Abstract
The structural features that govern broad-spectrum activity of broadly neutralizing anti-ebolavirus antibodies (Abs) outside of the internal fusion loop epitope are currently unknown. Here we describe the structure of a broadly neutralizing human monoclonal Ab (mAb), ADI-15946, which was identified in a human survivor of the 2013–2016 outbreak. The crystal structure of ADI-15946 in complex with cleaved Ebola virus glycoprotein (EBOV GPCL) reveals that binding of the mAb structurally mimics the conserved interaction between the EBOV GP core and its glycan cap β17–β18 loop to inhibit infection. Both endosomal proteolysis of EBOV GP and binding of mAb FVM09 displace this loop, thereby increasing exposure of ADI-15946’s conserved epitope and enhancing neutralization. Our work also mapped the paratope of ADI-15946, thereby explaining reduced activity against Sudan virus, which enabled rational, structure-guided engineering to enhance binding and neutralization of Sudan virus while retaining the parental activity against EBOV and Bundibugyo virus.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Coordinates and structure factors have been deposited in the Protein Data Bank under accession number 6MAM. Other data are available from corresponding author upon reasonable request.
References
Qiu, X. et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514, 47–53 (2014).
Corti, D. et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science 351, 1339–1342 (2016).
Pascal, K. E. et al. Development of clinical-stage human monoclonal antibodies that treat advanced Ebola virus disease in non-human primates. J. Infect. Dis. 218, S612–S626 (2018).
Cox, E. et al. Notes for the Record: Consultation on Monitored Emergency Use of Unregistered and Investigational Interventions for Ebola Virus Disease http://www.who.int/emergencies/ebola/MEURI-Ebola.pdf?ua=1 (World Health Organization, 2018).
Sivapalasingam, S. et al. Safety, pharmacokinetics, and immunogenicity of a co-formulated cocktail of three human monoclonal antibodies targeting Ebola virus glycoprotein in healthy adults: a randomised, first-in-human phase 1 study. Lancet. Infect. Dis. 18, 884–893 (2018).
Lee, J. E. et al. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature 454, 177–182 (2008).
Volchkov, V. E., Feldmann, H., Volchkova, V. A. & Klenk, H. D. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc. Natl Acad. Sci. USA 95, 5762–5767 (1998).
Gregory, S. M. et al. Structure and function of the complete internal fusion loop from Ebola virus glycoprotein 2. Proc. Natl Acad. Sci. USA 108, 11211–11216 (2011).
Lee, J. et al. Structure of the Ebola virus envelope protein MPER/TM domain and its interaction with the fusion loop explains their fusion activity. Proc. Natl Acad. Sci. USA 114, E7987–E7996 (2017).
Weissenhorn, W., Carfí, A., Lee, K. H., Skehel, J. J. & Wiley, D. C. Crystal structure of the Ebola virus membrane fusion subunit, GP2, from the envelope glycoprotein ectodomain. Mol. Cell 2, 605–616 (1998).
Malashkevich, V. N. et al. Core structure of the envelope glycoprotein GP2 from Ebola virus at 1.9 Å resolution. Proc. Natl Acad. Sci. USA 96, 2662–2667 (1999).
Wang, H. et al. Ebola viral glycoprotein bound to its endosomal receptor Niemann–Pick C1. Cell 164, 258–268 (2016).
Schornberg, K. et al. Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein. J. Virol. 80, 4174–4178 (2006).
Chandran, K., Sullivan, N. J., Felbor, U., Whelan, S. P. & Cunningham, J. M. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 308, 1643–1645 (2005).
Hood, C. L. et al. Biochemical and structural characterization of cathepsin L-processed Ebola virus glycoprotein: implications for viral entry and immunogenicity. J. Virol. 84, 2972–2982 (2010).
Bornholdt, Z. A. et al. Host-primed Ebola virus GP exposes a hydrophobic NPC1 receptor-binding pocket, revealing a target for broadly neutralizing antibodies. mBio 7, e02154–15 (2016).
Côté, M. et al. Small molecule inhibitors reveal Niemann–Pick C1 is essential for Ebola virus infection. Nature 477, 344–348 (2011).
Carette, J. E. et al. Ebola virus entry requires the cholesterol transporter Niemann–Pick C1. Nature 477, 340–343 (2011).
Miller, E. H. et al. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J. 31, 1947–1960 (2012).
Wec, A. Z. et al. Antibodies from a human survivor define sites of vulnerability for broad protection against ebolaviruses. Cell 169, 878–890 (2017).
West, B. R. et al. Structural basis of pan-ebolavirus neutralization by a human antibody against a conserved, yet cryptic epitope. mBio 9, e01674–18 (2018).
