Abstract
Transcription by RNA polymerase II (Pol II) can be repressed by noncoding RNA, including the human RNA Alu. However, the mechanism by which endogenous RNAs repress transcription remains unclear. Here we present cryogenic-electron microscopy structures of Pol II bound to Alu RNA, which reveal that Alu RNA mimics how DNA and RNA bind to Pol II during transcription elongation. Further, we show how distinct domains of the general transcription factor TFIIF control repressive activity. Together, we reveal how a noncoding RNA can regulate mammalian gene expression.
Similar content being viewed by others
Main
RNA polymerase II (Pol II) is the 12-subunit eukaryotic enzyme that generates messenger RNA and is thus a focal point for the regulation of transcription. The activity of Pol II is tightly regulated throughout the transcription cycle by association with various accessory factors, including single proteins, multi-subunit protein complexes and RNAs1. Whereas protein regulators of transcription have been extensively studied, RNA-based mechanisms for regulation remain poorly understood.
Human Alu RNA has been identified as a natural inhibitor of transcription, blocking transcription of specific genes during heat shock2. Alu RNA is transcribed by Pol III as single units from short interspersed elements, abundant genomic repeats. These free Alu RNAs are present at low levels under normal conditions, but levels increase in response to cellular stress3. Elevated levels of Alu RNA are able to repress Pol II transcription and, after heat shock, Alu RNA can be found at the promoters of repressed genes, consistent with the direct binding to Pol II observed in vitro2. Biochemical analysis revealed that Alu RNA binds directly to two molecules Pol II via independent interactions with the two halves of Alu RNA, Alu-left arm (Alu-LA, also known as scAlu) and Alu-right arm (Alu-RA)2 (Fig. 1a). Although both Alu-LA and Alu-RA bind to Pol II with high affinity, only Alu-RA is able to inhibit transcription in the presence of the heterodimeric general transcription factor TFIIF, comprising the proteins RAP74 and RAP30. It has been shown by low-resolution (~25 Å) cryogenic-electron microscopy (cryo-EM) reconstructions4 that both Alu-LA and Alu-RA localize to the Pol II DNA-binding cleft. Because the RNA secondary structure could not be resolved, it remains unknown how Alu RNAs are able to form high-affinity contacts with Pol II, thus allowing them to repress within an endogenous context. Additionally, the mechanism by which the repression of only one of these very similar Alu RNA halves is affected by TFIIF remains to be elucidated.
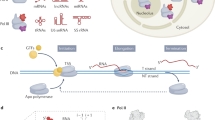
a, Schematic showing the secondary structure and domain organization of Alu RNA. Alu-LA (orange shading), Alu-RA (purple shading), Alu-RA minimal region (gray shading) and Alu-RA loose region (gray outline) are indicated. The 5′ and 3′ regions of Alu-LA and Alu-RA are indicated with orange and purple bars. Guanosine, green; cytidine, cyan; adenosine, blue; uridine, orange. b, Top-front view of Pol II–Alu-LA and -Alu-RA reconstructions (not B-factor sharpened), with 8-Å-filtered Alu-LA (orange) and Alu-RA (purple) difference densities overlaid. Pol II is colored by subunit. c, Side view of 8-Å-filtered Alu-LA and Alu-RA densities compared to the path of nucleic acids in a transcription EC (PDB ID 5OIK)27. d–h, Structure of Pol II–minimal Alu-RA. d, Top-front view of the locally filtered Pol II–minimal Alu-RA alternative conformation density. e, A rotated view of the modeled region of minimal Alu-RA overlaid with the electron microscopy density (DeepEMhancer postprocessed for visualization). f, Top-front view of the Pol II–minimal Alu-RA structure. g, A view of the DNA–RNA hybrid-like region of minimal Alu-RA and the same region of a transcription EC (PDB ID 5OIK)27. h, As in g, but focused on the downstream DNA region.
Results and discussion
Structural analysis of Pol II bound to Alu RNA
To investigate the principles determining Pol II inhibitory activity of Alu RNA, we reconstituted complexes of mammalian Pol II bound to in vitro transcribed and refolded Alu-LA and Alu-RA (Extended Data Fig. 1). Our initial attempts at cryo-EM sample preparation using holey carbon or thin carbon-coated electron microscopy grids revealed that the samples displayed biased particle orientations, hindering high resolution analysis. Mild BS3 or glutaraldehyde crosslinking, which improved particle orientations for the Pol II elongation complex (EC)5, resulted in dissociation of Alu RNA. Preparation of sample using graphene oxide-coated holey carbon grids6 resulted in less biased particle orientations and allowed structure determination of Pol II–Alu-LA and Pol II–Alu-RA to nominal resolutions of 2.4 and 2.5 Å (Table 1). For the Pol II–Alu-LA complex, the RNA could be resolved to approximately 5–8 Å. Similarly, Alu-RA within the Pol II–Alu-RA complex was resolved to approximately 5–8 Å. In both complexes, the Pol II clamp and stalk domains could be well resolved with further classification (Extended Data Figs. 2 and 3).
