics and Learning Laboratory (HIBALL), which focuses on integrating and sharing multiscale and multimodal neuroimaging data.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All figures can be reproduced using the tutorials presented in the online HippoMaps Tutorials available at https://hippomaps.readthedocs.io/en/latest/tutorials.html. This includes checkpoints of intermediate processed data. Most of the original datasets are also open-source and can be found online via BigBrain at https://bigbrain.loris.ca/main.php; AHEAD via figshare at https://uvaauas.figshare.com/articles/dataset/Ahead_Brain_122017_-_Original_stains/16844500 (ref. 107) and https://uvaauas.figshare.com/articles/dataset/Ahead_Brain_152017_-_Original_stains/16843039 (ref. 108); MICs at https://osf.io/j532r/; PNI at https://osf.io/mhq3f/ and iEEG at https://mni-open-ieegatlas.research.mcgill.ca/.
Code availability
To facilitate broad adoption and continued development, we made a toolbox of relevant functions available via GitHub at http://github.io/MICA-MNI/hippomaps and associated data available via the Open Science Framework at https://osf.io/92p34/, openly available under a GLP-3.0 license, and we provide expandable online tutorials and guidelines at http://hippomaps.readthedocs.io.
References
Duvernoy, H. M., Cattin, F. & Risold, P.-Y. The Human Hippocampus (Springer, 2013).
Olsen, R. K. et al. Progress update from the hippocampal subfields group. Alzheimers. Dement. 11, 439–449 (2019).
Poppenk, J., Evensmoen, H. R., Moscovitch, M. & Nadel, L. Long-axis specialization of the human hippocampus. Trends Cogn. Sci. 17, 230–240 (2013).
Strange, B. A., Witter, M. P., Lein, E. S. & Moser, E. I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 15, 655–669 (2014).
Genon, S., Bernhardt, B. C., La Joie, R., Amunts, K. & Eickhoff, S. B. The many dimensions of human hippocampal organization and (dys)function. Trends Neurosci. 44, 977–989 (2021).
Insausti, R., & Amaral, D. G. in The Human Nervous System (eds Paxinos, G. & Mai, J. K.) 871–914 (Elsevier, 2004).
Paquola, C. et al. Convergence of cortical types and functional motifs in the human mesiotemporal lobe. eLife 9, e60673 (2020).
DeKraker, J., Köhler, S. & Khan, A. R. Surface-based hippocampal subfield segmentation. Trends Neurosci. 44, 856–863 (2021).
Andrews-Hanna, J. R., Reidler, J. S., Huang, C. & Buckner, R. L. Evidence for the default network’s role in spontaneous cognition. J. Neurophysiol. 104, 322–335 (2010).
Buckner, R. L., Andrews-Hanna, J. R. & Schacter, D. L. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124, 1–38 (2008).
Li, Q. et al. Atypical neural topographies underpin dysfunctional pattern separation in temporal lobe epilepsy. Brain 144, 2486–2498 (2021).
Vos de Wael, R. et al. Anatomical and microstructural determinants of hippocampal subfield functional connectome embedding. Proc. Natl Acad. Sci. USA 115, 10154–10159 (2018).
Puelles, L., Alonso, A., García-Calero, E. & Martínez-de-la-Torre, M. Concentric ring topology of mammalian cortical sectors and relevance for patterning studies. J. Comp. Neurol. 527, 1731–1752 (2019).
Sanides, F. Comparative architectonics of the neocortex of mammals and their evolutionary interpretation. Ann. N. Y. Acad. Sci. 167, 404–423 (1969).
Eichert, N. et al. Hippocampal connectivity patterns echo macroscale cortical evolution in the primate brain. Nat. Commun. 15, 5963 (2024).
Iglesias, J. E. et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage 115, 117–137 (2015).
Kulaga-Yoskovitz, J. et al. Multi-contrast submillimetric 3 Tesla hippocampal subfield segmentation protocol and dataset. Sci. Data 2, 150059 (2015).
Yushkevich, P. A. et al. Nearly automatic segmentation of hippocampal subfields in in vivo focal T2-weighted MRI. Neuroimage 53, 1208–1224 (2010).
DeKraker, J. et al. Automated hippocampal unfolding for morphometry and subfield segmentation with HippUnfold. eLife 11, e77945 (2022).