Saphire, E. O. et al. Systematic analysis of monoclonal antibodies against Ebola virus GP defines features that contribute to protection. Cell 174, 938–952 (2018).
Furuyama, W. et al. Discovery of an antibody for pan-ebolavirus therapy. Sci. Rep. 6, 20514 (2016).
Milligan, J. C. et al. Structural characterization of pan-ebolavirus antibody 6D6 targeting the fusion peptide of the surface glycoprotein. J. Infect. Dis. 219, 415–419 (2019).
Bornholdt, Z. A. et al. Isolation of potent neutralizing antibodies from a survivor of the 2014 Ebola virus outbreak. Science 351, 1078–1083 (2016).
Zhao, X. et al. Immunization-elicited broadly protective antibody reveals ebolavirus fusion loop as a site of vulnerability. Cell 169, 891–904 (2017).
Pallesen, J. et al. Structures of Ebola virus GP and sGP in complex with therapeutic antibodies. Nat Microbiol 1, 16128 (2016).
Zhao, Y. et al. Toremifene interacts with and destabilizes the Ebola virus glycoprotein. Nature 535, 169–172 (2016).
Keck, Z. et al. Macaque monoclonal antibodies targeting novel conserved epitopes within filovirus glycoprotein. J. Virol. 90, 279–291 (2015).
Howell, K. A. et al. Cooperativity enables non-neutralizing antibodies to neutralize Ebola virus. Cell Rep. 19, 413–424 (2017).
Klein, F. et al. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell 153, 126–138 (2013).
Russi, S., Song, J., McPhillips, S. E. & Cohen, A. E. The Stanford automated mounter: pushing the limits of sample exchange at the SSRL macromolecular crystallography beamlines. J. Appl. Crystallogr. 49, 622–626 (2016).
Cohen, A. E., Ellis, P. J., Miller, M. D., Deacon, A. M. & Phizackerley, R. P. An automated system to mount cryo-cooled protein crystals on a synchrotron beam line, using compact sample cassettes and a small-scale robot. J. Appl. Crystallogr. 35, 720–726 (2002).
Soltis, S. M. et al. New paradigm for macromolecular crystallography experiments at SSRL: automated crystal screening and remote data collection. Acta Crystallogr. D 64, 1210–1221 (2008).
McPhillips, T. M. et al. Blu-Ice and the distributed control system: software for data acquisition and instrument control at macromolecular crystallography beamlines. J. Synchrotron. Radiat. 9, 401–406 (2002).
Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D 66, 133–144 (2010).
Kabsch, W. XDS. Acta Crystallogr. D 66, 125–132 (2010).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D . 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, (213–221 (2010).
Brünger, A. T. Free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature 355, 472–475 (1992).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Robert, X. & Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 (2014).
Ng, M. et al. Cell entry by a novel European filovirus requires host endosomal cysteine proteases and Niemann–Pick C1. Virology 468-470, 637–646 (2014).
Wong, A. C., Sandesara, R. G., Mulherkar, N., Whelan, S. P. & Chandran, K. A forward genetic strategy reveals destabilizing mutations in the Ebolavirus glycoprotein that alter its protease dependence during cell entry. J. Virol. 84, 163–175 (2010).
Wec, A. Z. et al. A ‘Trojan horse’ bispecific-antibody strategy for broad protection against ebolaviruses. Science 354, 350–354 (2016).
Ilinykh, P. A. et al. Chimeric filoviruses for identification and characterization of monoclonal antibodies. J. Virol. 90, 3890–3901 (2016).
Jahrling, P. B. et al. Evaluation of immune globulin and recombinant interferon-alpha2b for treatment of experimental Ebola virus infections. J. Infect. Dis. 179, S224–S234 (1999).
Maruyama, T. et al. Ebola virus can be effectively neutralized by antibody produced in natural human infection. J. Virol. 73, 6024–6030 (1999).
Acknowledgements
X-ray diffraction data were collected at the Stanford Synchrotron Radiation Lightsource (SSRL), SLAC National Accelerator Laboratory. SSRL is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (including P41GM103393). Opinions, conclusions, interpretations, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army. The mention of trade names or commercial products does not constitute endorsement or recommendation for use by the Department of the Army or the Department of Defense. We acknowledge National Institutes of Health grants no. U19 AI109762 (E.O.S., K.C., J.M.D.), no. R01 AI132256 (K.C.), no. U19 AI109711 (A.B.), no. R01 AI132204 (E.O.S., M.J.A.) and no. R01 AI126587 (M.J.A.), the Defense Threat Reduction Agency HDTRA1-13-1-0034 (A.B.) and the Viral Hemorrhagic Fever Immunotherapeutic Consortium for support. This is manuscript number 29630 from The Scripps Research Institute.
