Abstract
Ceramides are bioactive sphingolipids crucial for regulating cellular metabolism. Ceramides and dihydroceramides are synthesized by six ceramide synthase (CerS) enzymes, each with specificity for different acyl-CoA substrates. Ceramide with a 16-carbon acyl chain (C16 ceramide) has been implicated in obesity, insulin resistance and liver disease and the C16 ceramide-synthesizing CerS6 is regarded as an attractive drug target for obesity-associated disease. Despite their importance, the molecular mechanism underlying ceramide synthesis by CerS enzymes remains poorly understood. Here we report cryo-electron microscopy structures of human CerS6, capturing covalent intermediate and product-bound states. These structures, along with biochemical characterization, reveal that CerS catalysis proceeds through a ping-pong reaction mechanism involving a covalent acyl–enzyme intermediate. Notably, the product-bound structure was obtained upon reaction with the mycotoxin fumonisin B1, yielding insights into its inhibition of CerS. These results provide a framework for understanding CerS function, selectivity and inhibition and open routes for future drug discovery.
Similar content being viewed by others
Main
Ceramides are the precursors for the synthesis of complex sphingolipids and are bioactive signaling lipids. In particular, ceramides have been proposed as key metabolic sensors to promote fatty acid use and storage during excessive fatty acid availability1. Abnormal ceramide accumulation is associated with metabolic dysfunction and elevated levels of ceramides have been observed in obesity-related metabolic disorders such as diabetes, nonalcoholic fatty liver disease and nonalcoholic steatohepatitis (NASH)2,3,4.
Ceramides are composed of a sphingosine (d18:1) long-chain base with an N-linked acyl chain, the length of which is critical to the lipids’ biological functions and roles in pathophysiology5. For example, C16:0 ceramide is the most common ceramide in adipose tissue and its levels are elevated in this tissue of obese humans2. In addition, insulin resistance is correlated with plasma C16:0 and C18:0 ceramides, subcutaneous adipose tissue C16:0 ceramides and hepatic C16:0 and C18:0 ceramides6,7,8. Moreover, total ceramides and C16:0, C22:0 and C24:1 dihydroceramides were found to be elevated in the liver of insulin-resistant patients with NASH3.
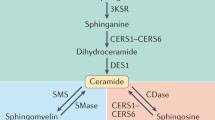
In mammals, de novo synthesis of ceramides is preceded by synthesis of dihydroceramides through the N-acylation of the sphingoid long-chain base sphinganine (dihydrosphingosine; d18:0) by one of six ceramide synthases (CerS1–CerS6)9. Alternatively, CerS can directly reacylate recycled sphingosine in the salvage pathway10. Of these enzymes, recent observations from human and mouse studies highlighted CerS6 as a target for treating obesity-associated metabolic disease, including type 2 diabetes and NASH2,5,11,12,13. Deletion of CerS6 in mice granted protection against diet-induced obesity, steatohepatitis and insulin resistance2,13 and liver-specific deletion improved glucose tolerance and mitochondrial morphology13. Strikingly, this occurs in CerS6Δ/Δ mice but not in CerS5Δ/Δ mice, although both show a preference for C16-CoA and, therefore, primarily produce C16 ceramides14, and hepatic C16:0 ceramide levels were reduced in both knockouts13. Resulting from the specific interaction of CerS6-derived C16:0 sphingolipids in mitochondria with the mitochondrial fission factor13, this revealed that the subcellular localization of ceramide production can lead to drastically different physiological outcomes13. In further support of the therapeutic potential of inhibiting CerS6, ablation of the protein’s expression in an obese insulin resistance mouse model led to improved body fat, oral glucose tolerance and insulin sensitivity11.
Central to their enzymatic function, CerSs contain a TRAM–Lag1–CLN8 (TLC) homology domain15, including a 52-residue Lag1p motif with conserved histidines and aspartates required for activity16,17. CerS2–CerS6 also contain a nonessential Hox-like domain between transmembrane (TM) domains 1 and 2 (ref. 18), flanked by two essential and conserved positively charged residues19. Regulators of CerS activity include phosphorylation20, glycosylation21, dimerization22 and other protein–protein interactions23,24. The six mammalian CerSs also have different acyl chain-length specificities and tissue expression patterns, further influencing the tissue-specific distribution of the different ceramides5,9,25. However, the molecular mechanisms underlying synthesis and regulation of ceramides and dihydroceramides remain unclear.
Despite the interest in pharmacologically reducing CerS6 activity, no specific inhibitors have been described for this family member. The best-characterized CerS inhibitor is fumonisin B1 (FB1), the most prevalent member of the fumonisin family of mycotoxins produced by Fusarium species. FB1 is of notable concern as it is a common carcinogenic and teratogenic contaminant of maize, rice, other cereals and cereal-based food stocks26,27,28. FB1 is a potent nonselective inhibitor of all human CerSs, being competitive toward both sphinganine and acyl-CoA substrates29. In addition, FTY720 (fingolimod), a prodrug administered to treat multiple sclerosis by modulating sphingosine-1-phosphate receptor 1, is also a nonselective CerS inhibitor30,31. Furthermore, a nonphosphorylatable analog of FTY720, P053, was recently shown to potently and selectively inhibit CerS1 (ref. 32), showcasing the possibility of achieving isoform-specific CerS inhibition.
Here, to understand the molecular basis of ceramide and dihydroceramide synthesis by the CerSs, we used a combination of cryo-electron microscopy (cryo-EM), intact protein mass spectrometry (MS) and small-molecule high-resolution MS (HRMS) and tandem MS (MS/MS) to probe the catalytic mechanism of human CerS6.
Results
Cryo-EM structure of human CerS6
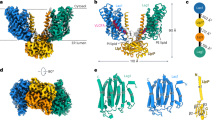
We expressed the human CerS6 protein, truncated at Asp350 to remove the predicted disordered C terminus, and purified the enzyme in glyco-diosgenin (GDN), yielding a mixture of monomeric and dimeric species on size-exclusion chromatography (SEC) (Extended Data Fig. 1a–d). While we selected the dimer species for structural studies to maximize particle size and possible symmetry for single-particle cryo-EM, its relatively low molecular weight (84 kDa) is still challenging for current methods. We, therefore, used a camelid nanobody to solve the structure of a CerS6 dimer at a nominal resolution of 3.2 Å (Fig. 1a,b, Table 1 and Extended Data Fig. 2). The CerS6–Nb22 complex was well resolved throughout the membrane-spanning region but the Hox-like domain was less well defined. This allowed for unambiguous tracing of CerS6 and Nb22 with the exception of the Hox-like domains, where residues 72–119 were fitted into the envelope of the cryo-EM map as rigid bodies (Methods and Extended Data Figs. 2e and 3a).
a, Cryo-EM map of the CerS6 dimer (blue) with one copy of Nb22 (gray) bound to each CerS6 monomer. b, Overall cartoon cylinder representation of the CerS6 dimer structure. One of the monomers is rainbow-colored from purple (N terminus) to red (C terminus). LH, luminal helix. c, Schematic representation of the CerS6 seven-TM helix topology. d, Cartoon representation of the transmembrane domain of a CerS6 monomer. The covalent acyl–imidazole species is shown in stick representation (acyl chain, pink carbon atoms) and the Coulombic potential map for this covalent species is shown as a pink transparent surface. The Hox-like domain was omitted for clarity. e, Denaturing intact protein MS analysis of purified CerS6 protein, revealing the presence of a covalent modification (+238.45 Da), which matches the expected mass shift corresponding to covalent attachment of a palmitoyl (C16:0) chain. This adduct peak was present in all purifications tested (n = 6). AMU, atomic mass units. Cyto, cytoplasm.
The cryo-EM structure reveals a CerS6 homodimer with one nanobody bound per monomer on the luminal face and a lipid adjacent to the dimer interface (Extended Data Fig. 3e and Supplementary Note 1). Each monomer has seven TM helices (Fig. 1b,c), with the dimer interface formed by TM1a, the C-terminal end of TM3 and the TM3–TM4 loop. The TLC domain (TM2–TM7) forms a barrel containing two sets of three-TM units arranged as inverted repeats (Extended Data Fig. 4a), while TM1 is attached to the outside of the barrel through contacts with TM2 and TM3. The TM2–TM7 barrel is assembled around a central cavity, which is open to the cytoplasm and closed to the endoplasmic reticulum (ER) lumen by a loop between TM6 and TM7. This central cavity has a large cytoplasmic opening (approximately 23 Å × 12 Å), creating a vestibule that funnels toward the midpoint of the membrane, where it forms a ~5-Å-wide tunnel that stretches toward the ER side.
Interestingly, the fold of the TM2–TM7 barrel of CerS6 resembles that of the six-TM barrels found in very-long-chain fatty acid elongase 7 (ELOVL7)33 and transmembrane proteins (TMEMs) 120A and 120B (refs. 34,35). Additionally, a structurally homologous six-TM barrel is predicted in the AlphaFold2 (AF2) models of 3-hydroxyacyl-CoA dehydratases (HACDs) 1–4 (Extended Data Fig. 4b), which participate in the same four-enzyme acyl-CoA elongation cycle as ELOVLs36. The observation that different acyl-CoA-modifying enzymes share a similar six-TM barrel structure is intriguing, suggesting that this architecture is likely optimized for the recognition and reaction with acyl-CoA substrates.