The resolution of the RNA precluded building of an atomic model, but did reveal helical structure and the binding location of Alu RNA. Both Alu-LA and Alu-RA bind in the Pol II DNA-binding cleft, adopting a conformation similar to that of DNA and RNA within the Pol II EC (Fig. 1b,c). Comparison to the Pol II EC revealed helical Alu RNA density overlapping the positions of the DNA–RNA hybrid, specific to transcribing complexes, and downstream DNA. Additional density partially overlapped the positions of upstream DNA and exiting RNA within the Pol II EC. This can explain how Alu RNA would block productive engagement with promoter DNA during transcription initiation, but could still allow assembly of a preinitiation complex of altered topology through TFIIB- and TBP-promoter DNA contacts7.
Unlike in the EC, the Pol II clamp domain did not close over the nucleic acids in a stable conformation. Fork loop 2 within the DNA-binding cleft, which interacts with the melted nontemplate DNA strand in the EC, was rearranged from the conformation observed in the EC to that observed in Pol II lacking DNA or RNA5, consistent with the absence of a melted nucleic acid strand in the Alu RNA structures (Extended Data Fig. 3g). Altogether, despite Alu RNAs adopting an EC-like conformation, the Pol II enzyme was observed in an inactive state, consistent with an enzyme–nucleic acid complex not competent for elongation.
If Alu RNA resolution was restricted by intrinsic flexibility, we reasoned that a shorter RNA construct could overcome that limitation. To that end, we prepared a minimal version of Alu-RA (Fig. 1a) containing the structured elements previously shown to be sufficient for both Pol II binding and repression2. We solved the structure of the Pol II–minimal Alu-RA complex to a nominal resolution of 3.1 Å, and observed that the quality of the RNA density in the initial map was greatly improved. Three-dimensional (3D) classification revealed two minimal Alu-RA conformations (Extended Data Fig. 4). The first RNA conformation could be resolved to an intermediate resolution of approximately 5–8 Å, and corresponded to an Alu-RA–RNA-like conformation. This allowed the putative assignment of RNA regions to the Alu-RA density (Extended Data Fig. 4i). The second conformation could be resolved to a higher resolution of 3–6 Å, in which individual bases could be distinguished (Fig. 1d,e). A model of Pol II and the well-resolved region of minimal Alu-RA in the alternative conformation was generated using a combination of de novo model building with DeepTracer8 and secondary structure-based manual building9 (Fig. 1f). The alternative conformation even more closely mimicked EC nucleic acids (Fig. 1g,h). Comparison of the Pol II–minimal Alu-RA structure to that of Saccharomyces cerevisiae Pol II bound to Fc aptamer10, a synthetic RNA inhibitor, revealed that although both RNAs bind to a region overlapping that bound by the DNA–RNA hybrid, only minimal Alu-RA forms essentially the same interactions as those found within the EC (Extended Data Fig. 5a,b). Thus, stable binding of mammalian Pol II to structured RNA likely involves contacts similar to those formed within an EC.
Analogous to Alu RNA, bacterial 6S RNA is a natural noncoding RNA that directly binds the bacterial RNA polymerase and inhibits transcription from σ70-dependent housekeeping promoters. Both Alu and 6S RNA inhibit promoter DNA engagement. A previous near-atomic structure of Escherichia coli RNA polymerase in complex with 6S RNA showed that this RNA adopted a conformation mimicking that of open promoter DNA11. This contrasts with our observation that Alu RNA mimics EC nucleic acids (Fig. 1c). Previous results have shown the importance of a loosely structured region within Alu-RA for repressive activity2 (Fig. 1a). We conclude that loosely base paired regions within Alu RNAs are important not as a mimic of open promoter DNA (Extended Data Fig. 5c), but speculate that it allows the RNA the flexibility to adopt a conformation matching that of the EC DNA–RNA hybrid and downstream DNA. This is consistent with previous observations that, unlike bacterial promoter complexes that can form a stable complex with just RNA polymerase and a sigma factor12,13, eukaryotic initiation complexes require a large number of transcription factors to stabilize the interaction with DNA14 and are dynamic15. Promoter complexes lacking accessory factors or a nascent RNA chain are unstable16. Thus, the more stable EC16,17,18 would be an effective mammalian intermediate to mimic.
TFIIF domains differentially affect transcriptional repression by Alu RNA
Although Alu-LA and Alu-RA form similar structures, previous studies have shown that Alu-LA is more labile in the presence of TFIIF19. To investigate these differences in Pol II–Alu RNA complex binding, we first measured binding affinities of Alu-LA and Alu-RA to Pol II using fluorescence anisotropy. We observed similarly high affinities for Alu-LA and Alu-RA when investigated in the presence of 150 mM monovalent salt (Fig. 2a). The similar affinities were consistent with previous reports in which the complex was investigated in low salt conditions2, despite a decrease of the apparent binding strength by approximately tenfold.