Author information
Authors and Affiliations
Contributions
B.R.W., A.Z.W., C.L.M., M.L.F., P.A.I., K.H., A.S.W., R.M.J., A.S.H., S.H., E.G., K.A.H. and S.K. carried out the research. B.R.W., A.Z.W., K.C. and E.O.S. designed the study. M.J.A. contributed materials. L.M.W., J.M.D., A.B., K.C. and E.O.S. supervised the research. B.R.W., A.Z.W., K.C. and E.O.S. drafted the manuscript. B.R.W., A.Z.W., K.C. and E.O.S edited the manuscript. All authors analyzed data and commented on the drafts.
Corresponding authors
Ethics declarations
Competing interests
M.J.A. has stock in Integrated Biotherapeutics, a company developing antibody therapeutics for ebolavirus disease. A.Z.W., E.G. and L.M.W. are employees and equity holders of Adimab. K.C. and J.M.D. are members of the Scientific Advisory Board of Integrum Scientific, LLC.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Structural modeling of potential interactions between ADI-15946 and the glycan cap.
(a) The structure of uncleaved GP (PDB 5JQ3) showing the position of the β17-β18 loop bound to the 310 pocket. The connecting residues between the bound peptide and the β-sheets are unresolved in this structure and are indicated here as dotted lines. GP1 is shown in teal, GP2 in light cyan, and the glycan cap is shown in light green. (b) Structural alignment of uncleaved GP to the EBOV GPCL–ADI-15946 complex shows that the mAb might make favorable interactions with the glycan cap even though binding displaces the glycan cap β17-β18 loop from the 310 pocket (arrow showing displacement of loop). The heavy and light chains of ADI-15946 are colored dark orange and light orange respectively. (c) The heavy and light chain of ADI-15946 may form favorable interactions with the glycan cap. For example, HC R100G and LC Y92 are oriented such that they could potentially form hydrogen bonds with the 100% conserved GP1 residue, Q251. (d) Cathepsin access to the β13-β14 loop, shown as a green cartoon with the unresolved continuation of the loop as a dotted line, is likely inhibited by the steric bulk of ADI-15946 upon binding to GP. The structural alignment shows that the β13-β14 loop passes within close proximity to ADI-15946 and highlights a possible interaction between GP1 residue S211 and ADI-15946 LC residues F67 and D70.
Supplementary Figure 2 Conformational changes influence binding of ADI-15946.
(a) Cartoon illustration of GP with mucin-like domains (MLD) and glycan cap domains (CAP) illustrated as green ovals. The β17-β18 loop of the glycan cap descends to cover the ADI-15946 epitope (orange). This loop may exist in a dynamic equilibrium between ‘tethered’ and ‘loose’ positions that mask and expose the ADI-15946 epitope. (b) The W291R point mutation in the β17-β18 loop results in enhanced exposure of the ADI-15946 epitope. (c) Antibody FVM09 (gray) which binds to the β17-β18 loop appears to lift it up and away30, also better exposing the ADI-15946 epitope. (d) Enzymatic cleavage of GP deletes the glycan cap and the β17-β18 loop, better exposing the ADI-15946 epitope. Deletion of the glycan cap and β17-β18 loop enhances neutralization by ~100-fold. Mutation of the loop and binding of the loop by FVM09 enhance ADI-15946 neutralization by ~10- and 15-fold, respectively.
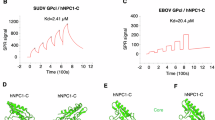
Supplementary Figure 3 Binding kinetics ADI-15946 and inferred germline progenitors against EBOV GPFL/GPCL.
Somatic hypermutation of ADI-15946 LC improves binding to GPFL and GPCL.
Supplementary Figure 4 FVM09 competition biolayer interferometry assays.
EBOV GP was loaded onto Nickel-NTA biosensors followed by two association steps of test antibodies. Pre-binding of FVM09 does not interfere with subsequent association of ADI-15946 (a) or KZ52 (c). In a converse experiment, pre-binding of ADI-15946 (b) or KZ52 (d) to GP does not interfere with subsequent binding of FVM09. In the second association steps, the first binder is maintained at the same concentration as in the first association step with addition of the competing antibody. All measurements were performed with antibodies at 330 nM concentration.
Supplementary Figure 5 Clashes between the β17-β18 loop and ADI-15946, c2G4, c4G7, or KZ52.