CerS6 copurifies with a covalently bound C16:0 acyl chain
The cryo-EM density map revealed the presence of a long density extending from near the membrane midpoint to the occluded ER end of the central cavity, spanning the entire length of the narrow tunnel (Figs. 1d and 2a). This density was continuous between protein and the unknown molecule, suggesting a covalent modification by a lipid. To probe the presence of covalent adducts, we performed denaturing intact protein MS analysis of purified protein samples and identified a +238.45-Da mass shift (Fig. 1e and Extended Data Fig. 1f,g). Notably, this adduct mass agrees with a covalently attached C16:0 acyl chain (theoretical: +238.41 Da). Strikingly, the shape and length of the density in the cryo-EM map is also consistent with a bound C16 acyl chain. A covalent bond is seen linking the acyl chain to the imidazole Nε of His211 (TM4) (Fig. 2b and Extended Data Fig. 3b), an absolutely conserved histidine that is required for CerS activity16,17. To further confirm this covalent adduct, we determined an additional CerS6 structure in complex with a second nanobody, Nb02, to a nominal resolution of 3.0 Å (Table 1 and Extended Data Fig. 5). The overall CerS6 structure is unchanged between nanobody complexes (TM region backbone root-mean-square deviation (r.m.s.d.) = 0.39 Å), with the improved local resolution in the active site validating the His211–acyl link (Extended Data Fig. 5g). Thus, on the basis of the extended density in the cryo-EM map and the observed mass adduct, we modeled a palmitoyl (C16:0) chain covalently attached to His211.
a, Cutaway molecular surface representation of the CerS6 TM region, revealing the presence of a long central cavity spanning the entire length of the protein. The palmitoyl chain covalently attached to His211 is bound in a narrow tunnel on the ER luminal half of the central cavity. b, Coulombic potential map in the region around the covalent linkage of the acyl chain to His211. c–e, CerS6’s acyl-binding tunnel, viewed from the membrane plane (c and d) or the ER face (e). Side chains lining and capping the tunnel are shown as sticks. Hydrogen bonds in the active site are shown as dashed lines.
The acyl chain is surrounded in the narrow tunnel primarily by hydrophobic side chains from TM4, TM5 and TM7 (Fig. 2c–e). The tunnel is sealed at the ER end by the TM6–TM7 loop (Fig. 2e), revealing how the chain-length preference of CerS6 for C16-CoA is determined by limiting the number of carbons that can fit in the acyl-binding tunnel, in agreement with earlier reports that this loop determines CerS acyl chain specificity21.
Catalysis by CerS6 proceeds through a ping-pong mechanism
Acyltransferases catalyze two-substrate, two-product reactions that can proceed through either ternary-complex or double-displacement (ping-pong) mechanisms37. In a ternary-complex mechanism, both the acyl donor and the acyl acceptor bind simultaneously and the enzyme catalyzes the direct acyl transfer from one substrate to the other. In contrast, a ping-pong mechanism involves two independent steps. Initially, reaction with the first substrate (the acyl donor) results in the transfer of the acyl chain to a nucleophilic residue, forming a covalent acyl–enzyme intermediate. This acyl chain is then transferred to the second substrate (the acyl acceptor) in the second step of the reaction.
The CerS6 structure has insufficient space for both substrates to access the active site at the same time because of the pathway from the cytoplasm narrowing to ~5 Å before the active site, thereby indicating that a ternary-complex mechanism is unlikely. Furthermore, the C16:0 chain covalently attached to the conserved His211 fits with CerS6’s selectivity for palmitoyl-CoA and suggests that this species could correspond to the acyl–enzyme intermediate of a ping-pong type reaction mechanism. To investigate whether the acylated enzyme species corresponds to a real catalytic intermediate, we incubated the purified protein with the enzyme’s second substrate, the long-chain base sphinganine (Fig. 3a). Using denaturing intact protein MS, we observed the complete loss of the C16:0-modified protein after sphinganine addition (Fig. 3b and Extended Data Fig. 6a). Furthermore, HRMS revealed that incubation of the acylated protein with sphinganine led to the production of C16:0 dihydroceramide (observed m/z: 540.5380; theoretical m/z: 540.5350; mass error: +5.55 ppm) (Fig. 3c). Overall, these results demonstrate that sphinganine reacts with the acyl–enzyme intermediate to deacylate the enzyme and generate the expected reaction product of dihydroceramide, thus supporting a ping-pong reaction mechanism for CerS6. Furthermore, the higher mass accuracy of HRMS also validates our identification of CerS6’s acyl modification as C16:0. Taken together with the structural data, this implies that His211 acts as the nucleophile in the first step of the reaction.
a, Chemical structures of the natural long-chain base substrate sphinganine (dihydrosphingosine) and the drug FTY720 (fingolimod). The homologous primary amines are highlighted in salmon. b, Intact mass analysis of protein samples after incubation in the absence of substrates (black) or in the presence of sphinganine (green) (n = 3 biological replicates). Replicate traces are provided in Extended Data Fig. 6. Deconvoluted mass peaks are indicated as follows: unmodified enzyme, orange icon; covalent acyl–enzyme species, orange and pink icon; background species present in all traces, gray circle. c, LC–HRMS detection of the reaction products. EICs are shown for the expected [M + H]+ ions of each of the reaction products after incubation of the acyl–enzyme intermediate with sphinganine (C16:0 dihydroceramide, green) or FTY720 (N-palmitoyl FTY720, orange). EICs obtained after incubation in the absence of substrates are shown in black. d, CerS6’s active site, viewed from the plane of the membrane. The central cavity is shown as a transparent gray surface, highlighting the presence of a side pocket (highlighted in green) adjacent to the acyl carbonyl. Hydrogen bonds are shown as dashed lines. e, Mutational analysis of the CerS6 active site by comparison of the C16:0 dihydroceramide synthase activity of WT and active site mutants (n = 3 independent biological replicates). Gray bars correspond to the mean of the biological replicates. Data points represent each biological replicate, corresponding to the mean of four technical replicates. Error bars show the s.e.m.
Notably, the CerS substrate sphinganine and FTY720 are chemically similar (Fig. 3a), with potential clinical consequences to drug pharmacokinetics as 15% of FTY720 becomes N-acylated in human subjects38. Although the major N-acyl FTY720 metabolites identified were N-stearoyl and N-2-hydroxystearoyl FTY720, there is evidence suggesting that a small amount of N-palmitoyl (C16:0) FTY720 is formed38. Which enzyme catalyzes the N-acylation of FTY720 has not been determined but a role for CerS enzymes has been proposed38. Incubation of acyl–CerS6 with FTY720 results in a small increase in the protein’s thermostability, suggesting that it binds to the purified enzyme (Extended Data Fig. 1e). To investigate whether purified CerS6 can N-acylate FTY720 in vitro, we incubated the enzyme with this compound and subsequently identified C16:0 FTY720 in the reaction mixture (observed m/z: 546.4869; theoretical m/z: 546.4881; mass error: −2.20 ppm) (Fig. 3c), the expected product of a reaction between FTY720 and the CerS6-bound acyl chain. The proposed structure of this C16:0 FTY720 reaction product was further validated on the basis of the product ion MS/MS spectrum of its [M + H]+ ion (Extended Data Fig. 6c,d). However, no obvious loss of the acyl–enzyme mass peak was observed when the reaction mixture was analyzed by denaturing intact protein MS (Extended Data Fig. 6b), suggesting that FTY720 is a poor acyl acceptor for CerS6.
Highly conserved residues line the catalytic site
CerS6’s Lag1p motif (Arg202–Tyr253) (TM4–TM5) contains the highly conserved His211/His212 (TM4) and Asp239/Asp242 (L5a-b/TM5), which are suggested to be in the catalytic site and have been shown to be essential for function in mammalian CerS16 and Saccharomyces cerevisiae Lag1p17.
Within the CerS6 structure, these residues line the central cavity near the midpoint of the membrane (Fig. 3d). Notably, the copurified covalently linked C16:0 chain is covalently attached to the first histidine of the Lag1p motif (His211) (Fig. 2a–d), which appears to act as the nucleophile in the first step of the reaction and is required for CerS6 function (Fig. 3e). Although it is remarkable that CerS6 uses a nucleophilic histidine, as cysteines or serines are more commonly used as nucleophiles, there are multiple well-documented examples of other enzymes with nucleophilic histidines in their catalytic mechanisms33,39,40,41,42,43.
Histidine nucleophiles are typically oriented and activated by hydrogen bonding between a proton at Nδ of the imidazole ring and a hydrogen-bond acceptor, ensuring that Nε remains unprotonated33,40,44. Examining our structure, we noted the proximity of His212 to the acylated His211 in CerS6, suggesting that this residue is the hydrogen-bond acceptor. While the Nδ of His212 is located 3.84 Å away from the Nδ of His211, it is plausible that the two side chains interact in the acyl-CoA-bound state. Indeed, we found that substitution of His212 to alanine results in the complete loss of activity (Fig. 3e). Substitution of residues corresponding to His212 of CerS6 also caused loss of function in the homologous mouse and human CerS1 (refs. 16,45) and yeast Lag1p17. This demonstrates that the second histidine has a key role in catalysis and explains the pathogenic effect of substituting the equivalent histidine of CerS1 to a glutamine in persons with progressive myoclonic epilepsy and dementia45. Moreover, we recently demonstrated that the reaction of human ELOVL7 with acyl-CoA substrates also involves a double-histidine motif, where the first histidine acts as a nucleophile and is activated by hydrogen bonding to the second33. Strikingly, structural alignment of CerS6 and ELOVL7 places the histidine pairs in equivalent positions (Extended Data Fig. 4c), suggesting that these residues have similar roles in the first step of their respective reactions.
Formation of the acyl–enzyme intermediate proceeds through an oxyanion transition state, which is usually stabilized by hydrogen bonding. Within the acyl–CerS6 structure, the carbonyl oxygen of the covalently attached C16:0 chain is within hydrogen-bonding distance (2.8 Å) of the Nδ of Asn315 (TM7). On the basis of the proximity of these moieties, we hypothesized that this interaction stabilizes the formation of the oxyanion transition state during catalysis. Consistent with this notion, an asparigine is present at the equivalent position in CerS1, whereas CerS2–CerS5, Lag1p and Lac1p have a histidine at this position (Extended Data Fig. 7), which could likewise act as a hydrogen-bond donor. Indeed, we found that an Asn315His mutant preserves activity. Surprisingly, however, substitution of Asn315 to an alanine or aspartic acid did not result in loss of activity (Fig. 3e), which does not support our initial hypothesis for the mechanism of oxyanion transition-state stabilization.