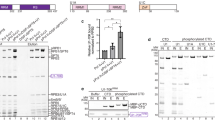
a, Fluorescence anisotropy experiments reveal Alu-LA and Alu-RA binding affinity to Pol II. Results represent analysis of three independently prepared reactions. Data are plotted as mean values ± standard deviation. b, Alu-LA density overlaid on Pol II-TFIIF as found within a transcription initiation open complex (PDB ID 5IYB)25. Pol II subunits are shown as semitransparent ribbons. The mutated regions of the TFIIF constructs used in c are indicated. c, Transcription assays carried out in the presence of the indicated TFIIF variant and either no RNA, Alu-LA or Alu-RA. Results represent analysis of three independently prepared reactions. Data are presented as mean values ± standard deviation, with individual data points shown as black circles. Achieved significance level relative to the wild-type TFIIF condition was calculated using an unpaired, two-tailed t-test: ***P ≤ 0.001; **P ≤ 0.01; *P ≤ 0.05; NS, P > 0.05. Exact P values are as follows: TFIIF (W164A), Alu-LA P = 0.0169, Alu-RA P = 0.2798; TFIIF (RAP30ΔC), Alu-LA P = 0.0002, Alu-RA P = 0.0040; TFIIF (RAP74ΔC), Alu-LA P = 0.0030, Alu-RA P = 0.0089. d, Schematic representation of the effects of TFIIF domains on the repressive activity of Alu-LA and Alu-RA.
Inspection of the Alu structures shows that TFIIF would not sterically clash with the resolved RNA elements (Fig. 2b)20. TFIIF has been shown to inhibit nonspecific DNA binding by Pol II, with the small subunit of TFIIF implicated as particularly important21. It has also been suggested that a negatively charged region of the large subunit of TFIIF may be responsible for repelling nonspecific DNA from the active site cleft22. To investigate how these regions of TFIIF may differentially affect Alu-LA and Alu-RA, we performed transcription inhibition assays in the presence of TFIIF or TFIIF mutants (Fig. 2c and Extended Data Fig. 6a–e). As expected, we observed that in the presence of TFIIF, Alu-RA inhibits transcription more strongly than Alu-LA or an unrelated, unstructured RNA (Extended Data Fig. 6d)23. A TFIIF mutant within the charged region helix of the large subunit, TFIIF (W164A), known to be defective in transcription initiation but maintaining both domains thought to be important in the ability of TFIIF to inhibit nonspecific DNA binding24, behaved similar to wild-type TFIIF. We next investigated the effect of the winged helix domain of the small TFIIF subunit, a domain that binds upstream promoter DNA within the transcription initiation complex25 and is important for transcription initiation activity26. TFIIF (RAP30ΔC) was unable to relieve transcriptional repression by either Alu construct. Last, we tested a TFIIF mutant lacking the C terminus of the largest subunit, TFIIF (RAP74ΔC). The deleted region contained the negatively charged region and a winged helix domain. In the presence of TFIIF (RAP74ΔC), Alu-RA transcriptional repression was partially relieved relative to wild type, suggesting that either the charged low complexity domain or the winged helix, or both together, can enhance Alu-RA repressive activity. In contrast, Alu-LA transcriptional repression activity increased slightly, suggesting that the C-terminal region is deleterious for the repressive activity of this Alu construct.
To test whether direct interactions between TFIIF and Alu RNA could be involved in transcriptional derepression, we investigated the ability of TFIIF to interact with Alu-LA and Alu-RA and found that wild-type TFIIF bound to both Alu halves. Furthermore, both TFIIF (RAP30ΔC) and TFIIF (RAP74ΔC) could interact with Alu-LA and Alu-RA, with TFIIF (RAP74ΔC) displaying reduced binding relative to wild-type TFIIF (Extended Data Fig. 6f,g). Based on previously reported initiation complex structures25, the RAP30 winged helix domain would localize to the upstream DNA region, in close proximity to the putative location of the more conserved 5′ regions of Alu-LA and Alu-RA. Consistent with a localization near conserved RNA regions, we observed similar effects of TFIIF (RAP30ΔC) on Alu-LA and Alu-RA repression. We speculate that the shorter, RNA bulge-containing 3′ region of Alu-LA may sensitize the Pol II–Alu-LA complex to disruption by the RAP74 C terminus, whereas the longer 3′ region of Alu-RA could allow association of the RAP74 C terminus with RNA without Pol II–Alu-RA disruption. Altogether, these results indicate that the winged helix of the small TFIIF subunit RAP30 acts similarly on the repressive and nonrepressive RNAs, whereas the C terminus of the large subunit RAP74 contributes to the distinct activities of Alu-LA and Alu-RA.
In summary, we found that Alu RNA is able to tightly bind Pol II via mimicry of EC nucleic acids, in contrast to the promoter-like mimicry reported in the bacterial system11. Although both halves of Alu RNA adopt the same EC-like structure when bound to Pol II, transcriptional repressive activity of Alu RNA relies on the differential response to distinct TFIIF domains, potentially through TFIIF–RNA binding activity. We propose that EC mimicry and TFIIF domain-dependent activity may be a general mechanism by which structured noncoding RNAs regulate mammalian transcription.
