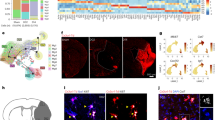
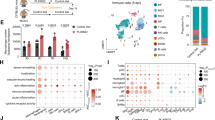
Abstract
Age is a major nonmodifiable risk factor for ischemic stroke. Central nervous system-associated macrophages (CAMs) are resident immune cells located along the brain vasculature at the interface between the blood circulation and the parenchyma. By using a clinically relevant thromboembolic stroke model in young and aged male mice and corresponding human tissue samples, we show that during aging, CAMs acquire a central role in orchestrating immune cell trafficking after stroke through the specific modulation of adhesion molecules by endothelial cells. The absence of CAMs provokes increased leukocyte infiltration (neutrophils and CD4+ and CD8+ T lymphocytes) and neurological dysfunction after stroke exclusively in aged mice. Major histocompatibility complex class II, overexpressed by CAMs during aging, plays a significant role in the modulation of immune responses to stroke. We demonstrate that during aging, CAMs become central coordinators of the neuroimmune response that ensure a long-term fine-tuning of the immune responses triggered by stroke.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Mouse macrophage RNA-seq data are available at the Gene Expression Omnibus under accession number GSE267623. All data necessary for the conclusions of the study are available in the main text, figures and Extended Data figures. Source data are provided with this paper.
References
Iadecola, C., Buckwalter, M. S. & Anrather, J. Immune responses to stroke: mechanisms, modulation, and therapeutic potential. J. Clin. Invest. 130, 2777–2788 (2020).
Drieu, A. et al. Parenchymal border macrophages regulate the flow dynamics of the cerebrospinal fluid. Nature 611, 585–593 (2022).
Amann, L., Masuda, T. & Prinz, M. Mechanisms of myeloid cell entry to the healthy and diseased central nervous system. Nat. Immunol. 24, 393–407 (2023).
Kida, S., Steart, P. V., Zhang, E. T. & Weller, R. O. Perivascular cells act as scavengers in the cerebral perivascular spaces and remain distinct from pericytes, microglia and macrophages. Acta Neuropathol. 85, 646–652 (1993).
Mendes-Jorge, L. et al. Scavenger function of resident autofluorescent perivascular macrophages and their contribution to the maintenance of the blood–retinal barrier. Invest. Ophthalmol. Vis. Sci. 50, 5997–6005 (2009).
Fabriek, B. O. et al. CD163-positive perivascular macrophages in the human CNS express molecules for antigen recognition and presentation. Glia 51, 297–305 (2005).
Faraco, G. et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J. Clin. Invest. 126, 4674–4689 (2016).
Park, L. et al. Brain perivascular macrophages initiate the neurovascular dysfunction of Alzheimer Aβ peptides. Circ. Res. 121, 258–269 (2017).
Pedragosa, J. et al. CNS-border associated macrophages respond to acute ischemic stroke attracting granulocytes and promoting vascular leakage. Acta Neuropathol. Commun. 6, 76 (2018).
Drieu, A. et al. Alcohol exposure-induced neurovascular inflammatory priming impacts ischemic stroke and is linked with brain perivascular macrophages. JCI Insight 5, e129226 (2020).
Popa-Wagner, A. et al. Ageing as a risk factor for cerebral ischemia: underlying mechanisms and therapy in animal models and in the clinic. Mech. Ageing Dev. 190, 111312 (2020).
Virani, S. S. et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 141, e139–e596 (2020).
Mozaffarian, D. et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133, e38–e360 (2016).
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C. & Santoro, A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590 (2018).
Jordão, M. J. C. et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363, eaat7554 (2019).
Prinz, M., Masuda, T., Wheeler, M. A. & Quintana, F. J. Microglia and central nervous system-associated macrophages-from origin to disease modulation. Annu. Rev. Immunol. 39, 251–277 (2021).
Masuda, T. et al. Specification of CNS macrophage subsets occurs postnatally in defined niches. Nature 604, 740–748 (2022).
Orset, C. et al. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke 38, 2771–2778 (2007).
Gauberti, M., Fournier, A. P., Docagne, F., Vivien, D. & Martinez de Lizarrondo, S. Molecular magnetic resonance imaging of endothelial activation in the central nervous system. Theranostics 8, 1195–1212 (2018).
Martinez de Lizarrondo, S. et al. Tracking the immune response by MRI using biodegradable and ultrasensitive microprobes. Sci. Adv. 8, eabm3596 (2022).
Fournier, A. P. et al. Prediction of disease activity in models of multiple sclerosis by molecular magnetic resonance imaging of P-selectin. Proc. Natl Acad. Sci. USA 114, 6116–6121 (2017).