The cartoon representation on the left shows the various antibodies’ Fragment variable (Fv) aligned to unbound, uncleaved EBOV GP (PDB 5JQ3). The zoomed-in panel shows a stereoview of each alignment. On the right is a molecular surface of GPCL with the footprints of each antibody highlighted by selecting all atoms within 4 Å of the Fv. (a) The position of the β17-β18 loop (green) in unbound, uncleaved GP sterically overlaps the position of ADI-15946 in the EBOV GPCL–ADI-15946 crystal structure. (b-c) The position of the β17-β18 loop also interferes with the binding of c2G4 (PDB 5KEL), and c4G7 (PDB 5KEN). (d) The CDRs of KZ52 (PDB 3CSY), however, do not clash with the β17-β18 loop, but rather may form favorable interactions with the loop in its tethered position.
Supplementary Figure 6 Germline neutralization data.
(a-c) Neutralization of rVSVs bearing EBOV GP, BDBV GP, and SUDV GP, respectively, by wild-type ADI-15946 (black circles), heavy chain germline-revertant (HCIGL, gray squares), light chain germline-revertant (LCIGL; light blue triangles) and the HCIGL-LCIGL combination (purple diamonds). (d-f) Neutralization of rVSV bearing GPCL of EBOV, BDBV and SUDV, respectively, by the same ADI-15946 variants. (g) Neutralization of rVSV-EBOV GP bearing a W291R point mutation by wild-type and germline-revertant ADI-15946. For comparison, neutralization of rVSV-EBOV bearing uncleaved GP by wild-type ADI-15946 (orange open circles) is also shown. Virions bearing GPCL are better neutralized by wild-type ADI-15946 and its heavy chain germline-revertant (HCIGL; gray squares). (a-g) Mean±SD; n=6 biologically independent samples in each figure panel.
Supplementary Figure 7 Neutralization and binding of rVSVs bearing ebolavirus GPs or expressed ectodomains by engineered variants of mAb ADI-15946.
(a-e) Neutralization of rVSVs bearing GP from EBOV, BDBV, SUDV, TAFV, and RESTV respectively. Wild-type ADI-15946 is in black, structure-guided mutants 46M1, 46M2 and 46M3 in light, medium and dark blue, respectively. (f-h) Neutralization of rVSV bearing GPCL from EBOV, BDBV and SUDV, respectively. Neutralization of GPCL-bearing virions by wild-type ADI-15946 is in orange. Mutants 46M1-46M3 are in light to dark blue. Neutralization of virions bearing full-length GP by wild-type ADI-15946 is shown in black for comparison. (i) Antibody variants were tested for binding to soluble EBOV, BDBV (j), or SUDV (k) GP ectodomain immobilized on ELISA plates. Mutation of the neighboring ADI-15946 residue Y100A to alanine decreases binding to SUDV GPecto. (a-h) Mean±SD; n=6 biologically independent samples in each figure panel. (i-k) mean±SD; n=3 biologically independent samples in each figure panel.
Supplementary Figure 8 Neutralization of rVSV-EBOV GP and rVSV-EBOV GPΔβ17-β18 by ADI-15946 and affinity variants.
(a) ADI-15946 shows increased neutralization of an rVSV-EBOV GP variant bearing the deletion of residues 187-198 (GPΔβ17-β18) compared to rVSV bearing wild-type GP (WT)—same as figure 3C. (b) Deletion of the β17-β18 loop does not have a major impact on neutralization by KZ52. (c-d) ADI-15946 affinity variants 46M1, 46M2, and 46M3 also show enhanced neutralization of rVSV-EBOV GPΔβ17-β18. (f) The IC50 values are tabulated from each of the experiments graphed in a-e. (a-e) Mean ± SD; n = 6 biologically independent samples in each figure panel.
Supplementary information
Supplementary Figures, Supplementary Notes and Supplementary Tables
Supplementary Figures 1–8 and Supplementary Notes 1–3 and Supplementary Tables 1–3
Rights and permissions
About this article
Cite this article
West, B.R., Wec, A.Z., Moyer, C.L. et al. Structural basis of broad ebolavirus neutralization by a human survivor antibody. Nat Struct Mol Biol 26, 204–212 (2019). https://doi.org/10.1038/s41594-019-0191-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41594-019-0191-4
This article is cited by
-
Potent neutralization of Marburg virus by a vaccine-elicited antibody
Nature (2026)
-
Rational design of next-generation filovirus vaccines combining glycoprotein stabilization and nanoparticle display with glycan modification
Nature Communications (2025)
-
Longitudinal proteome-wide antibody profiling in Marburg virus survivors identifies wing domain immunogen for vaccine design
Nature Communications (2024)
-
Variant-proof vaccines — invest now for the next pandemic
Nature (2021)
-
Ebola virus glycoprotein GP1—host cell-surface HSPA5 binding site prediction
Cell Stress and Chaperones (2020)