Methods
Cloning and constructs
The full-length Alu sequence from complementary DNA clone TS 103 (refs. 2,28) containing an A10 poly(A) tail was purchased as a gBlock (IDT) and cloned into a modified pSP64 plasmid (Promega) containing an autocleavable 3′ terminal hepatitis delta virus ribozyme29. Alu-LA (nucleotides 1–118) was PCR amplified from the full-length construct and Alu-RA (nucleotides 134–291 containing a 5′ hammerhead ribozyme sequence30) was purchased as a gBlock (IDT). Both constructs were cloned into the modified pSP64 plasmids. The minimal Alu-RA sequence (nucleotides 183–216, 230–238 and 249–281) was ordered as a duplexed DNA containing a 5′ T7 promoter (IDT). A plasmid encoding wild-type His10-Arg7-3C-SUMO-TFIIF was a gift from D. Slade31. TFIIF mutants were created by round-the-horn site-directed mutagenesis. The TFIIF mutant W164A contains a single point mutation, W164A, in the charged region of GTF2F1 (RAP74)32. In TFIIF (RAP74∆C), amino acids 181–517, encompassing the unstructured region and winged helix of GTF2F1 (RAP74), were deleted. In TFIIF (RAP30∆C), amino acids 175–249, encompassing the GTF2F2 winged helix, were deleted, with a short, vector-derived short C-terminal extension remaining (‘LSVFFLFPFSPRSPAFPVKL’). The sequences of all oligonucleotide primers used in this study can be found in the Supplementary Information.
RNA production
Plasmids were used to generate a PCR product containing Alu-LA or Alu-RA sequences and ribozyme as detailed above, which was then used as a template for in vitro transcription using T7 RNAP (prepared in-house33). The templates for an unstructured 151-nucleotide-long RNA (RNA 151) (sequence 5′- GAACGACAUCAUAACAUUUGAACAAGAAUACCAAUACAUAAAUCCAUUAAGAACGACAUCAUAACAUUUGAACAACAAUAAACAUACAUAAAUCCAUCAAGAACGAGAUCAUAACAUUUGAACAAGAAUAUAUAUACAUAAAUCCAUCAUA-3′) and minimal Alu-RA were produced by PCR, and RNA was produced via runoff transcription. The PCR template was removed by RQ1 RNase-free DNase I treatment (Promega). RNAs were gel purified and ethanol precipitated. Hepatitis delta virus ribozyme cleavage resulted in 3′ cyclic phosphates, which were removed by T4 PNK (NEB) treatment (100 mM MES-NaOH pH 5.5 at room temperature, 10 mM MgCl2, 10 mM 2-mercaptoethanol, 300 mM NaCl, 1 U µl−1 RiboLock RNase Inhibitor (Thermo Scientific)) as previously described34. RNA was again gel purified and ethanol precipitated. RNA 151 was subjected to an additional round of DNase I treatment and repurified using a Monarch RNA clean-up kit (NEB).
RNA labeling
RNAs were 3′ end-labeled by ligation of pCp-ATTO-488. RNA (100 pmol in water) was heated for 3 min at 95 °C, cooled at 25 °C for 10 min and labeled in 50 µl of reaction containing a fivefold molar excess of pCp-ATTO-488 (Jena Bioscience), 1 mM ATP, 5% (v/v) PEG 8000, 40 U of RiboLock RNase Inhibitor (Thermo Scientific), 20 U of T4 RNA ligase 1 and 1× T4 RNA ligase reaction buffer (NEB). Labeling reactions were carried out for 16 h at 16 °C, and RNA was subsequently purified using a Monarch RNA clean-up kit (NEB). RNA integrity was verified by denaturing urea–PAGE, with the gel imaged using an Amersham Typhoon RGB 9400 scanner (Cytiva).
Protein preparation
Sus scrofa Pol II was purified as previously described5. The porcine and human enzymes are 99.9% identical, with only four changes within the 4,587-amino-acid sequence. TFIIF was recombinantly expressed in BL21 DE3-RIL E. coli. Cultures were grown to an optical density at 600 nm of 0.9 and induced by addition of 0.5 mM IPTG at 37 °C for 4 h. Cells were collected by centrifugation, resuspended in lysis buffer (50 mM HEPES-NaOH pH 7.5 at 25 °C, 350 mM NaCl, 20 mM imidazole, 10% (v/v) glycerol, 1 mM dithiothreitol (DTT)) plus protease inhibitors (1 mM PMSF, 2 mM benzamidine, 1 µM leupeptin and 2 µM pepstatin) and lysed by sonication. TFIIF was purified by affinity chromatography using a HisTrap HP column (Cytiva) equilibrated with lysis buffer. Protein was eluted with lysis buffer containing 400–500 mM imidazole, dialyzed (50 mM HEPES-NaOH pH 7.5 at 25 °C, 150 mM NaCl, 10% (v/v) glycerol, 1 mM DTT) and cleaved by 3C protease at 4 °C overnight. Cleaved protein was diluted to 100 mM NaCl and purified using a HiTrap SP column equilibrated with ion exchange buffer (50 mM HEPES-NaOH pH 7.5 at 25 °C, 10% (v/v) glycerol, 1 mM DTT) containing 100 mM NaCl. Protein was eluted using a linear gradient of 0.1–1 M NaCl in ion exchange buffer and further purified using a MonoQ (TFIIF wild type, W164A and RAP30ΔC) or a MonoS (TFIIF RAP74ΔC) column equilibrated with ion exchange buffer containing 100 mM NaCl. Protein was eluted as before and subjected to size exclusion chromatography using a Superdex 200 10/300 column (TFIIF wild type, W164A and RAP30ΔC) or a Superdex 75 10/300 column (TFIIF RAP74ΔC) (Cytiva) equilibrated with 50 mM HEPES-NaOH pH 7.5 at 25 °C, 150 mM NaCl, 10% (v/v) glycerol and 1 mM DTT. Purified TFIIF was concentrated using a Amicon Ultra-4 concentrator with a 30 kDa (TFIIF wild type, W164A and RAP30ΔC) or 10 kDa (TFIIF RAP74ΔC) cutoff (Amicon) and stored at −80 °C until use.