Mato, M. et al. Involvement of specific macrophage-lineage cells surrounding arterioles in barrier and scavenger function in brain cortex. Proc. Natl Acad. Sci. USA 93, 3269–3274 (1996).
Mrdjen, D. et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395 (2018).
Kierdorf, K., Masuda, T., Jordão, M. J. C. & Prinz, M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat. Rev. Neurosci. 20, 547–562 (2019).
Se, H. et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 9, 1512–1519 (2006).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290 (2017).
Hickman, S. E. et al. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 16, 1896–1905 (2013).
Knox, E. G., Aburto, M. R., Clarke, G., Cryan, J. F. & O’Driscoll, C. M. The blood–brain barrier in aging and neurodegeneration. Mol. Psychiatry 27, 2659–2673 (2022).
Yousef, H. et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat. Med. 25, 988–1000 (2019).
Paolicelli, R. C. et al. Microglia states and nomenclature: a field at its crossroads. Neuron 110, 3458–3483 (2022).
Schonhoff, A. M. et al. Border-associated macrophages mediate the neuroinflammatory response in an α-synuclein model of Parkinson disease. Nat. Commun. 14, 3754 (2023).
Garcia-Bonilla, L. et al. Analysis of brain and blood single-cell transcriptomics in acute and subacute phases after experimental stroke. Nat. Immunol. 25, 357–370 (2024).
Frosch, M., Amann, L. & Prinz, M. CNS-associated macrophages shape the inflammatory response in a mouse model of Parkinson’s disease. Nat. Commun. 14, 3753 (2023).
Drieu, A. et al. Parenchymal border macrophages regulate tau pathology and tau-mediated neurodegeneration. Life Sci. Alliance 6, e202302087 (2023).
Schmidt, A. et al. Targeting different monocyte/macrophage subsets has no impact on outcome in experimental stroke. Stroke 48, 1061–1069 (2017).
Drieu, A., Levard, D., Vivien, D. & Rubio, M. Anti-inflammatory treatments for stroke: from bench to bedside. Ther. Adv. Neurol. Disord. 11, 1756286418789854 (2018).
Vogelgesang, A. et al. Siponimod (BAF312) treatment reduces brain infiltration but not lesion volume in middle-aged mice in experimental stroke. Stroke 50, 1224–1231 (2019).
Llovera, G. et al. The choroid plexus is a key cerebral invasion route for T cells after stroke. Acta Neuropathol. 134, 851–868 (2017).
Benakis, C. et al. T cells modulate the microglial response to brain ischemia. eLife 11, e82031 (2022).
Roth, S., Yang, J., Cramer, J. V., Malik, R. & Liesz, A. Detection of cytokine-induced sickness behavior after ischemic stroke by an optimized behavioral assessment battery. Brain Behav. Immun. 91, 668–672 (2021).
Iadecola, C. et al. Cell autonomous role of border associated macrophages in ApoE4 neurovascular dysfunction and susceptibility to white matter injury. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-3222611/v1 (2023).
Gervois, P. & Lambrichts, I. The emerging role of triggering receptor expressed on myeloid cells 2 as a target for immunomodulation in ischemic stroke. Front. Immunol. 10, 1668 (2019).
Atagi, Y. et al. Apolipoprotein E is a ligand for triggering receptor expressed on myeloid cells 2 (TREM2). J. Biol. Chem. 290, 26043–26050 (2015).
Krumbholz, M. et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 129, 200–211 (2006).
Polfliet, M. M. J. et al. The role of perivascular and meningeal macrophages in experimental allergic encephalomyelitis. J. Neuroimmunol. 122, 1–8 (2002).
Alves de Lima, K., Rustenhoven, J. & Kipnis, J. Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu. Rev. Immunol. 38, 597–620 (2020).
Lehenkari, P. P. et al. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol. Pharmacol. 61, 1255–1262 (2002).
Quenault, A. et al. Molecular magnetic resonance imaging discloses endothelial activation after transient ischaemic attack. Brain 140, 146–157 (2017).
Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Bindea, G. et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25, 1091–1093 (2009).