DeKraker, J., Ferko, K. M., Lau, J. C., Köhler, S. & Khan, A. R. Unfolding the hippocampus: an intrinsic coordinate system for subfield segmentations and quantitative mapping. Neuroimage 167, 408–418 (2018).
DeKraker, J. et al. Evaluation of surface-based hippocampal registration using ground-truth subfield definitions. eLife 12, RP88404 (2023).
Dale, A. M., Fischl, B. & Sereno, M. I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194 (1999).
Lyttelton, O., Boucher, M., Robbins, S. & Evans, A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage 34, 1535–1544 (2007).
DeKraker, J., Lau, J. C., Ferko, K. M., Khan, A. R. & Köhler, S. Hippocampal subfields revealed through unfolding and unsupervised clustering of laminar and morphological features in 3D BigBrain. Neuroimage 206, 116328 (2020).
Haast, R. A. M. et al. Insights into hippocampal perfusion using high-resolution, multi-modal 7T MRI. Proc. Natl Acad. Sci. USA 121, e2310044121 (2024).
Ngo, A. et al. Associations of cerebral blood flow patterns with gray and white matter structure in patients with temporal lobe epilepsy. Neurology 103, e209528(2) (2024).
Karat, B. G., DeKraker, J., Hussain, U., Köhler, S. & Khan, A. R. Mapping the macrostructure and microstructure of the in vivo human hippocampus using diffusion MRI. Hum. Brain Mapp. 44, 5485–5503 (2023).
Ripart, M. et al. Automated and interpretable detection of hippocampal sclerosis in temporal lobe epilepsy: AID-HS. Ann. Neurol. 97, 62–75 (2025).
Hanson, J. L., Adkins, D. J., Nacewicz, B. M. & Barry, K. R. Impact of socioeconomic status on amygdala and hippocampus subdivisions in children and adolescents. Preprint at bioRxiv https://doi.org/10.1101/2023.03.10.532071 (2023).
Cabalo, D. G. et al. Differential reorganization of episodic and semantic memory systems in epilepsy-related mesiotemporal pathology. Brain 147, 3918–3932 (2024).
Leferink, C. A. et al. Organization of pRF size along the AP axis of the hippocampus and adjacent medial temporal cortex is related to specialization for scenes versus faces. Cereb. Cortex 34, bhad429 (2024).
Alexander-Bloch, A. F. et al. On testing for spatial correspondence between maps of human brain structure and function. Neuroimage 178, 540–551 (2018).
Amunts, K., Mohlberg, H., Bludau, S. & Zilles, K. Julich-Brain: a 3D probabilistic atlas of the human brain’s cytoarchitecture. Science 369, 988–992 (2020).
Amunts, K. et al. BigBrain: an ultrahigh-resolution 3D human brain model. Science 340, 1472–1475 (2013).
Axer, M. et al. High-resolution fiber tract reconstruction in the human brain by means of three-dimensional polarized light imaging. Front. Neuroinform. 5, 34 (2011).
Alkemade, A. et al. A unified 3D map of microscopic architecture and MRI of the human brain. Sci. Adv. 8, eabj7892 (2022).
Schleicher, A., Amunts, K., Geyer, S., Morosan, P. & Zilles, K. Observer-independent method for microstructural parcellation of cerebral cortex: a quantitative approach to cytoarchitectonics. Neuroimage 9, 165–177 (1999).
Coifman, R. R. et al. Geometric diffusions as a tool for harmonic analysis and structure definition of data: diffusion maps. Proc. Natl Acad. Sci. USA 102, 7426–7431 (2005).
Margulies, D. S. et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl Acad. Sci. USA 113, 12574–12579 (2016).
Vos de Wael, R. et al. BrainSpace: a toolbox for the analysis of macroscale gradients in neuroimaging and connectomics datasets. Commun. Biol. 3, 103 (2020).
Ding, S.-L. & Van Hoesen, G. W. Organization and detailed parcellation of human hippocampal head and body regions based on a combined analysis of cyto- and chemoarchitecture. J. Comp. Neurol. 523, 2233–2253 (2015).
Biswal, B. B., Van Kylen, J. & Hyde, J. S. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 10, 165–170 (1997).
Smith, S. M. et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl Acad. Sci. USA 106, 13040–13045 (2009).
Golesorkhi, M. et al. The brain and its time: intrinsic neural timescales are key for input processing. Commun. Biol. 4, 970 (2021).
Zang, Y., Jiang, T., Lu, Y., He, Y. & Tian, L. Regional homogeneity approach to fMRI data analysis. Neuroimage 22, 394–400 (2004).