Mass photometry
RNA was refolded by heating to 95 °C for 5 min followed by snap cooling on ice in 50 mM KCl binding buffer (20 mM HEPES-KOH pH 7.5 at 25 °C, 50 mM KCl, 10 μM ZnCl2, 4 mM MgCl2, 10 mM DTT). Pol II and refolded RNA were diluted to 1 µM in the same buffer. The complex was prepared by mixing 2.5 µl diluted Pol II with 2.5 µl diluted RNA, followed by incubation at 30 °C for 30 min. Samples were placed on ice and diluted fivefold in 50 mM KCl binding buffer. Measurements were carried out using a Refeyn TwoMP mass photometer (Refeyn Ltd). The instrument was focused with 10 µl of buffer and 1 µl of sample was used for each measurement. Movies were collected for 1 min and processed with DiscoverMP software. Calibration of the instrument was performed before each data collection session.
Cryo-EM sample preparation
Pol II was diluted in 150 mM NaCl polymerase buffer (5 mM HEPES-KOH pH 7.25 at 25 °C, 150 mM NaCl, 10 μM ZnCl2, 10 mM DTT) and mixed with a 1.5-fold excess of refolded RNA, which had been heated to 95 °C for 5 min and snap cooled on ice in 150 mM NaCl polymerase buffer. The complex was incubated at 30 °C for 30 min, diluted to a salt concentration of 50 mM NaCl and placed on ice. The final concentration of Pol II was 400 nM.
Four microliters of sample were applied to graphene oxide-coated Quantifoil R1.2/1.3 holey carbon grids6 that had been glow discharged for 5 s (23 mA current, 7.0 × 10−1 mbar vacuum). Using a Vitrobot Mark IV set to 100% humidity and 4 °C, grids were blotted for 13 s and immediately plunge frozen in liquid ethane.
Cryo-electron microscopy
Grids were screened for particle density, particle orientation and graphene oxide coverage using a Thermo Fisher Glacios transmission electron microscope (200 kV) equipped with a Falcon III direct electron detector. Grids were transferred to a Thermo Fisher Titan Krios G3i transmission electron microscope (300 kV) equipped with a Gatan K3 BioQuantum direct electron detector (energy slit width 10 eV) for data collection. Datasets were recorded using SerialEM v.3.8 and EPU v.2.11 (Pol II–Alu-RA), SerialEM v.3.9 (Pol II–Alu-LA) and EPU v.2.11 (Pol II–minimal Alu-RA). Micrographs were collected at a nominal magnification of ×105,000, corresponding to a counting mode object scale pixel size of 0.835 Å. For Pol II–Alu-LA, the dataset consisted of 16,470 micrographs collected using a defocus range of −0.2 to −2.0 μm with an electron exposure rate of 18.7 e−/Å2/s and an exposure time 2.14 s. The total electron exposure was 40 e−/Å2 distributed over 40 frames. For Pol II–Alu-RA, the dataset consisted of 29,262 micrographs collected using a defocus range of −0.2 to −2.2 μm with an average electron exposure rate of 18.2 e−/Å2/s and an average exposure time of 2.2 s. The total electron exposure was 40 e−/Å2 distributed over 40 frames. For Pol II–minimal Alu-RA, the dataset consisted of 7,588 micrographs collected using a defocus range of −0.1 to −2.2 μm with an electron exposure rate of 22.7 e−/Å2/s and an exposure time 1.81 s. The total electron exposure was 41 e−/Å2 distributed over 40 frames.
Micrographs were first processed with Warp35 and visually screened to remove micrographs with poorly visible Thon rings or multiple layers of graphene oxide. Micrographs were motion corrected and dose weighted using RELION v.3.1 (ref. 36), followed by contrast transfer function estimation using CTFFIND4 (ref. 37). Particle coordinates were selected with Topaz38 using a very permissive threshold to ensure all particles were identified. Datasets were subsequently cleaned using a combination of 2D and 3D classification in RELION v.3.1 and 2D classification in CryoSPARC39 (Extended Data Figs. 2–4). Final reconstructions were obtained using 3D refinement with defocus refinement, beam tilt refinement and particle polishing in RELION v.3.1 and a final binned pixel size of 1.0 Å. Local resolution was calculated in CryoSPARC using an FSC cutoff of 0.5. Densities displaying well-resolved clamp and stalk domains could be obtained after additional 3D classification with appropriate masks (Extended Data Figs. 2 and 3).