On the opposite side of the active site, the essential conserved Asp242 (TM5b) participates in a hydrogen-bonding network with Arg275 (L6a-b) and Asn315 (TM7) (Fig. 3d). We found that an Arg275Lys mutant retained only approximately 20% of wild-type (WT) activity (Fig. 3e), suggesting that the guanidino group of Arg275 is important for function and may facilitate the alignment of neighboring residues in the active site.
There is a third histidine on TM5a that is conserved in mammalian CerS but is a methionine in yeast Lag1p and Lac1p, thus being of ambiguous importance to the enzymes’ reaction cycle. In the CerS6 structure, His238 is located above the acyl–imidazole carbonyl and lays against the hydrocarbon chain (Fig. 3d). We found that substitution of this His238 to alanine reduced activity by over 50% (Fig. 3e), thus suggesting that this residue may participate in aligning the acyl chain.
Lastly, we noticed the presence of a side pocket in the active site of CerS6, adjacent to His211 (Fig. 3d) and lined by Tyr142 (TM2), Tyr189 (TM3), His212 (TM4), Asp239 (L5a-b), Asp242 (TM5b) and Arg275 (L6a-b). The position and chemistry of this pocket relative to the carbonyl of the acyl–enzyme intermediate suggest that this is likely where the amino alcohol moiety of the second (long-chain base) substrate binds. Supporting this notion, the residues of this pocket are highly conserved (Extended Data Fig. 7). This pocket, therefore, likely ensures that the primary amine of the long-chain base is ideally located for a nucleophilic attack on the carbonyl carbon of the acyl–imidazole intermediate in the second step of the reaction.
Structure of N-acyl FB1-bound CerS6
Having captured the intermediate state of the CerS6 enzyme, we next set out to examine the structural basis of CerS inhibition by FB1. To this end, we incubated purified Nb22-bound acyl–CerS6 with FB1 and determined the complex’s structure to a nominal resolution of 2.95 Å (Fig. 4a,b and Extended Data Fig. 8). In comparison to the covalent intermediate state, the overall structure of each CerS6 monomer did not change (TM region backbone r.m.s.d. = 0.39 Å), although the flexible Hox-like domains rotated by approximately 10o.
a, Cartoon representation of CerS6 with bound N-palmitoyl FB1 (shown as sticks; cyan carbon atoms). The cryo-EM density of the bound product is shown as a transparent cyan surface. b, Cutaway molecular surface representation, revealing that the N-palmitoyl FB1 species occupies the entire length of the central cavity. The Hox-like domain was omitted for clarity. c, Intact mass analysis of protein samples after incubation in the absence of substrates (black) or in the presence of the mycotoxin FB1 (blue) (n = 3 biological replicates). Replicate traces are provided in Extended Data Fig. 6. d, LC–HRMS detection of N-palmitoyl FB1. The EIC for its expected [M + H]+ ion is shown after incubation of the acyl–enzyme intermediate with FB1 (blue) or in the absence of the toxin (black). Inset, chemical structure of FB1. Its primary amine (salmon circle) and TCA (gray circles) groups are highlighted.
The cryo-EM map of the putative FB1-bound complex contains a long, continuous nonprotein density starting within the ER luminal end of the narrow tunnel and extending to the cytoplasmic entrance to the central cavity (Fig. 4a and Extended Data Fig. 3c,d). The observed density is too long to be FB1 alone. Notably, incubation of palmitoyl–CerS6 with FB1 completely removed the acyl group from the enzyme (Fig. 4c and Extended Data Fig. 6a) and generated C16:0–FB1 (observed m/z: 960.6260; theoretical m/z: 960.6254; mass error: +0.62 ppm) (Fig. 4d). Noting that the covalent bond between the enzyme and the palmitoyl group was replaced by a bond to the toxin, we modeled this density as C16:0–FB1 (Fig. 5a and Extended Data Fig. 3c). These results indicate that FB1 reacted with the covalent acyl–enzyme intermediate, whereby the palmitoyl group was released from His211 and attached to the toxin, generating the N-acyl FB1 product. This product remains bound to the enzyme, thus acting as an inhibitor. This finding is consistent with earlier reports that CerS enzymes can N-acylate FB1 (refs. 46,47).
a, Close-up view of the CerS6 active site in the covalent acyl–enzyme intermediate and N-palmitoyl FB1-bound states, showing the transfer of the palmitoyl chain from His211 to the toxin. b, Cytoplasmic portion of the central cavity, viewed from the membrane plane. Residues lining the cavity are shown as sticks. Polar interactions between the carboxylates of the TCA groups of FB1 and positively charged residues on TM2 and TM7 are shown as dashed lines. c, Active site, viewed from the plane of the membrane. d, Polar and nonpolar surfaces on the cytoplasmic half of the central cavity. Cutaway molecular surface view, showing that the hydrocarbon chain of FB1 interacts with the large hydrophobic face formed by TM5–TM7.
The N-acyl FB1 species spans the entire length of the central cavity, with the acyl chain remaining bound in the narrow tunnel at the occluded ER end of the cavity and the FB1 portion sitting in the wider cytoplasmic entrance (Fig. 4b and Fig. 5b,c). The newly formed amide bond sits in the active site (Fig. 5c), with the carbonyl oxygen forming a hydrogen bond with Tyr189 (TM3) and the amide NH forming a hydrogen bond with the Nε of His211 (TM4). The 3-hydroxyl group of FB1, which is also present in sphingoid base substrates, forms a hydrogen bond with the strictly conserved and catalytically essential Asp242 (TM5b)16,17. We hypothesize that this interaction with the 3-hydroxyl group of sphingoid base substrates would optimally place the sphingoid primary amine to attack the carbonyl carbon.
Below the active site, the cytoplasmic vestibule of the central cavity has both polar and nonpolar surfaces, where we noted that the hydrocarbon chain of FB1 interacts with the long hydrophobic face formed by TM5–TM7 (Fig. 5b,d). This hydrocarbon chain lies against the conserved Trp318 of TM7 just beneath the active site. Substituting the corresponding residue in CerS5 to an alanine abolishes enzymatic activity48. In contrast, a W318F substitution in CerS6 retained approximately WT levels of activity (Fig. 3e). Therefore, we hypothesize that the conserved tryptophan orients the sphingoid base substrate to place its amino alcohol moiety in the active site’s side pocket for the second step of the reaction.
The face of the central cavity opposite the FB1 hydrocarbon-binding surface displays a polar character and is lined by conserved charged residues that may participate in CoA binding (Fig. 5b,d). Accordingly, the positive charges provided by Lys125 and Arg131, located at the cytoplasmic entrance to the cavity, adjacent to the Hox-like domain, are important for the function of CerS5 and CerS6 (ref. 19). Furthermore, substitution of Lys131 in CerS3, equivalent to CerS6’s Arg131, to alanine was implicated in autosomal recessive congenital ichthyosis49, a disorder that can be caused by loss of ≥C26 ceramides because of mutations in CERS3 (refs. 50,51).
The two tricarballylic acid (TCA) groups of FB1 span the width of the cytoplasmic end of the central cavity, where one of the groups interacts with Arg131 (TM2) and the other interacts with Lys328 (TM7) (Fig. 5b). Thus, through these charge–charge interactions, FB1 links the first helix of the first three-TM bundle and the last helix of the second. Supporting the notion that FB1 acts to restrain the structure, atomistic molecular dynamics (MD) simulations of covalent intermediate and N-acyl FB1-bound CerS6 monomers indicated that a bound N-palmitoyl FB1 reduces overall protein flexibility, including TM6, TM7 and the Hox-like domain (Extended Data Fig. 9a–c). Moreover, while Lys125 was not resolved in our cryo-EM maps, in the simulations, Lys125 formed a salt bridge with the same TCA group that contacts Arg131 (Extended Data Fig. 9d). Furthermore, the simulations showed that this TCA also contacts Lys249 (TM5b), Tyr253 (TM5b) and Arg118 and Arg121 (last helix of the Hox-like domain). Therefore, FB1 appears to mimic several CoA interactions through the binding of one of its TCA moieties at the polar face of the cytoplasmic cavity. Lastly, on the opposite side of the cavity, the other TCA moiety can form salt bridges with Lys328 or Arg332 (TM7) (Extended Data Fig. 9d). Overall, our cryo-EM structure and simulations revealed that FB1 is anchored by polar interactions at opposite sides of the six-TM barrel, which likely hinder product release, arresting the enzyme in an inhibitory product-bound state.
Discussion
CerSs carry out an essential step in sphingolipid biosynthesis and are promising drug targets. Yet, our limited understanding of their structures and biochemical mechanism has hindered their therapeutic targeting. Here, we provide structural snapshots of CerS6 at two stages of its reaction cycle, revealing its ping-pong (double-displacement) reaction mechanism that uses a histidine nucleophile to attack the acyl-CoA thioester to form a stable acyl–imidazole intermediate. This intermediate subsequently reacts with the sphingoid long-chain base substrate to yield the final ceramide or dihydroceramide reaction product (Fig. 6). This sequential reaction and single substrate entry path contrasts with the ternary-complex reaction mechanism and lateral entry gate proposed for CerS2 and Lac1p (Supplementary Discussion).
Initially, the acyl-CoA substrate binds with the acyl chain buried deep within the central tunnel and the CoA moiety sitting near the cytoplasmic entrance to the central cavity. In the first step, the nucleophilic attack of His211 on the acyl-CoA thioester carbonyl results in thioester cleavage, covalent acyl–imidazole intermediate formation and release of CoA. Subsequently, the long-chain sphingoid base substrate binds with its hydrocarbon chain interacting with the hydrophobic face of the central cavity and its amino alcohol moiety sitting in the side pocket in the active site. In the second step of the reaction, the primary amine of the long-chain base attacks the acyl–imidazole intermediate, leading to covalent intermediate breakdown and formation of the final N-acyl sphingoid base (ceramide) product.