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement number 813294 (ENTRAIN). This work was also supported by grants from the Ministère de l’Enseignement Supérieur et de la Recherche and INSERM (French National Institute for Health and Medical Research; HCERES U1237-2017/2022), Throne (ANR-22-CE14-002), Eranet-Neuron MeniPSYs (ANR-22-NEU2-0005) and the Fondation pour la Recherche Médicale (ARF202005011926; E.L.). The work of A.M. is supported by the UK Dementia Research Institute (DRI), which receives its funding from UK DRI, funded by the UK Medical Research Council, Alzheimer’s Society and Alzheimer’s Research UK. A.M. also holds a UKRI Medical Research Council fellowship (Career Development Award MR/V032488/1) and a Foundation for Research on Alzheimer’s disease award. A.P.F. is funded by the WINNINGNormandy Program supported by the Normandy Region and the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement number 101034329. Some illustrations were created using BioRender.com.
Author information
Authors and Affiliations
Contributions
D.L. designed and performed the experiments, analyzed and interpreted the data, created the figures and wrote the manuscript. C. Seillier performed the flow cytometry experiments. M.B.-S. performed the quantification of human sample staining and analyzed and interpreted the data. A.P.F. performed the molecular MRI experiments. E.L. and C.D. performed immunohistological experiments and data analyses. G.R. performed the cell sorting experiments. A.M., K.M., C. Smith and C.M. performed the human sample staining experiments and participated in methods writing. A.M., M.P. and D.V. provided intellectual contributions. L.A. and M.P. performed the mouse RNA-seq data analyses and participated in methods writing. D.V. provided resources and intellectual contributions. M.R. designed and performed the experiments, analyzed and interpreted the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Ádám Dénes, Robert Harris and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Immunofluorescent characterization of CAMs in young and aged mice cortex.
a) Representative immunofluorescence images of CD206+ CAMs (red), P2Y12R+ microglia (green) and collagen IV in the basal lamina of blood vessels (gray) in young male mice brain cortex. Scale bar: 100 µm. b-d) Representative immunofluorescence images of CD206+ CAMs (red), P2Y12R+ microglia (green), GFAP+ astrocytes (cyan), AQP4+ astrocytic endfeet (cyan) in young mice brain cortex. Scale bar: 100 µm. e) Representative immunofluorescence images of CD206+ CAMs (red), Iba1+ microglia (green) and collagen IV in the basal lamina of blood vessels (gray) in young and aged male mice brain cortex. Scale bar: 100 µm. f) Representative immunofluorescence images of CD206+ and Lyve1+ CAMs (red) and podocalyxin-labelled blood vessels (gray), in young or aged male mice brain cortex. Scale bar: 100 µm. g) Representative immunofluorescence images of Iba1+ microglia (green) and CD68+ macrophage/phagocytic microglia (red) in young or aged male mice brain cortex. Scale bar: 100 µm. h) Immunohistological quantification of Iba1+ microglia and Iba1+/CD68+ phagocytic microglia or macrophages in young or aged male mice brain cortex. n=6 mice/groupe. Two-sided Mann-Whitney U-test. Data are presented as mean values +/− SEM.
Extended Data Fig. 2 Flow cytometry dot-plots and gating strategies.
a) Representative flow cytometry dot-plots and gating strategy used for quantification of CAMs, microglia or macrophages and neutrophils from young and aged male mice brain. b) Representative flow cytometry dot-plots and gating strategy used for quantification of CD4 and CD8 T cells from young and aged mice brain. c) Representative flow cytometry dot-plots and gating strategy used for quantification of CD19+ B220+ B cells from young and aged mice brain. d) Representative flow cytometry dot-plots and gating strategy used for isolation of CD11b+ CD45+ CD206+ CAMs by fluorescence activated cell sorting (FACS) from young and aged mice brain to analyze the RNA expression profile.
Extended Data Fig. 3 Immunohistochemical analysis of microglia in young and aged human brain cortex.
a) Table representing the information about the gender, the age and the Broadmann area of interest of the subjects within the two groups "Young controls" and "Aged controls". b) Representative IHC staining of human brain cortex within grey matter area. Hematoxylin (Blue), Iba1 (DAB), HLA (green). Scale bar: 50 µm. c) Representative IHC staining of human brain cortex within grey matter area. Hematoxylin (Blue), Iba1 (DAB), CD74 (green) Scale bar: 50 µm. d) Quantification of Iba1+, Iba1+ HLA+ and Iba1+ CD74+ microglia in the human grey matter. n= 5 young controls/ 5 aged controls. Two-sided Mann-Whitney Utest. Data are presented as mean values +/− SEM.