Modeling and refinement
A model for the Pol II core lacking the stalk and clamp domains, generated from the Pol II DSIF-EC structure (Protein Data Bank (PDB) ID 5OIK)27, was rigid body fit into the Pol II–Alu-RA map using UCSF Chimera40. The conformation of fork loop 2 (RBP2 487–499) was manually adjusted in Coot41, and the resulting model was adjusted using Isolde42. The model was real space refined in Phenix43 using Isolde-suggested parameters (global minimization with reference restraints and ADP refinement). The minimal Alu-RA RNA was de novo modeled into an initial Pol II–minimal Alu-RA map (before classification of the two RNA conformations) using DeepTracer8. Using the final classified, B-factor-sharpened minimal Alu-RA alternative conformation map, the model was adjusted in Coot41 and the sequence register was assigned, guided by the minimum free energy RNAfold9 secondary structure prediction generated using energy parameters rescaled to 4 °C, as energy parameters at 37 °C yielded the canonical Alu-RA conformation. Base G52 was not built, despite potential density for a base stacked on residue RBP1 Y859, due to density quality. Clamp and adjusted core Pol II models were rigid body fitted into the Pol II–minimal Alu-RA alternative conformation map, regions lacking any density in the unsharpened electron microscopy map were deleted and the combined Pol II-RNA model was further adjusted in Isolde. The model was real space refined in Phenix43 using Isolde-suggested parameters (global minimization with reference restraints and ADP refinement). Densities and models were visualized using UCSF ChimeraX44. Difference densities for Pol II–Alu-LA and Pol II–Alu-RA reconstructions were visualized after subtraction of individually fitted Pol II core, clamp and stalk domains. For visualization purposes, the Pol II–minimal Alu-RA alternative conformation map was postprocessed with DeepEMhancer45.
Fluorescence anisotropy binding experiments
ATTO-488-labeled RNA was folded by heating to 65 °C for 5 min followed by controlled cooling to 4 °C (1 °C per 30 s) in 5 mM HEPES-NaOH pH 7.25 at 25 °C, 150 mM NaCl and 1 mM MgCl2. Pol II–Alu RNA complexes were prepared using 4 nM RNA and increasing concentrations of Pol II (0–500 nM). Reactions were prepared in a final volume of 20 µl (5 mM HEPES-NaOH pH 7.25 at 25 °C, 150 mM NaCl, 10 μM ZnCl2, 1 mM MgCl2, 1 mM DTT) and incubated at 30 °C for 30 min. All experiments were performed in triplicate. Fluorescence anisotropy was measured in 384-well plates and data were collected using a Plate Reader Synergy H1-MF (Bio-TEK). Data were analyzed in GraphPad Prism v.9 using a single site quadratic binding equation accounting for ligand depletion46. Values represent the mean and error bars represent the standard deviation.
Transcription and inhibition assays
Pol II was diluted in transcription buffer (20 mM HEPES-NaOH pH 7.5 at 25 °C, 50 mM NaCl, 5 µM ZnCl2, 4 mM MgCl2, 4 (v/v) % glycerol, 1 mM DTT) and mixed with 5 pmol of a tailed DNA template (template strand sequence 5′-ACAAATTACTGGGAAGTCGACTATGCAATACAGGCATCATTTGATCAAGCTCAAGTACTTAATCATAACCATA-3′, nontemplate strand sequence 5′-TAAGTACTTGAGCTTGATCAAATGATGCCTGTATTGCATAGTCGACTTCCCAGTAATTTGT-3′) and bovine serum albumin (BSA, 40 µg ml−1). Reactions were incubated for 10 min at 30 °C. The final concentration of Pol II was 0.12 µM. An NTP mix containing radioactive CTP (625 µM ATP, GTP, UTP; 17 µM CTP and 0.3 µM [α-32P] CTP) was added to the reaction and incubated for 10 min at 37 °C. Transcription was stopped by adding an equal volume of stop buffer (8 M urea, 20 mM EDTA), incubated for 5 min at 95 °C and snap cooled on ice. The reaction was incubated with Proteinase K (0.2 mg ml−1) for 20 min at 37 °C, then heat inactivated for 5 min at 95 °C. RNA was resolved on a denaturing gel (20% acrylamide, 8 M Urea).
For inhibition assays, folded Alu RNA or RNA 151 was preincubated with Pol II for 30 min at 30 °C (protein:RNA molar ratio of 1:4). DNA template was added and transcription was carried out as described above. For reactions containing TFIIF, Pol II was preincubated with TFIIF or TFIIF mutants (Pol II:TFIIF molar ratio of 1:4) for 1 h at 30 °C, then Alu RNA was added to the reaction. Assays were carried out as described above. For detection of radioactivity, gels were exposed to a storage phosphor screen overnight at 4 °C and imaged with an Amersham Typhoon RGB 9400 scanner (Cytiva). Data were analyzed using ImageQuant TL v.10.2 analysis software (Cytiva).
Electrophoretic mobility shift assays
RNA was folded as in the fluorescence anisotropy binding experiments. RNA (5 nM) was mixed with increasing concentrations of wild-type or mutant TFIIF (0, 15, 30 and 60 nM) in a final buffer containing 20 mM HEPES-NaOH pH 7.25 at 25 °C, 150 mM NaCl, 1 mM MgCl2, 5% (v/v) glycerol, 2 U of RiboLock RNase Inhibitor (Thermo Scientific), 40 µg ml−1 BSA and 1 mM DTT. Reactions were incubated for 30 min at 30 °C, and loaded directly to a NativePAGE, 3 to 12%, Bis-Tris gel (Invitrogen). Gels were run in 1× NativePAGE running buffer (Invitrogen) at 4 °C (1 h, constant 150 V). Experiments were performed in triplicate. Labeled RNA was visualized using an Amersham Typhoon RGB 9400 scanner. Data were analyzed using ImageJ (v.154.g). Free RNA band intensities were quantified and normalized to the no protein control of the respective experiment to obtain a quantification of unbound RNA, which was subsequently plotted as fraction of bound RNA (1-unbound RNA).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Cryo-EM maps for Pol II–Alu-LA, Pol II–Alu-RA, Pol II–minimal-Alu-RA canonical conformation and Pol II–minimal-Alu-RA alternative conformation were deposited to the Electron Microscopy Data Bank under the accession codes EMD-18367, EMD-18375, EMD-18371 and EMD-18376. Model coordinates for the Pol II–Alu-RA polymerase core and Pol II–minimal Alu-RA complex were deposited to the PDB under the accession codes 8QEP and 8QEQ. The analysis presented here used previously published and publicly accessible model coordinates, which are available at the PDB under the accession codes 5OIK and 5IYB. Detailed plasmid maps are available on request. Source data are provided with this paper.