Although CoA and the hydrocarbon chain of long-chain bases likely interact at distinct faces of the cytoplasmic vestibule, there is not enough space for the acyl-CoA and long-chain base substrates to bind at the same time. Therefore, upon thioester cleavage and covalent intermediate formation, the CoA product needs to exit the central cavity before the long-chain base substrate can bind. We hypothesize that the amino alcohol group of the long-chain base would bind in a discrete side pocket lined by polar residues conserved across the entire CerS family. This long-chain base is further oriented for the second step of the reaction by hydrophobic residues located at the entrance to this pocket, particularly by the conserved Trp318 (TM7). The primary amine of the long-chain base then attacks the acyl–enzyme intermediate to form the final reaction product. Release of this product into the membrane likely occurs between two TM helices and the two halves of the six-TM barrel immediately suggest an egress route between TM4 and TM7. Indeed, we found that the TCA groups of N-palmitoyl FB1 attach to both halves of the barrel and restrict the movement of TM7, suggesting that these interactions likely prevent that product’s release. This proposed role for the TCA groups of FB1 is also consistent with previous reports that the hydrolyzed form of FB1, lacking the TCA moieties, is a weaker CerS inhibitor52. However, FB1 is competitive toward both substrates29. This suggests that FB1’s mode of inhibition of CerS is likely to be multifaceted, sterically blocking substrate binding to the apo state and locking the protein in a product-bound state as shown here.
Lastly, the acylation of FTY720 has important consequences for the clinical use of compounds that mimic sphingoid bases. The action of CerS partially explains the N-acylated FTY720 species found during the FTY720 drug trials38 and, by extension, this enzyme family may have a role in the pharmacokinetics of other sphingoid analogs. Overall, our findings support the notion that unintended substrates can lead to the formation of CerS-inhibiting products, as shown by FB1’s ability to arrest the protein in a product-bound state (Extended Data Fig. 10). This suggests that ceramide or N-acyl FB1 mimetics could provide novel chemical scaffolds for new CerS inhibitors.
Methods
CerS6 cloning and expression
The Homo sapiens CERS6 gene, encoding the CerS6 protein (Uniprot Q6ZMG9), was cloned into the baculovirus transfer vector pHTBV1.1-CT10H-SIII-LIC (adapted from the BacMam vector pHTBV1.1; provided by F. Boyce, Massachusetts General Hospital) upstream of C-terminal tobacco etch virus (TEV)-cleavable 10xHis and Twin-Strep tags. The construct used for structural determination lacked the final 42 amino acids, which are predicted to be disordered. For in vitro biotinylation, the Avi-tagged CerS6 construct contained a Gly-Gly-Gly-Ser linker between CerS6 and the Avi tag, located upstream of the TEV cleavage site. Site-directed mutagenesis was performed using the Q5 site-directed mutagenesis kit (New England Biolabs).
Baculoviral DNA was generated by transposition of DH10Bac with the baculovirus transfer vector. Baculovirus was produced by transfecting Sf9 cells (Thermo Fisher Scientific) with the baculoviral DNA using the Insect GeneJuice transfection reagent (Merck Milipore). The virus was amplified by infecting Sf9 cells in the presence of 2% FBS and incubating for 72 h at 27 °C. For large-scale protein expression in Expi293F GnTI− cells (Thermo Fisher Scientific) in Freestyle 293 expression medium (Thermo Fisher Scientific), cells were transduced with baculovirus and supplemented with 5 mM sodium butyrate. Cells were incubated in a humidity-controlled orbital shaker at 37 °C with 8 % CO2 for 48 h and subsequently harvested by centrifugation at 900g for 15 min. Pelleted cells were washed with PBS, pelleted again by centrifugation, flash-frozen in liquid N2 and stored at −80 °C. To compare mutant activity, WT and mutant proteins were expressed in Expi293F cells (Thermo Fisher Scientific) using the same protocol.
Large-scale CerS6 purification
CerS6-overexpressing cells were resuspended in buffer A (50 mM HEPES pH 7.5, 200 mM NaCl and 5% (v/v) glycerol) and solubilized with 1% (w/v) lauryl maltose neopentyl glycol (LMNG) (Anatrace) and 0.1% (w/v) cholesteryl hemisuccinate (CHS) (Sigma-Aldrich). Insoluble material was removed by centrifugation at 35,000g and the protein was purified from the supernatant by batch binding to Strep-Tactin XT Superflow resin (IBA Lifesciences). The resin was washed initially with buffer A containing 0.05% (w/v) GDN and subsequently with buffer A supplemented with 1 mM adenosine triphosphate (ATP; Sigma-Aldrich), 10 mM MgCl2 and 0.05 % (w/v) GDN. CerS6 was eluted from the resin using buffer A supplemented with 100 mM d-biotin (Fluorochem) and 0.02% (w/v) GDN, while the C-terminal tag was cleaved by TEV protease digestion overnight. The His-tagged TEV protease was removed by binding to Co2+-charged TALON resin (Clontech) and the flowthrough was concentrated using a concentrator with a 100-kDa molecular weight cutoff (Corning). The protein was finally purified by SEC on a Superdex 200 Increase 10/300 GL column (GE Healthcare), pre-equilibrated in SEC buffer (20 mM HEPES pH 7.5, 200 mM NaCl and 0.01 % (w/v) GDN).
For nanobody generation and selection, GDN was replaced in the purification buffers with 0.003% (w/v) LMNG and 0.0003% (w/v) CHS. For in vitro biotinylation of C-terminally Avi-tagged CerS6, during the TEV cleavage step, the protein sample was further supplemented with 15 mM MgCl2, 15 mM ATP, 50 mM bicine pH 8.3 and BirA at a 1:15 (w/w) BirA:CerS ratio. Biotinylation efficiency was routinely monitored by denaturing intact protein MS.
For structural determination, CerS6 was mixed with a 1.5× molar excess of nanobody (either Nb22 or Nb02) and incubated for 1 h on ice. The CerS6–nanobody complexes were purified by SEC, concentrated to 5 mg ml−1 using a concentrator with a 100-kDa molecular weight cutoff (Sartorius) and processed immediately for cryo-EM.
Nanobody library generation and selection
Purified CerS6 was reconstituted at a 1:20 (protein:lipid (w/w) ratio into liposomes composed of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol, 1,2-dimyristoyl-sn-glycero-3-phosphocholine and 1,2-dimyristoyl-sn-glycero-3-phosphoglycerol (7:3:7:3 (w/w)) as previously described53.
To obtain anti-CerS6 nanobodies, alpacas were immunized and the nanobody library was generated as previously described54, with the exception that 200 µg of purified CerS6 in proteoliposomes was used for each immunization. All the procedures concerning alpaca immunization were approved by the Cantonal Veterinary Office of Zurich (license no. ZH 198/17). The resulting nanobody library was screened by biopanning against CerS6. Subsequently, 190 single clones from the enriched nanobody library were analyzed by ELISA for binding to CerS6. A total of 96 ELISA-positive clones were Sanger-sequenced and grouped in families according to their complementarity-determining region length and sequence diversity55. Of these, 42 unique nanobodies were identified as belonging to 26 nanobody families and were taken for further validation.
Nanobodies were expressed in 50-ml scale in WK6 cells and purified from periplasmic extracts using Ni2+-NTA resin, as previously described55. Unique nanobodies were screened by biolayer interferometry (BLI) in an Octet Red 384 system (Sartorius) using Streptavidin SA biosensors (Sartorius) loaded with 40 µg ml−1 biotinylated CerS6 in SEC buffer containing 0.003% (w/v) LMNG and 0.0003% (w/v) CHS. Nanobodies with slow off rates were identified and nanobodies Nb22 and Nb02 were prioritized for structural studies.
Thermal stability measurements
Thermal unfolding experiments were carried out by measuring intrinsic tryptophan fluorescence on a Prometheus NT.48 instrument (NanoTemper Technologies) using 5 µM CerS6 solubilized in 0.003% LMNG and 0.0003% CHS. The protein was incubated for 1 h at 4 °C in the presence or absence of 20 or 100 µM FTY720 (Sigma-Aldrich) or FB1 (Sigma-Aldrich). Proteins were heated from 20 °C to 95 °C, at a rate of 1 °C min−1, and unfolding was monitored by the ratio of fluorescence emission at 350 nm and 330 nm. The melting temperature was determined from the inflection point of the transition using the PR.ThermControl software (NanoTemper Technologies).
Cryo-EM sample preparation and data acquisition
The purified CerS6–Nb22 and CerS6–Nb02 complexes (5 mg ml−1) were applied to freshly glow-discharged Quantifoil 200-mesh Au R1.2/1.3 grids. Plunge-freezing in liquid ethane was carried out using a Vitrobot Mark IV (Thermo Fisher Scientific) set to 4 °C and 100 % humidity. For the CerS6–Nb22–FB1 complex, the SEC-purified CerS6–Nb22 complex was incubated with 120 µM FB1 (Sigma-Aldrich) for 10 min on ice before protein concentration and subsequently for an additional 2 h on ice before grid preparation. EM grids were screened on a Glacios (Oxford Particle Imaging Center (OPIC)) and high-resolution data were collected on a Titan Krios G3 microscope (Leicester Institute of Structural and Chemical Biology (LISCB)) operating at 300 kV and equipped with a Bioquantum energy filter (Gatan) (operated at 20-eV slit width) and a K3 direct electron detector (Gatan) at ×130,000 nominal magnification, in super-resolution mode (2× binning; physical pixel size: 0.656 Å per pixel), using a defocus range between −0.8 µm and −2.4 µm. The total exposure dose was 56.3 e− per Å2, fractionated over 50 frames. In total, 14,309, 18,386 and 14,656 videos were collected for the CerS6–Nb22, CerS6–Nb22–FB1 and CerS6–Nb02 datasets, respectively.