Honey, C. J. et al. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl Acad. Sci. USA 106, 2035–2040 (2009).
van den Heuvel, M. P. & Sporns, O. Network hubs in the human brain. Trends Cogn. Sci. 17, 683–696 (2013).
Paquola, C. et al. Microstructural and functional gradients are increasingly dissociated in transmodal cortices. PLoS Biol. 17, e3000284 (2019).
Schaefer, A. et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex 28, 3095–3114 (2018).
Przeździk, I., Faber, M., Fernández, G., Beckmann, C. F. & Haak, K. V. The functional organisation of the hippocampus along its long axis is gradual and predicts recollection. Cortex 119, 324–335 (2019).
Vogel, J. W. et al. A molecular gradient along the longitudinal axis of the human hippocampus informs large-scale behavioral systems. Nat. Commun. 11, 960 (2020).
Frauscher, B. et al. Atlas of the normal intracranial electroencephalogram: neurophysiological awake activity in different cortical areas. Brain 141, 1130–1144 (2018).
Paquola, C. et al. A multi-scale cortical wiring space links cellular architecture and functional dynamics in the human brain. PLoS Biol. 18, e3000979 (2020).
Stark, S. M., Kirwan, C. B. & Stark, C. E. L. Mnemonic similarity task: a tool for assessing hippocampal integrity. Trends Cogn. Sci. 23, 938–951 (2019).
Blümcke, I., Coras, R., Miyata, H. & Ozkara, C. Defining clinico-neuropathological subtypes of mesial temporal lobe epilepsy with hippocampal sclerosis. Brain Pathol. 22, 402–411 (2012).
Blümcke, I. et al. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia 54, 1315–1329 (2013).
Steve, T. A. et al. Hippocampal subfield measurement and ILAE hippocampal sclerosis subtype classification with in vivo 4.7 tesla MRI. Epilepsy Res. 161, 106279 (2020).
O’Keefe, J. & Nadel, L. Précis of O’Keefe & Nadel’s The hippocampus as a cognitive map. Behav. Brain Sci. 2, 487–494 (1979).
Knierim, J. J. & Neunuebel, J. P. Tracking the flow of hippocampal computation: pattern separation, pattern completion, and attractor dynamics. Neurobiol. Learn. Mem. 129, 38–49 (2016).
Leutgeb, J. K., Leutgeb, S., Moser, M.-B. & Moser, E. I. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315, 961–966 (2007).
Glasser, M. F. et al. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178 (2016).
Robinson, E. C. et al. MSM: a new flexible framework for multimodal surface matching. Neuroimage 100, 414–426 (2014).
Van Essen, D. C., Drury, H. A., Joshi, S. & Miller, M. I. Functional and structural mapping of human cerebral cortex: solutions are in the surfaces. Proc. Natl Acad. Sci. USA 95, 788–795 (1998).
Gorgolewski, K. J. et al. The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci. Data 3, 160044 (2016).
Glasser, M. F. et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124 (2013).
Reiser, L., Harper, L., Freeling, M., Han, B. & Luan, S. FAIR: a call to make published data more findable, accessible, interoperable, and reusable. Mol. Plant 11, 1105–1108 (2018).
Huntenburg, J. et al. Loading and plotting of cortical surface representations in Nilearn. Res. Ideas Outcomes 3, e12342 (2017).
González-Arnay, E. et al. Immunohistochemical field parcellation of the human hippocampus along its antero-posterior axis. Brain Struct. Funct. 229, 359–385 (2024).
Henriksen, E. J. et al. Spatial representation along the proximodistal axis of CA1. Neuron 68, 127–137 (2010).
Igarashi, K. M., Ito, H. T., Moser, E. I. & Moser, M.-B. Functional diversity along the transverse axis of hippocampal area CA1. FEBS Lett. 588, 2470–2476 (2014).
Yushkevich, P. A. et al. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: towards a harmonized segmentation protocol. Neuroimage 111, 526–541 (2015).
Mountcastle, V. B. The columnar organization of the neocortex. Brain 120, 701–722 (1997).
Duncan, J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn. Sci. 14, 172–179 (2010).
Buckner, R. L. The role of the hippocampus in prediction and imagination. Annu. Rev. Psychol. 61, 27–48 (2010).