References
Schier, A. C. & Taatjes, D. J. Structure and mechanism of the RNA polymerase II transcription machinery. Genes Dev. 34, 465–488 (2020).
Mariner, P. D. et al. Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mol. Cell 29, 499–509 (2008).
Liu, W. M., Chu, W. M., Choudary, P. V. & Schmid, C. W. Cell stress and translational inhibitors transiently increase the abundance of mammalian SINE transcripts. Nucleic Acids Res. 23, 1758–1765 (1995).
Kassube, S. A. et al. Structural insights into transcriptional repression by noncoding RNAs that bind to human Pol II. J. Mol. Biol. 425, 3639–3648 (2013).
Bernecky, C., Herzog, F., Baumeister, W., Plitzko, J. M. & Cramer, P. Structure of transcribing mammalian RNA polymerase II. Nature 529, 551–554 (2016).
Palovcak, E. et al. A simple and robust procedure for preparing graphene-oxide cryo-EM grids. J. Struct. Biol. 204, 80–84 (2018).
Yakovchuk, P., Goodrich, J. A. & Kugel, J. F. B2 RNA and Alu RNA repress transcription by disrupting contacts between RNA polymerase II and promoter DNA within assembled complexes. Proc. Natl Acad. Sci. USA 106, 5569–5574 (2009).
Pfab, J., Phan, N. M. & Si, D. DeepTracer for fast de novo cryo-EM protein structure modeling and special studies on CoV-related complexes. Proc. Natl Acad. Sci. USA 118, e2017525118 (2021).
Lorenz, R. et al. ViennaRNA package 2.0. Algorithms Mol. Biol. 6, 26 (2011).
Kettenberger, H. et al. Structure of an RNA polymerase II-RNA inhibitor complex elucidates transcription regulation by noncoding RNAs. Nat. Struct. Mol. Biol. 13, 44–48 (2006).
Chen, J. et al. 6S RNA mimics B-form DNA to regulate Escherichia coli RNA polymerase. Mol. Cell 68, 388–397 e6 (2017).
Roe, J. H., Burgess, R. R. & Record, M. T. Jr Kinetics and mechanism of the interaction of Escherichia coli RNA polymerase with the lambda PR promoter. J. Mol. Biol. 176, 495–522 (1984).
Saecker, R. M. et al. Structural origins of Escherichia coli RNA polymerase open promoter complex stability. Proc. Natl Acad. Sci. USA 118, e2112877118 (2021).
Buratowski, S., Hahn, S., Guarente, L. & Sharp, P. A. Five intermediate complexes in transcription initiation by RNA polymerase II. Cell 56, 549–561 (1989).
Treutlein, B. et al. Dynamic architecture of a minimal RNA polymerase II open promoter complex. Mol. Cell 46, 136–146 (2012).
Cai, H. & Luse, D. S. Transcription initiation by RNA polymerase II in vitro. Properties of preinitiation, initiation, and elongation complexes. J. Biol. Chem. 262, 298–304 (1987).
Cheung, A. C., Sainsbury, S. & Cramer, P. Structural basis of initial RNA polymerase II transcription. EMBO J. 30, 4755–4763 (2011).
Liu, X., Bushnell, D. A., Silva, D. A., Huang, X. & Kornberg, R. D. Initiation complex structure and promoter proofreading. Science 333, 633–637 (2011).
Wagner, S. D., Kugel, J. F. & Goodrich, J. A. TFIIF facilitates dissociation of RNA polymerase II from noncoding RNAs that lack a repression domain. Mol. Cell. Biol. 30, 91–97 (2010).
Chen, X. et al. Structural insights into preinitiation complex assembly on core promoters. Science 372, eaba8490 (2021).
Killeen, M. T. & Greenblatt, J. F. The general transcription factor RAP30 binds to RNA polymerase II and prevents it from binding nonspecifically to DNA. Mol. Cell. Biol. 12, 30–37 (1992).
Chen, Z. A. et al. Architecture of the RNA polymerase II-TFIIF complex revealed by cross-linking and mass spectrometry. EMBO J. 29, 717–726 (2010).
Pai, D. A. et al. RNAs nonspecifically inhibit RNA polymerase II by preventing binding to the DNA template. RNA 20, 644–655 (2014).
Ren, D., Lei, L. & Burton, Z. F. A region within the RAP74 subunit of human transcription factor IIF is critical for initiation but dispensable for complex assembly. Mol. Cell. Biol. 19, 7377–7387 (1999).