Cryo-EM data processing and model building
Movies were motion-corrected using MotionCor2 (ref. 56) and contrast transfer function (CTF) estimation was carried out in cryoSPARC57 (version 3.3.1). Micrographs with bad CTF fitting (>5 Å) were excluded, yielding 14,196, 16,647 and 14,596 micrographs for further analysis for the CerS6–Nb22, CerS6–Nb22–FB1 and CerS6–Nb02 datasets, respectively.
For the CerS6–Nb22 covalent acyl–enzyme intermediate dataset, particles were initially blob-picked and extracted from a subset of micrographs; then, representative well-resolved two-dimensional (2D) classes were used for template-based picking of the entire dataset. A total of 3,400,118 template-picked particles were extracted in a 360-pixel box Fourier-cropped to 120 pixels, corresponding to a pixel size of 1.968 Å per pixel, and classified in two rounds of reference-free 2D classification yielding 1,261,928 good particles. Four ab initio models were generated and used as reference models in heterogeneous refinement. Particles belonging to the well-resolved 2:2 CerS6–Nb22 dimer complex class (53% of input particles) were selected and subjected to a further round of heterogeneous refinement. The best-resolved particles (497,504) were then subjected to nonuniform refinement58 with C2 symmetry imposed, yielding a 3.21-Å reconstruction (Fourier shell correlation (FSC) = 0.143). These aligned particles were imported into RELION using the csparc2star.py script from the University of California, San Francisco pyem package59 and re-extracted in a 360-pixel box Fourier-cropped to 270 pixels, corresponding to a pixel size of 0.875 Å per pixel. In RELION, CTF parameters were refined, followed by Bayesian particle polishing60. The polished particles were subjected to three-dimensional (3D) classification without image alignment (k = 10, T = 12). Certain classes displayed worse side-chain density and local deviations from C2 symmetry. Therefore, to improve map quality, the 93,680 particles belonging to the two highest-resolution C2-symmetric classes were reimported into cryoSPARC and subjected to a final nonuniform refinement with C2 symmetry, yielding a 3.22-Å reconstruction (FSC = 0.143). Importantly, even though CTF refinement, Bayesian polishing and 3D classification in RELION did not improve the nominal resolution, the final reconstruction revealed better-defined features, including improved side-chain density in the CerS6 active site (Extended Data Fig. 2f,g).
The CerS6–Nb22 N-acyl FB1 dataset was processed using the same workflow as the covalent acyl–enzyme intermediate dataset, with some changes. Briefly, 3,998,935 template-picked particles were classified in two rounds of reference-free 2D classification, yielding 1,353,329 good particles. Four ab initio models were generated and used as reference models in heterogeneous refinement. A total of 641,462 particles belonging to the well-resolved 2:2 CerS6–Nb22 dimer complex class (48% of input particles) were subjected to nonuniform refinement with C2 symmetry imposed, yielding a 3.17-Å reconstruction. After CTF refinement, Bayesian polishing and 3D classification without image alignment in RELION, the 154,239 particles belonging to the three highest-resolution C2-symmetric classes were selected. These particles were reimported into cryoSPARC and subjected to a final nonuniform refinement with C2 symmetry, yielding a 2.95-Å reconstruction (FSC = 0.143).
The CerS6–Nb02 covalent acyl–enzyme intermediate dataset was processed similarly to the other datasets, with some modifications. Briefly, 4,497,470 template-picked particles underwent two rounds of reference-free 2D classification, leading to the identification of 1,025,813 good particles. Following heterogenous refinement, 507,128 particles belonging to the well-resolved 2:2 CerS6–Nb02 dimer complex class (50% of input particles) were subjected to nonuniform refinement with C2 symmetry imposed, yielding a 3.22-Å reconstruction. Particles were imported into RELION and re-extracted in a 432-pixel box Fourier-cropped to 324 pixels, corresponding to a pixel size of 0.875 Å per pixel. After CTF refinement, Bayesian polishing and 3D classification without alignment in RELION, the 153,485 particles belonging to the two highest-resolution C2-symmetric classes were selected. These particles were then reimported into cryoSPARC for a final nonuniform refinement with C2 symmetry applied, yielding a 3.02-Å reconstruction (FSC = 0.143).
Atomic models were generated by fitting the AF2 (ref. 61) prediction of CerS6 (AF-Q6ZMG9-F1) and Phyre2 (ref. 62) nanobody homology models into the cryo-EM maps and subsequently manually adjusted in Coot63. In all datasets, residues 72–119, corresponding to the Hox-like domains, were poorly resolved in the sharpened maps. However, their position was evident in the unsharpened and blurred maps (Extended Data Figs. 2e and 3a). To model this region, we used tight restraints to the AF2 prediction for this domain and docked it into the envelope of the blurred maps (Bblur = 200 Å2) and surface-exposed side chains were truncated at Cβ. The atomic models were refined using PHENIX real-space refinement64 with secondary structure and Ramachandran restraints and noncrystallographic symmetry constraints. Restraint dictionaries for FB1 and the covalently attached C16:0 chain were generated using AceDRG65. The final models comprised residues 2–330 (CerS6–Nb22 and CerS6–Nb02 covalent intermediate state) or 2–334 (CerS6–Nb22 N-palmitoyl FB1-bound state) of CerS6, residues 1–124 of Nb22 or residues 1–123 of Nb02, the C16:0 chain covalently attached to His211 of CerS6 or the N-palmitoyl FB1 product, one POPC molecule and the first N-acetylglucosamine (GlcNac) residue of the N-linked glycan visible on Asn18. Weighted Fo − Fc ligand difference maps were calculated using Servalcat66, available in CCPEM67, by omitting the ligands from the atomic models. All descriptions and figures are based on the structures of the CerS6–Nb22 complex unless otherwise stated. Structural similarity to other acyl-CoA-binding proteins was identified using DALI68.
Denaturing intact protein MS
All MS experiments were carried out on purified CerS6 in the absence of nanobodies. The intact masses of purified protein samples were analyzed by denaturing intact protein MS, conducted using an Agilent 1290 Infinity liquid chromatography (LC) system in line with an Agilent 6530 accurate-mass quadrupole time-of-flight MS instrument (Agilent Technologies), as previously described33. Typically, 5–8 μg of purified protein (at 1.5–2.0 mg ml−1), diluted to 20 μl in 30% methanol in 0.1% formic acid, was used per injection. Data were acquired between 100 and 3,200 m/z and analyzed using MassHunter Qualitative Analysis version B.07.00 (Agilent Technologies) software. Peaks between 650 and 3,200 m/z in the sum of the mass spectra obtained during protein elution were deconvoluted using the maximum entropy charge deconvolution algorithm.
The identity of the deconvoluted mass peaks was assigned initially on the basis of the expected mass of the purified proteins and subsequently the observed mass shifts between peaks in the deconvoluted mass spectra. The lower-molecular-weight CerS6 peak corresponded to loss of the initiator methionine (theoretical mass shift of −131.20 Da), acetylation of the new N terminus (theoretical mass shift of +42.04 Da) and addition of an N-linked GlcNAc (theoretical mass shift of +203.19 Da). However, the major glycosylation species observed contained the complete core N-linked glycan (theoretical mass shift of +1217.05 Da), as expected for protein expressed in Expi293F GnTI− cells. In addition, we observed mass shifts of +237.47 and +238.51 Da relative to these two glycosylated species, respectively, which we interpreted as palmitoylation at a single site (theoretical mass shift of +238.41 Da), corresponding to the covalent acyl–imidazole intermediate observed in the cryo-EM structure. One additional modification was observed (approximately +264 Da) but its identity could not be assigned. This unknown modification occurred at a distinct site to that of the palmitoylation, as both modifications could occur simultaneously in the same protein molecule (Extended Data Fig. 1f,g), thus excluding the possibility of this unknown modification occurring in the active site.
To monitor the reaction of the covalent intermediate species with the second substrates, before denaturing intact protein MS analysis, the protein (2 mg ml−1) was incubated with 200 µM sphinganine (Avanti Polar Lipids), 200 µM FB1 (Sigma-Aldrich) or 600 µM FTY720 (Sigma-Aldrich) for 90 min at 37 °C. All intact mass experiments were conducted at least twice using distinct biological samples. Replicate deconvoluted mass spectra are shown in Extended Data Fig. 6.
Product detection by LC–HRMS
Product detection by LC–HRMS was conducted on a nanoElute LC system in line with a timsTOF Pro 2 MS instrument (Bruker). The reactions were set up as described for the denaturing intact protein MS experiments, diluted 1:40 (v/v) in 30% methanol in 0.1% formic acid, and 1 µl of each sample was injected onto an IonOpticks C18 nano ultrahigh-performance LC column (1.6-μm particle size; 0.075 mm × 250 mm).
The flow rate was set to 0.5 µl min−1 and the solvent system consisted of 0.1% Optima LC–MS-grade formic acid (Fisher Chemical) in high-performance LC electrochemical-grade water (Fisher Chemical) (solvent A) and 0.1% formic acid in Optima LC–MS-grade methanol (Fisher Chemical) (solvent B). The initial condition was 60% solvent B and a linear gradient from 60% to 95% solvent B was applied over 17.8 min to elute the samples. This was then followed by a final 2.2-min isocratic elution with 95% solvent B before the system was re-equilibrated between samples for 5 min with 60% solvent B.
The MS instrument was operated in positive ion mode with a capillary voltage of 1,600 V and the drying gas was supplied at 180 °C with a flow rate of 3 L min−1. Additional parameters were as follows: deflection delta, 70 eV; funnel 1 radiofrequency (RF), 350 Vpp; funnel 2 RF, 600 Vpp; multipole RF, 500 Vpp. Data were acquired between 150 and 2,200 m/z and analyzed using the Bruker Compass DataAnalysis 5.3.556 software. The extracted ion chromatograms (EICs) are presented in Figs. 3c and 4d and correspond to the theoretical [M + H]+ ions (tolerance: ±0.005 m/z) of the reaction products N-palmitoyl dihydrosphingosine (C16:0 ceramide) (theoretical m/z: 540.5350), N-palmitoyl FB1 (theoretical m/z: 960.6254) and N-palmitoyl FTY720 (theoretical m/z: 546.4881).