Wagner, H. H. & Dray, S. Generating spatially constrained null models for irregularly spaced data using Moran spectral randomization methods. Methods Ecol. Evol. 6, 1169–1178 (2015).
Koussis, N. C. et al. Generation of surrogate brain maps preserving spatial autocorrelation through random rotation of geometric eigenmodes. Imaging Neurosci. (Camb). 3, IMAG.a.71 (2025).
Norman, Y., Raccah, O., Liu, S., Parvizi, J. & Malach, R. Hippocampal ripples and their coordinated dialogue with the default mode network during recent and remote recollection. Neuron 109, 2767–2780 (2021).
Ward, A. M. et al. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum. Brain Mapp. 35, 1061–1073 (2014).
Bellana, B., Liu, Z.-X., Diamond, N. B., Grady, C. L. & Moscovitch, M. Similarities and differences in the default mode network across rest, retrieval, and future imagining. Hum. Brain Mapp. 38, 1155–1171 (2017).
Christoff, K., Irving, Z. C., Fox, K. C. R., Spreng, R. N. & Andrews-Hanna, J. R. Mind-wandering as spontaneous thought: a dynamic framework. Nat. Rev. Neurosci. 17, 718–731 (2016).
Fox, K. C. R., Spreng, R. N., Ellamil, M., Andrews-Hanna, J. R. & Christoff, K. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage 111, 611–621 (2015).
Ross, T. W. & Easton, A. The hippocampal horizon: constructing and segmenting experience for episodic memory. Neurosci. Biobehav. Rev. 132, 181–196 (2022).
Schacter, D. L., Benoit, R. G. & Szpunar, K. K. Episodic future thinking: mechanisms and functions. Curr. Opin. Behav. Sci. 17, 41–50 (2017).
Yang, Y., Chen, Z., Zhang, R., Xu, T. & Feng, T. Neural substrates underlying episodic future thinking: a voxel-based morphometry study. Neuropsychologia 138, 107255 (2020).
Axer, H., Axer, M., Krings, T. & Keyserlingk, D. G. Quantitative estimation of 3-D fiber course in gross histological sections of the human brain using polarized light. J. Neurosci. Methods 105, 121–131 (2001).
Dammers, J. et al. Automatic identification of gray and white matter components in polarized light imaging. Neuroimage 59, 1338–1347 (2012).
Chaudhuri, R., Bernacchia, A. & Wang, X.-J. A diversity of localized timescales in network activity. eLife 3, e01239 (2014).
Larivière, S. et al. BrainStat: a toolbox for brain-wide statistics and multimodal feature associations. Neuroimage 266, 119807 (2023).
Markello, R. D. et al. neuromaps: structural and functional interpretation of brain maps. Nat. Methods 19, 1472–1479 (2022).
Schmidt, B., Marrone, D. F. & Markus, E. J. Disambiguating the similar: the dentate gyrus and pattern separation. Behav. Brain Res. 226, 56–65 (2012).
Guzman, S. J., Schlögl, A., Frotscher, M. & Jonas, P. Synaptic mechanisms of pattern completion in the hippocampal CA3 network. Science 353, 1117–1123 (2016).
Leutgeb, S. & Leutgeb, J. K. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn. Mem. 14, 745–757 (2007).
Chen, J., Olsen, R. K., Preston, A. R., Glover, G. H. & Wagner, A. D. Associative retrieval processes in the human medial temporal lobe: hippocampal retrieval success and CA1 mismatch detection. Learn. Mem. 18, 523–528 (2011).
Berron, D. et al. Strong evidence for pattern separation in human dentate gyrus. J. Neurosci. 36, 7569–7579 (2016).
Ly, M. et al. Mesoscale diffusion magnetic resonance imaging of the ex vivo human hippocampus. Hum. Brain Mapp. 41, 4200–4218 (2020).
Hebb, D. O. The Organization of Behavior: A Neuropsychological Theory (Psychology, 2005).
Ackley, D. H., Hinton, G. E. & Sejnowski, T. J. A learning algorithm for Boltzmann machines. Cogn. Sci. 9, 147–169 (1985).
Whittington, J. C. R. et al. The Tolman–Eichenbaum machine: unifying space and relational memory through generalization in the hippocampal formation. Cell 183, 1249–1263 (2020).
Gandolfi, D. et al. Full-scale scaffold model of the human hippocampus CA1 area. Nat. Comput. Sci. 3, 264–276 (2023).
Royer, J. et al. An open MRI dataset for multiscale neuroscience. Sci. Data 9, 569 (2022).