Extended Data Fig. 4 CAMs depletion by intracerebroventricular injection of clodronate-liposomes.
a) Schematic representation of the experimental protocol for CAMs depletion and quantification. b) Representative flow cytometry dot-plots and gating strategy used for quantification of CAMs, microglia, activated microglia or macrophages and neutrophils from young and aged male mice brain treated with vehicle or clodronate-liposomes. c) Flow cytometry quantification of CAMs, microglia, activated microglia or macrophages and neutrophils from young and aged mice brain treated with vehicle or clodronate-liposomes. n=4 mice/group. Two-sided Mann-Whitney U test. d) Representative immunofluorescence images of CD206+ CAMs (red), P2Y12R+ or Iba1+ microglia (green) and collagen IV in the basal lamina of blood vessels (gray), in vehicle or clodronate-liposomes treated mice cortex. Scale bar: 100 µm. e) Immunohistological quantification of CD206+ CAMs and Iba1+ microglia in vehicle or clodronate-liposomes treated mice cortex. n=5, Two-sided Mann-Whitney U-test. Data are presented as mean values +/− SEM (c, e).
Extended Data Fig. 5 Immunohistochemical analysis of P-selectin expression and fibrinogen extravasation.
a) Representative immunofluorescence images of Psel+ blood vessels in mice brain cortex at 5 days after stroke in vehicle or clodronate-treated young male mice. Scale bar: 50 µm. b) Representative immunofluorescence images of Psel+ blood vessels in mice brain cortex at 5 days after stroke in vehicle or clodronate-treated aged mice. Scale bar: 50 µm. c) Immunohistological quantification of the number of Psel+ blood vessels in mice brain cortex at 5 days after stroke. n=5 mice/group. d) Immunohistological quantification of the Psel+ area in mice brain cortex at 5 days after stroke. n=5 mice/group. e) Immunofluorescence images of Psel+ (magenta) blood vessels (gray) and Iba1+ microglia (green) in mice brain cortex at 5 days after stroke in vehicle or clodronate-treated aged mice. Scale bar: 50 µm. f) Representative immunofluorescence images of fibrinogen extravasation in mice brain cortex 1 day after stroke in young and aged mice treated with PBS (Vehicle) or clodronate (CLO). Scale bar 100µm. g) Quantification of the fibrinogen extravasation area. n=6 vehicle, young / n=6 CLO, young / n=5 vehicle, aged / n=5 CLO, aged. Multiple Mann-Whitney U-test with two-stage step up method for false discovery rate (c, d, g). Data are presented as mean values +/− SEM (c, d, g).
Extended Data Fig. 6 CAMs and microglia characterization after stroke in young and aged mice.
a) Representative flow cytometry dot-plots and gating strategy used for quantification of CAMs, microglia, macrophages and neutrophils 2 days after stroke in vehicle or clodronate-treated young and aged male mice brain. b-c) Flow cytometry quantification of CAMs, microglia, macrophages and neutrophils 2 days after stroke in vehicle or clodronate-treated young and aged mice brain. n=7 mice/group. d) Representative immunofluorescence images of CD206+ CAMs and Iba1+ microglia in perilesional area at 5 days after stroke in vehicle or CLO treated young and aged mice. Scale bar: 100 µm. e) Immunohistological quantification of CD206+ CAMs 1 day (left) and 5 days (right) after stroke. n=5 mice/group. f) Representative immunofluorescence images of Iba1+/CD68+ phagocytic microglia in perilesional area 1 day after stroke in vehicle or clodronate-treated young or aged mice. Scale bar: 100 µm. g) Immunohistological quantification of Iba1+ microglia 1 day and 5 days after stroke. n=5 mice/group. h) Immunohistological quantification of Iba1+/CD68+ phagocytic microglia 1 day and 5 days after stroke. n=5 mice/group. Multiple Mann-Whitney U-test with two-stage step up method for false discovery rate (b, c, e, g, h). Data are presented as mean values +/− SEM (b, c, e, g, h).
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Levard, D., Seillier, C., Bellemain-Sagnard, M. et al. Central nervous system-associated macrophages modulate the immune response following stroke in aged mice. Nat Neurosci 27, 1721–1733 (2024). https://doi.org/10.1038/s41593-024-01695-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41593-024-01695-3
This article is cited by
-
Molecular and cellular characteristics of cerebrovascular cell types and their contribution to neurodegenerative diseases
Molecular Neurodegeneration (2025)
-
Border-associated macrophages: an emerging perspective from physiological basis and multi-disease roles to the mechanism of vascular cognitive impairment and dementia
Journal of Neuroinflammation (2025)
-
Immunosenescence: signaling pathways, diseases and therapeutic targets
Signal Transduction and Targeted Therapy (2025)
-
Microglia–neuron crosstalk through Hex–GM2–MGL2 maintains brain homeostasis
Nature (2025)
-
Dynamic fibroblast–immune interactions shape recovery after brain injury
Nature (2025)