LC–electrospray ionization (ESI)-MS/MS characterization of N-palmitoyl FTY720
To structurally characterize the proposed N-palmitoyl FTY720 reaction product, purified protein was incubated with 600 μM FTY720 as before and the reaction mixture was initially analyzed by LC–ESI-MS on an Agilent 1290 Infinity LC system in line with an Agilent 6530 accurate-mass quadrupole time-of-flight MS instrument (Agilent Technologies) as described above. This enabled the identification of the putative [M + H]+ ion of the N-palmitoyl FTY720 product (observed m/z: 546.4849; theoretical m/z: 546.4881). Its product ion spectrum was then obtained by LC–ESI-MS/MS. For this purpose, the MS instrument was operated in positive ESI mode (4 GHz). MS parameters were as follows: capillary voltage, 4,000 V; fragmentor voltage, 175 V. Data were acquired between 100 and 1,700 m/z. The targeted parent ion (546.4881 m/z; retention time, 8.713 min) was fragmented using a collision energy of 14 V.
Dihydroceramide synthase activity measurements
For activity assays, WT or mutant CerS6 proteins were overexpressed in Expi293F cells and membranes were prepared as follows. The cell pellet from 0.5 L of culture was thawed in PBS and lysed using an Emulsiflex C5 homogenizer (Avestin); then, cell debris was removed by centrifugation. Membranes were subsequently isolated by ultracentrifugation at 160,000g for 90 min, resuspended in assay buffer (20 mM HEPES pH 7.5, 25 mM KCl, 1 mM MgSO4 and 0.1% (v/v) glycerol), flash-frozen in liquid N2 and stored at −80 °C.
On the day of the assay, membranes were thawed and diluted to 0.25 mg ml−1. Next, 20 µl of diluted membranes were dispensed per well on flat-bottom polystyrene 384-well Lumitrac plates (Greiner Bio One). Then, 50 µM sphinganine (2.5 µl of 500 µM sphinganine in assay buffer containing 10% ethanol) and 50 µM palmitoyl-CoA (Sigma-Aldrich) (2.5 µl of 500 µM palmitoyl-CoA in assay buffer) were added to each well for a final reaction volume of 25 µl. For untreated controls, 5 µl of assay buffer were added instead of the substrates. The plates were then incubated for 1 h at room temperature and the reaction was terminated by the addition of 40 µl of butanol spiked with N-palmitoyl(d9) dihydrosphingosine (Avanti Polar Lipids) to yield a final concentration of 5 µM N-palmitoyl(d9) dihydrosphingosine as an internal standard. The plates were then shaken at 1,800 rpm for 2 min and centrifuged at 1,000g for 30 s. Finally, 40 µl of the organic (upper) phase was transferred into 384-well polypropylene deep-well plates (Greiner Bio One) and diluted with 40 µl of butanol.
The analytical sample handling was performed by a rapid-injecting RapidFire autosampler system (Agilent) coupled to a triple-quadrupole MS instrument (Triple Quad 6500, AB Sciex) as previously described69, with some modifications. Briefly, the liquid sample was aspirated by a vacuum pump into a 10-µl sample loop for 6,000 ms and subsequently flushed for 3,000 ms onto a C4 cartridge (Agilent) with the aqueous mobile phase (99.5% water, 0.49% acetic acid and 0.01% trifluoroacetic acid; flow rate, 1.5 ml min−1). The multiple reaction monitoring transition for C16:0 dihydroceramide is 540.5 → 266.3 m/z (declustering potential, 130 V; collision energy, 38 V) and that for the internal standard C16:0 (d9)dihydroceramide is 549.5 → 266.3 m/z (declustering potential, 130 V; collision energy, 38 V). The MS instrument was operated in positive ion mode (curtain gas, 35 arbitrary units (AU); collision gas, medium; ion spray voltage, 4,200 V; temperature, 550 °C; ion source gas 1, 65 AU; ion source gas 2, 80 AU). MS data processing was performed in Gubbs Mass Spec Utilities and peak area ratios between C16:0 dihydroceramide and the internal standard were calculated.
Expression of each mutant in the membrane samples was evaluated through western blotting using 10 ng ml−1 Strep-Tactin conjugated with horseradish peroxidase (IBA Lifesciences), applying SYPRO ruby (Thermo Fisher Scientific) staining as a loading control (Fig. 3e). Band intensity was quantified using the GeneTools software (Syngene) and activity was normalized to protein expression.
MD simulations
Atomistic MD simulations were performed using the Desmond software package (D. E. Shaw Research) within the Maestro software suite (Schrödinger). The simulation setup and analysis were carried out as follows. For each of the two states, a CerS6 monomer was embedded in a lipid bilayer consisting of 99 POPC molecules after undergoing the protein preparation step as implemented in Maestro. The dimensions of the simulation cell were approximately 90 × 70 × 70 Å, with a minimum distance of 10 Å between the protein and the cell boundaries. The systems contained 8,855 (covalent intermediate bound state) or 9,368 (N-acyl FB1-bound state) water molecules and eight or six corresponding chloride counterions to maintain overall charge neutrality. The simulations were performed using the OPLS4 force field70 for the protein and lipid molecules and the simple point-charge water model for the water molecules. The system was set up using the Maestro software suite (Schrödinger). The simulations were run in Desmond using an NPT ensemble at a temperature of 300 K. A timestep of 2 fs was used for the integration of equations of motion. Four independent simulations were performed, each with a duration of 100 ns, resulting in a total simulation time of 400 ns. Snapshots of the system were recorded every 100 ps for further analysis.
Figures
Figures depicting molecular models were generated using PyMOL (Schrödinger) and ChimeraX71.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The cryo-EM maps were deposited to the EM Data Bank under accession codes EMD-18770 (CerS6–Nb22 covalent acyl–enzyme intermediate state), EMD-18771 (CerS6–Nb22 N-acyl FB1-bound state) and EMD-19869 (CerS6–Nb02 covalent acyl–enzyme intermediate state). The atomic models were deposited to the PDB under accession codes 8QZ6 (CerS6–Nb22 covalent acyl–enzyme intermediate state), 8QZ7 (CerS6–Nb22 N-acyl FB1-bound state) and 9EOT (CerS6–Nb02 covalent acyl–enzyme intermediate state). All raw MS data are available for download from Zenodo (https://doi.org/10.5281/zenodo.10604228)72. Source data are provided with this paper.
References
Summers, S. A., Chaurasia, B. & Holland, W. L. Metabolic messengers: ceramides. Nat. Metab. 1, 1051–1058 (2019).
Turpin, S. M. et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20, 678–686 (2014).
Apostolopoulou, M. et al. Specific hepatic sphingolipids relate to insulin resistance, oxidative stress, and inflammation in nonalcoholic steatohepatitis. Diabetes Care 41, 1235–1243 (2018).
Hajduch, E., Lachkar, F., Ferre, P. & Foufelle, F. Roles of ceramides in non-alcoholic fatty liver disease. J. Clin. Med. 10, 792 (2021).
Hammerschmidt, P. & Brüning, J. C. Contribution of specific ceramides to obesity-associated metabolic diseases. Cell. Mol. Life Sci. 79, 395 (2022).
Neeland, I. J. et al. Relation of plasma ceramides to visceral adiposity, insulin resistance and the development of type 2 diabetes mellitus: the Dallas Heart Study. Diabetologia 61, 2570–2579 (2018).
Błachnio-Zabielska, A. U. et al. Increased bioactive lipids content in human subcutaneous and epicardial fat tissue correlates with insulin resistance. Lipids 47, 1131–1141 (2012).
Luukkonen, P. K. et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J. Hepatol. 64, 1167–1175 (2016).
Levy, M. & Futerman, A. H. Mammalian ceramide synthases. IUBMB Life 62, 347–356 (2010).
Kitatani, K., Idkowiak-Baldys, J. & Hannun, Y. A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 20, 1010–1018 (2008).
Raichur, S. et al. The role of C16:0 ceramide in the development of obesity and type 2 diabetes: CerS6 inhibition as a novel therapeutic approach. Mol. Metab. 21, 36–50 (2019).
Raichur, S. Ceramide synthases are attractive drug targets for treating metabolic diseases. Front. Endocrinol. 11, 483 (2020).
Hammerschmidt, P. et al. CerS6-derived sphingolipids interact with Mff and promote mitochondrial fragmentation in obesity. Cell 177, 1536–1552.e23 (2019).
Mizutani, Y., Kihara, A. & Igarashi, Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem. J. 390, 263–271 (2005).
Winter, E. & Ponting, C. P. TRAM, Lag1 and CLN8: members of a novel family of lipid-sensing domains? Trends Biochem. Sci. 27, 381–383 (2002).
Spassieva, S. et al. Necessary role for the Lag1p motif in (dihydro)ceramide synthase activity. J. Biol. Chem. 281, 33931–33938 (2006).
Kageyama-Yahara, N. & Riezman, H. Transmembrane topology of ceramide synthase in yeast. Biochem. J. 398, 585–593 (2006).
Venkataraman, K. & Futerman, A. H. Do longevity assurance genes containing Hox domains regulate cell development via ceramide synthesis? FEBS Lett. 528, 3–4 (2002).
Mesika, A., Ben-Dor, S., Laviad, E. L. & Futerman, A. H. A new functional motif in Hox domain-containing ceramide synthases: identification of a novel region flanking the Hox and TLC domains essential for activity. J. Biol. Chem. 282, 27366–27373 (2007).
Sassa, T., Hirayama, T. & Kihara, A. Enzyme activities of the ceramide synthases CERS2–6 are regulated by phosphorylation in the C-terminal region. J. Biol. Chem. 291, 7477–7487 (2016).
Tidhar, R. et al. Eleven residues determine the acyl chain specificity of ceramide synthases. J. Biol. Chem. 293, 9912–9921 (2018).