Cabalo, D. G. et al. Multimodal precision neuroimaging of the individual human brain at ultra-high fields. Sci. Data. 12, 526 (2025).
Avants, B. B. et al. Advanced normalization tools (ANTS). Insight J. 2, 1–35 (2009).
Tournier, J.-D., Calamante, F. & Connelly, A. MRtrix: diffusion tractography in crossing fiber regions. Int. J. Imaging Syst. Technol. 22, 53–66 (2012).
Cruces, R. R. et al. Micapipe: a pipeline for multimodal neuroimaging and connectome analysis. Neuroimage 263, 119612 (2022).
Drouin, S. et al. IBIS: an OR ready open-source platform for image-guided neurosurgery. Int. J. Comput. Assist. Radiol. Surg. 12, 363–378 (2017).
Marcus, D. S. et al. Informatics and data mining tools and strategies for the human connectome project. Front. Neuroinform. 5, 4 (2011).
Bazin, P. L. E. A., Forstmann, B. U. & Alkemade, A. Ahead Brain 122017 - Original stains. Figshare https://uvaauas.figshare.com/articles/dataset/Ahead_Brain_122017_-_Original_stains/16844500 (2022).
Bazin, P. L. E. A., Forstmann, B. U. & Alkemade, A. Ahead Brain 152017 - Original stains. Figshare https://uvaauas.figshare.com/articles/dataset/Ahead_Brain_152017_-_Original_stains/16843039 (2022).
Acknowledgements
J.D. was supported by a Natural Science and Engineering Research Council of Canada Post Doctoral Fellowship award (NSERC-PDF), and the HIBALL, supported by the Helmholtz Association’s Initiative and Networking Fund and the Healthy Brains, Healthy Lives initiative at McGill University. B.G.K. was supported by a NSERC postgraduate scholarship. J.R. was supported by a Canadian Institute of Health Research Canada (CIHR) fellowship award. D.G.C. was supported by Fonds de recherche du Quebec-Sante (FRQS) and Savoy Foundation Doctoral fellowship award. A.N. acknowledges funding from FRQS and CIHR. R.C.-R. acknowledges that this research was undertaken thanks in part to funding from the Ministère de l’Economie, de l’Innovation et de l’Énergie du Québec and to funding from the Healthy Brains, Healthy Lives (HBHL) initiative at McGill University and FRQS. K.A. received funding from the European Union’s Horizon Europe Programme, grant agreement no. 101147319 (EBRAINS 2.0 Project, K.A.). M.A. was supported by computing time and was granted through VSR Computing Time Projects on the supercomputer JURECA at Jülich Supercomputing Centre (JSC), Germany, to analyze and 3D reconstruct the 3D-PLI dataset. B.F. was supported by a project grant from CIHR (grant no. PJT-175056). M.K. received funding from the Swiss National Science Foundation (grant no. SNF_219240). S.L.V. is supported by HIBALL and the Jacobs foundation. J.C.L. was supported by an NSERC Discovery grant (no. RGPIN-2023-05562), research start-up funding from the Department of Clinical Neurological Sciences at Western University and a McGill-Western Initiative for Translational Neuroscience Impact grant. B.C.B. acknowledges research support from NSERC (grant no. Discovery-1304413), CIHR (grant nos. FDN-154298, PJT-174995, PJT-191853), SickKids Foundation (grant no. NI17-039), HIBALL, HBHL, Brain Canada Foundation, the Centre of Excellence in Epilepsy of the Neuro at the Montreal Neurological Institute and the Tier-2 Canada Research Chairs Program. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the paper.