Laviad, E. L., Kelly, S., Merrill, A. H.Jr & Futerman, A. H. Modulation of ceramide synthase activity via dimerization. J. Biol. Chem. 287, 21025–21033 (2012).
Jensen, S. A. et al. Bcl2L13 is a ceramide synthase inhibitor in glioblastoma. Proc. Natl Acad. Sci. USA 111, 5682–5687 (2014).
Kim, J. L. et al. Fatty acid transport protein 2 interacts with ceramide synthase 2 to promote ceramide synthesis. J. Biol. Chem. 298, 101735 (2022).
Cingolani, F., Futerman, A. H. & Casas, J. Ceramide synthases in biomedical research. Chem. Phys. Lipids 197, 25–32 (2016).
Marasas, W. F. et al. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: a potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J. Nutr. 134, 711–716 (2004).
Riley, R. T. & Merrill, A. H.Jr Ceramide synthase inhibition by fumonisins: a perfect storm of perturbed sphingolipid metabolism, signaling, and disease. J. Lipid Res. 60, 1183–1189 (2019).
Riley, R. T. et al. Safety Evaluation of Certain Food Additives and Contaminants (World Health Organization, 2018).
Merrill, A. H. Jr, van Echten, G., Wang, E. & Sandhoff, K. Fumonisin B1 inhibits sphingosine (sphinganine) N-acyltransferase and de novo sphingolipid biosynthesis in cultured neurons in situ. J. Biol. Chem. 268, 27299–27306 (1993).
Berdyshev, E. V. et al. FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J. Biol. Chem. 284, 5467–5477 (2009).
Lahiri, S. et al. Ceramide synthesis is modulated by the sphingosine analog FTY720 via a mixture of uncompetitive and noncompetitive inhibition in an acyl-CoA chain length-dependent manner. J. Biol. Chem. 284, 16090–16098 (2009).
Turner, N. et al. A selective inhibitor of ceramide synthase 1 reveals a novel role in fat metabolism. Nat. Commun. 9, 3165 (2018).
Nie, L. et al. The structural basis of fatty acid elongation by the ELOVL elongases. Nat. Struct. Mol. Biol. 28, 512–520 (2021).
Niu, Y. et al. Analysis of the mechanosensor channel functionality of TACAN. eLife 10, e71188 (2021).
Ke, M. et al. Cryo-EM structures of human TMEM120A and TMEM120B. Cell Discov. 7, 77 (2021).
Ikeda, M. et al. Characterization of four mammalian 3-hydroxyacyl-CoA dehydratases involved in very long-chain fatty acid synthesis. FEBS Lett. 582, 2435–2440 (2008).
Cleland, W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim. Biophys. Acta 67, 104–137 (1963).
Zollinger, M. et al. Absorption and disposition of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in healthy volunteers: a case of xenobiotic biotransformation following endogenous metabolic pathways. Drug Metab. Dispos. 39, 199–207 (2011).
Wedekind, J. E., Frey, P. A. & Rayment, I. The structure of nucleotidylated histidine-166 of galactose-1-phosphate uridylyltransferase provides insight into phosphoryl group transfer. Biochemistry 35, 11560–11569 (1996).
Zhou, X. et al. Kinetic mechanism of human histidine triad nucleotide binding protein 1. Biochemistry 52, 3588–3600 (2013).
Trent, M. S., Worsham, L. M. S. & Ernst-Fonberg, M. L. HlyC, the internal protein acyltransferase that activates hemolysin toxin: roles of various conserved residues in enzymatic activity as probed by site-directed mutagenesis. Biochemistry 38, 9541–9548 (1999).
Dodds, A. W., Ren, X. D., Willis, A. C. & Law, S. K. The reaction mechanism of the internal thioester in the human complement component C4. Nature 379, 177–179 (1996).
Gadjeva, M. et al. The covalent binding reaction of complement component C3. J. Immunol. 161, 985–990 (1998).
Selvy, P. E., Lavieri, R. R., Lindsley, C. W. & Brown, H. A. Phospholipase D: enzymology, functionality, and chemical modulation. Chem. Rev. 111, 6064–6119 (2011).
Vanni, N. et al. Impairment of ceramide synthesis causes a novel progressive myoclonus epilepsy. Ann. Neurol. 76, 206–212 (2014).
Harrer, H., Laviad, E. L., Humpf, H. U. & Futerman, A. H. Identification of N-acyl-fumonisin B1 as new cytotoxic metabolites of fumonisin mycotoxins. Mol. Nutr. Food Res. 57, 516–522 (2013).
Harrer, H., Humpf, H. U. & Voss, K. A. In vivo formation of N-acyl-fumonisin B1. Mycotoxin Res. 31, 33–40 (2015).
Zelnik, I. D. et al. Computational design and molecular dynamics simulations suggest the mode of substrate binding in ceramide synthases. Nat. Commun. 14, 2330 (2023).
Youssefian, L. et al. Autosomal recessive congenital ichthyosis: CERS3 mutations identified by a next generation sequencing panel targeting ichthyosis genes. Eur. J. Hum. Genet. 25, 1282–1285 (2017).
Eckl, K. M. et al. Impaired epidermal ceramide synthesis causes autosomal recessive congenital ichthyosis and reveals the importance of ceramide acyl chain length. J. Invest. Dermatol. 133, 2202–2211 (2013).
Radner, F. P. et al. Mutations in CERS3 cause autosomal recessive congenital ichthyosis in humans. PLoS Genet. 9, e1003536 (2013).
Merrill, A. H.Jr, Wang, E., Gilchrist, D. G. & Riley, R. T. Fumonisins and other inhibitors of de novo sphingolipid biosynthesis. Adv. Lipid Res. 26, 215–234 (1993).
Geertsma, E. R., Nik Mahmood, N. A., Schuurman-Wolters, G. K. & Poolman, B. Membrane reconstitution of ABC transporters and assays of translocator function. Nat. Protoc. 3, 256–266 (2008).
Hutter, C. A. J. et al. The extracellular gate shapes the energy profile of an ABC exporter. Nat. Commun. 10, 2260 (2019).
Pardon, E. et al. A general protocol for the generation of nanobodies for structural biology. Nat. Protoc. 9, 674–693 (2014).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Asarnow, D., Palovcak, E. & Cheng, Y. UCSF pyem v0.5. Zenodo https://doi.org/10.5281/zenodo.3576630 (2019).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Long, F. et al. AceDRG: a stereochemical description generator for ligands. Acta Crystallogr. D 73, 112–122 (2017).
Yamashita, K., Palmer, C. M., Burnley, T. & Murshudov, G. N. Cryo-EM single-particle structure refinement and map calculation using Servalcat. Acta Crystallogr. D 77, 1282–1291 (2021).
Burnley, T., Palmer, C. M. & Winn, M. Recent developments in the CCP-EM software suite. Acta Crystallogr. D 73, 469–477 (2017).
Holm, L., Laiho, A., Törönen, P. & Salgado, M. DALI shines a light on remote homologs: one hundred discoveries. Protein Sci. 32, e4519 (2023).
Tautermann, C. S. et al. Allosteric activation of striatal-enriched protein tyrosine phosphatase (STEP, PTPN5) by a fragment-like molecule. J. Med. Chem. 62, 306–316 (2019).
Lu, C. et al. OPLS4: improving force field accuracy on challenging regimes of chemical space. J. Chem. Theory Comput. 17, 4291–4300 (2021).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Pascoa, T. C. et al. Structural basis of the mechanism and inhibition of a human ceramide synthase. Zenodo https://doi.org/10.5281/zenodo.10604228 (2024).
Acknowledgements
T.C.P. was supported by a Wellcome PhD studentship (102164/B/15/Z). Initial work on this project was funded by the Structural Genomics Consortium, a registered charity (number 1097737) that received funds from AbbVie, Bayer, Boehringer Ingelheim, the Canada Foundation for Innovation, Genome Canada, Janssen, Merck, Novartis, the Ontario Ministry of Economic Development and Innovation, Pfizer and Takeda, as well as the Wellcome Trust (106169/Z/14/Z). D.B.S., A.C.W.P. and G.C. were supported by the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement no. 875510. The JU receives support from the European Union’s Horizon 2020 research and innovation program, the European Federation of Pharmaceutical Industries and Associations, the Ontario Institute for Cancer Research, the Royal Institution for the Advancement of Learning McGill University, Kungliga Tekniska Hoegskolan and Diamond Light Source, Ltd. EM was provided through OPIC, an Instruct European Research Infrastructure Consortium center (funded by Wellcome Trust Joint Infrastructure Fund award (060208/Z/00/Z) and equipment grant (093305/Z/10/Z)) and the Midlands Regional Cryo-EM Facility at the LISCB, with major funding from the Medical Research Council (MC_PC_17136). We thank C. S. Savva (LISCB) for technical assistance with cryo-EM data collection and W. Greenland (Agilent) for technical advice on MS/MS. We thank W. Yue and C. Siebold for helpful discussions.
Author information
Authors and Affiliations
Contributions
T.C.P. expressed and purified protein samples, prepared proteoliposome samples for alpaca immunization and membranes for mutant activity testing, carried out biophysical characterizations including BLI experiments, designed and generated CerS6 mutants through site-directed mutagenesis, built and refined the atomic models and performed intact protein MS, product identification by HRMS and small-molecule structural characterization by LC–ESI-MS/MS. T.C.P. and G.C. prepared cryo-EM grids. T.C.P. and A.C.W.P. collected and processed the cryo-EM data. C.S.T. carried out the MD simulations and analysis. A.Q. was involved in the early stages of the project, including initial screening of expression and purification conditions. R.C. provided access to MS instruments for intact protein and small-molecule analysis and assisted with small-molecule MS experiments. S.Š. conducted alpaca immunizations, nanobody library generation, panning and identification of unique nanobody sequences. M.T. performed the CerS6 mutant activity assays, supervised by S.T. T.C.P. and D.B.S. wrote the original draft of the paper. All authors participated in the discussion and paper editing. A.P., E.P.C., G.S. and D.B.S. supervised the research.
Corresponding authors
Ethics declarations
Competing interests
C.S.T., M.T., S.T., A.P. and G.S. are employees of Boehringer Ingelheim Pharma, GmbH & Co. KG. The other authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Michael Airola and Daniel Raben for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Properties of purified CerS6.
a, Elution profile of CerS6 (Met1-Asp350). The collected dimer peak is shaded in gray. b, SDS-PAGE analysis of the purified dimeric (D) and monomeric (M) SEC peak fractions. Similar results were obtained for all purifications tested (n = 6). c, Elution profile of CerS6 (Met1-Asp350) in complex with nanobody 22. The putative dimer peak, shaded in gray, was pooled and concentrated for single particle cryo-EM. d, SDS-PAGE analysis of the SEC-purified CerS6 dimer and nanobody 22 complex. Similar results were obtained for all purifications tested (n = 3). e, NanoDSF screening of CerS inhibitors. n = 3 technical replicates. Error bars show the SEM. f-g, Denaturing intact protein MS analysis of purified CerS6. f, Deconvoluted mass spectra of wild-type CerS6 (Met1-Asp350). The expected mass of the untagged, unmodified, truncated enzyme based on the sequence is 42,373.53 Da. The observed lower mass peak (42,488.76 Da) corresponds to loss of the initiator methionine (−131.20 Da), acetylation of the new N-terminus ( + 42.04 Da), and addition of an N-linked GlcNAc ( + 203.19 Da). An additional, higher intensity, deconvoluted mass peak (43,502.71 Da) corresponds to the addition of a core N-linked glycan (theoretical +1217.09 Da) in place of simply the N-linked GlcNAc. Mass shifts corresponding to the mass of a palmitoyl group (theoretical +238.41 Da) are labelled in blue. Additional deconvoluted mass peaks corresponding to the addition of an unknown modification of approximately +264 Da on top of the glycosylation or glycosylation + palmitoylation peaks are labelled with gray dots. g, Deconvoluted mass spectra obtained for the purified CerS6 N18Q mutant which removes the only identified glycosylation site. The expected mass of the untagged, unmodified, truncated CerS6 N18Q enzyme based on the sequence is 42,387.56 Da. The observed lower mass peak (42,294.87 Da) corresponds to loss of the initiator methionine followed by acetylation of the new N-terminus. The +239.80 Da mass shift, corresponding to palmitoylated protein, is labelled in blue.
Extended Data Fig. 2 Cryo-EM data processing: CerS6-Nb22 covalent intermediate dataset.
a, Cryo-EM data processing flowchart. b, Representative micrograph. c, 2D classes obtained prior to 3D classification. d, Final 3D reconstruction, colored by local resolution. e, Overlay of the structural model (tan) and the AlphaFold2 monomer prediction (gray). The cryo-EM maps are shown as blue (unsharpened map) or outline (blurred map; Bblur = 200 Å2) surfaces. A purple arrow indicates the manual adjustment of the position of the Hox-like domain into the experimental blurred map. f-g, Cryo-EM map (f) before and (g) after CTF refinement, Bayesian polishing and 3D classification in Relion. h, Angular distribution of particles used in the final reconstruction. i, Fourier Shell Correlation (FSC) plots, indicating overall map resolution (GSFSC = 0.143) and a model-to-map FSC curve. j, Sharpened cryo-EM map (contoured at 4.6σ) overlaid on the final model.
Extended Data Fig. 3 Cryo-EM map quality in the CerS6-Nb22 datasets.
a, Fit of the Hox-like domain into the unsharpened cryo-EM map (blue surface). b-d, Cryo-EM density in the regions around the modelled ligands. The respective sharpened cryo-EM maps (blue mesh) and Servalcat Fo-Fc difference maps (obtained by omitting the acyl-imidazole and the N-palmitoyl fumonisin B1 species from the models; green mesh) are overlaid on the models. e, Cryo-EM density for the bound lipid molecule, modelled as phosphatidylcholine (purple carbon atoms).
Extended Data Fig. 4 Transmembrane helix topology of CerS6.
a, The 6-TM barrel formed by TM2-7 of CerS6 is composed of two 3-TM units (TM2-4, blue; TM5-7, red), arranged as inverted repeats. b, Comparison of the transmembrane helix topology of the 6-TM barrels of CerS6, ELOVL7, HACD4 and TMEM120A (TACAN). c, Structural alignment of CerS6 and ELOVL7 reveals that their histidine pairs are structurally homologous. The acyl chain linked to His211 in the CerS6 covalent intermediate structure is shown in pink.
Extended Data Fig. 5 Cryo-EM data processing: CerS6-Nb02 covalent intermediate dataset.
a, Cryo-EM data processing flowchart. b, Representative micrograph. c, 2D classes obtained prior to 3D classification. d, Final 3D reconstruction, colored by local resolution. e, Angular distribution of particles used in the final reconstruction. f, Fourier Shell Correlation (FSC) plots, indicating overall map resolution (GSFSC = 0.143) and a model-to-map FSC curve. g, Coulombic potential map in the region around the covalent linkage of the acyl chain to His211. h, Sharpened cryo-EM map (contoured at 4.6σ) overlaid on the final model.
Extended Data Fig. 6 Monitoring the reaction of the covalent acyl-enzyme intermediate by mass spectrometry.
a-b, Purified CerS6 was incubated in the presence and absence of substrates prior to liquid chromatography – electrospray ionization – mass spectrometry (LC-ESI-MS) intact mass analysis. a, Deconvoluted mass spectra are shown for CerS6 incubated in the absence of substrates (protein only), with 200 µM sphinganine, or with 200 µM fumonisin B1. Deconvoluted mass peaks are indicated as follows: unmodified enzyme, orange icon; covalent acyl-enzyme species, orange and pink icon; background species ( + 264 Da) present in all traces, gray circle. Spectra are shown for 3 biological replicates. b, Deconvoluted mass spectra shown for CerS6 incubated in the presence and absence of 600 µM FTY720. Spectra are shown for 2 biological replicates. c-d Structural characterization of N-palmitoyl FTY720 species by LC-ESI-MS/MS. c, Structure of the proposed N-palmitoyl FTY720 reaction product, annotated with the m/z values of the daughter ions observed in panel d. d, MS/MS spectrum obtained from the fragmentation of the [M + H]+ ion of N-palmitoyl FTY720 (m/z 546.4881) using a collision-induced dissociation energy of 14 V. Proposed relationships between the observed m/z peaks are indicated.
Extended Data Fig. 7 Multiple sequence alignment of human (CerS1-6) and S. cerevisiae (Lag1p, Lac1p) ceramide synthases.
Blue circles below the alignment indicate the active site residues.
Extended Data Fig. 8 Cryo-EM data processing: CerS6-Nb22 N-palmitoyl FB1-bound dataset.
a, Cryo-EM data processing flowchart. b, Representative micrograph. c, 2D classes obtained prior to 3D classification. d, Final 3D reconstruction, colored by local resolution. e, Fourier Shell Correlation (FSC) plots, indicating overall map resolution (GSFSC = 0.143) and a model-to-map FSC curve. f, Angular distribution of particles used in the final reconstruction. g, Sharpened cryo-EM map (contoured at 4.6σ) overlaid on the final model.
Extended Data Fig. 9 CerS6 dynamics and N-palmitoyl fumonisin B1 interactions in simulations.
a, Average root-mean-square deviation (RMSD) values of Cα atoms from atomistic simulations of the covalent intermediate and N-palmitoyl fumonisin B1-bound states. Traces where the Hox-like domain residues (70-126) were excluded from the RMSD calculation are shown to highlight the stability of the transmembrane region. b, Root-mean-square fluctuation (RMSF) values by residue (Cα atoms). RMSD and RMSF values shown are averaged over 4 ×100 ns atomistic simulations. c, N-palmitoyl FB1 stabilises the CerS6 structure. RMSF values from the simulations are mapped onto the structures, revealing a reduction in the overall dynamics of the protein upon binding the reaction product N-palmitoyl FB1, particularly in the Hox-like domain, the TM3-4 loop, TM6, and TM7. d, Analysis of the polar interactions between CerS6 and FB1 during the simulations. Values shown correspond to the percentage of snapshots (taken every 100 ps during the simulations) containing CerS6-FB1 polar interactions at each residue. Residues are coloured by the frequency of polar interactions with FB1 (cyan sticks).
Extended Data Fig. 10 Cartoon representation of the proposed mechanism of ceramide synthases and their inhibition by FB1.
a, CerS6 initially reacts with an acyl-CoA substrate, forming a covalent acyl-enzyme intermediate and releasing CoA as the first product. Subsequently, the reaction of the acyl-enzyme intermediate with sphinganine produces ceramide as the final product. Ceramide dissociates from the enzyme, which can then react with another acyl-CoA substrate to re-start the cycle. b, Reaction of the covalent acyl-enzyme intermediate species with FB1 results in the formation of an N-acyl FB1 product. This inhibitory product remains tightly bound in the central cavity, forming polar interactions near the cytoplasmic entrance, and preventing recycling of the enzyme.
Supplementary information
Source data
Source Data Fig. 1
Source data of traces.
Source Data Fig. 3
Source data of expression.
Source Data Fig. 3
Source data of traces.
Source Data Fig. 4
Source data of traces.
Source Data Extended Data Fig. 1
Source data of unprocessed gels.
Source Data Extended Data Fig. 1
Source data of traces.
Source Data Extended Data Fig. 6
Source data of traces.
Source Data Extended Data Fig. 9
Source data of traces.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pascoa, T.C., Pike, A.C.W., Tautermann, C.S. et al. Structural basis of the mechanism and inhibition of a human ceramide synthase. Nat Struct Mol Biol 32, 431–440 (2025). https://doi.org/10.1038/s41594-024-01414-3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41594-024-01414-3