Author information
Authors and Affiliations
Contributions
J.D. contributed to project conceptualization, planning, data acquisition, preprocessing, analyses, code development, writing and editing. D.G.C. contributed to data acquisition, analyses and code development, particularly for fMRI data and editing of the paper. J.R. contributed to data preprocessing and analyses, particularly for iEEG data. A.N. contributed to data acquisition, preprocessing and code development. A.R.K. contributed to project conceptualization, planning and code development, particularly for HippUnfold surface fitting and editing of the paper. B.G.K. contributed to data preprocessing, particularly DWI data and code development, particularly permutation testing. O.B. contributed to data preprocessing and code development, particularly for surface plotting, surface statistics and deployment in an open-source repository. R.C.-R. contributed to data preprocessing, particularly fMRI, DWI and structural MRI surface fitting, and advised on code development. B.F. contributed to data acquisition, preprocessing and planning, particularly for iEEG data. R.P. contributed to data acquisition and preprocessing, particularly for iEEG and structural MRI from patients with epilepsy. J.Y.H. contributed to project conceptualization and planning, particularly in the layout of the paper and online resources. B.M. contributed to project conceptualization and planning, particularly in the layout of the paper and online resources, and editing of the paper. S.L.V. contributed to project conceptualization, planning and editing of the paper. J.C.L. contributed to project conceptualization and editing of the paper, particularly for iEEG data. M.K. contributed to project conceptualization and editing of the paper. A.B. contributed to project conceptualization, particularly for iEEG and structural MRI from patients with epilepsy. N.B. contributed to project conceptualization, particularly for iEEG and structural MRI from patients with epilepsy. S.E.A.M. contributed to data acquisition and preprocessing, particularly for histology PLI. M.A. contributed to data acquisition and preprocessing, particularly for histology PLI. K.A. contributed to project conceptualization and planning, organization and quality control of histological data and editing of the paper. A.C.E. contributed to project conceptualization, planning, writing and editing of the paper, and project supervision. B.C.B. contributed to project conceptualization, planning, analyses, code development, writing, editing and project supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Birte Forstmann, Tehila Nugiel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Nina Vogt, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
Replication of a subset of structural MRI features. An indpenedent, 3 T cohort (n = 82) was used to replicate results from Fig. 3. FA, fractional anisotropy; ADC, apparent diffusivity coefficient; qT1, quantitative T1.
Extended Data Fig. 2
Replication of functional MRI properties. An independent 7 T cohort (n = 10) was used to replicate the results from Fig. 4.
Extended Data Fig. 3 Separation of iEEG properties in Fig. 5 into component datasets for replication.
Extended Data Fig. 4
Morphometric thickness, gyrification, and curvature across datasets. PNI and MICs refer to 7 T and 3 T MRI datasets, respectively.
Extended Data Fig. 5 Examples of HippoMaps usage in task-fMRI.
The Mnemonic Similarity Task (MST) is used to probe the haemodynamic response function (HRF) magnitudes during successful pattern separation and novel trials. These maps are then compared to all others (right), listing the top two strongest correlations (black lines). Consistency plot shows mean +/− S.D. (n = 3160 pairwise comparisons, all p < 0.001, one-sample, two-sided t-tests). Scale bars are arbitrary unless indicated otherwise.
Extended Data Fig. 6 Replication of task fMRI analyses from Extended Fig. 5 at 3 T and 7 T, and across the MST task and a common object pairing study-test paradigm.
a) Same data as shown in Extended Data Fig. 5, but with significance testing. b) Successfully trials during episodic encoding (subsequent memory paradigm) and retrieval. c) and d) same as a) and b) but performed with 7 T fMRI and a smaller cohort.
Extended Data Fig. 7 Morphological differences between ipsilateral temporal lobe epilepsy (TLE) patients and healthy controls.
Comparisons to other maps are again shown to the right. Consistency plot shows mean +/− S.D. (n = 190 pairwise comparisons, all p < 0.001, one-sample, two-sided t-tests). Scale bars are arbitrary unless indicated otherwise.
Extended Data Fig. 8 Alignment of microstructural profiles by vertical translations.
a) One slice from 3D PLI data illustrating surfaces at various depths through the cortical mantle (yellow, orange, and red), as well as surfaces extrapolated above and below the cortical mantle (green and violet). b) Profiles across equivalent vertices from all surfaces, shown for a set of 500 vertices. The segmented cortical mantle boundaries are indicated by the dotted lines. Image intensities more closely follow these boundaries after alignment.
Extended Data Fig. 9 Permutation tests for spatial correlation of hippocampal maps.
Example Neurite Density Index (NDI), thickness, and T1w over T2w ratios are shown on the left, with data from27). To the right, a null distribution of correlation with permuted data is shown for each method. The observed R value is indicated by the dotted line. If the observed R value is within the null distribution, then there is no significant correlation. The p-value can be calculated as the proportion of the null distribution greater than the observed R value. One example of a permuted thickness map is shown below for each method.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
DeKraker, J., Cabalo, D.G., Royer, J. et al. HippoMaps: multiscale cartography of human hippocampal organization. Nat Methods (2025). https://doi.org/10.1038/s41592-025-02783-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41592-025-02783-3