He, Y. et al. Near-atomic resolution visualization of human transcription promoter opening. Nature 533, 359–365 (2016).
Tan, S., Conaway, R. C. & Conaway, J. W. Dissection of transcription factor TFIIF functional domains required for initiation and elongation. Proc. Natl Acad. Sci. USA 92, 6042–6046 (1995).
Bernecky, C., Plitzko, J. M. & Cramer, P. Structure of a transcribing RNA polymerase II-DSIF complex reveals a multidentate DNA-RNA clamp. Nat. Struct. Mol. Biol. 24, 809–815 (2017).
Shaikh, T. H., Roy, A. M., Kim, J., Batzer, M. A. & Deininger, P. L. cDNAs derived from primary and small cytoplasmic Alu (scAlu) transcripts. J. Mol. Biol. 271, 222–234 (1997).
Martinez-Rucobo, F. W. et al. Molecular basis of transcription-coupled pre-mRNA capping. Mol. Cell 58, 1079–1089 (2015).
Mörl, M. & Hartmann, R. K. in Handbook of RNA Biochemistry (eds Hartmann, R. K., Bindereif, A., Schön, A. & Westhof, E.) 29–44 (Wiley, 2014).
Appel, L. M. et al. PHF3 regulates neuronal gene expression through the Pol II CTD reader domain SPOC. Nat. Commun. 12, 6078 (2021).
Zhang, C., Zobeck, K. L. & Burton, Z. F. Human RNA polymerase II elongation in slow motion: role of the TFIIF RAP74 alpha1 helix in nucleoside triphosphate-driven translocation. Mol. Cell. Biol. 25, 3583–3595 (2005).
Ichetovkin, I. E., Abramochkin, G. & Shrader, T. E. Substrate recognition by the leucyl/phenylalanyl-tRNA-protein transferase. Conservation within the enzyme family and localization to the trypsin-resistant domain. J. Biol. Chem. 272, 33009–33014 (1997).
Schurer, H., Lang, K., Schuster, J. & Morl, M. A universal method to produce in vitro transcripts with homogeneous 3′ ends. Nucleic Acids Res. 30, e56 (2002).
Tegunov, D. & Cramer, P. Real-time cryo-electron microscopy data preprocessing with Warp. Nat. Methods 16, 1146–1152 (2019).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166. (2018).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 66, 486–501 (2010).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D. Struct. Biol. 74, 519–530 (2018).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Vos, S. M. et al. Architecture and RNA binding of the human negative elongation factor. eLife 5, e14981 (2016).
Lei, L., Ren, D. & Burton, Z. F. The RAP74 subunit of human transcription factor IIF has similar roles in initiation and elongation. Mol. Cell. Biol. 19, 8372–8382 (1999).
Fang, S. M. & Burton, Z. F. RNA polymerase II-associated protein (RAP) 74 binds transcription factor (TF) IIB and blocks TFIIB-RAP30 binding. J. Biol. Chem. 271, 11703–11709 (1996).
Acknowledgements
We thank the members of the Bernecky laboratory for helpful discussions and A. Hlavata for providing Pol II for use in the fluorescence anisotropy binding assay. We thank V.-V. Hodirnau for SerialEM data collection and support with EPU data collection. We thank D. Slade (Max Perutz Laboratories and Medical University of Vienna, Vienna, Austria) for the wild-type TFIIF expression plasmid. We thank N. Thompson and R. Burgess (McArdle Laboratory for Cancer Research, University of Wisconsin-Madison, Madison, WI, USA) for the 8WG16 hybridoma cell line. We thank C. Plaschka and M. Loose for critical reading of the manuscript. This work was supported by Austrian Science Fund (FWF) grant no. P34185 (DOI 10.55776/P34185) (C.B.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. This research was further supported by the Scientific Service Units of ISTA through resources provided by the Laboratory Support Facility, Electron Microscopy Facility, Scientific Computing and the Preclinical Facility.
Author information
Authors and Affiliations
Contributions
K.T., B.K., C.B. and A.S. performed experiments and analyzed the data. K.T., B.K. and A.S. prepared protein and RNA reagents. C.B. designed and supervised research. K.T. and C.B. processed cryo-EM data. K.T., B.K. and C.B. prepared the manuscript, with input from A.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural and Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Dimitris Typas, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Preparation of Pol II-Alu RNA complexes.
a, SDS-PAGE analysis (Coomassie) of purified Pol II b, Denaturing urea-PAGE analysis of purified Alu RNAs. Unlabeled RNAs were visualized by SYBR Gold staining, and fluorescently labeled RNAs were visualized using a Typhoon RGB scanner. c, Mass photometry analysis of Pol II-Alu complexes. The mass of the monomeric Pol II mass photometry peak was measured in technical triplicate, with each data point shown as a black circle. Bar heights represent the average and error bars represent the standard deviation from the average.
Extended Data Fig. 2 Cryo-EM analysis of Pol II-Alu-LA.
a, Cryo-EM micrograph representative field of view, -1.9 μm defocus. One of a total of 16,470 micrographs is shown. b, Representative 2D classes. c, Processing tree outlining the steps taken to generate the Pol II-Alu-LA reconstruction. Classes with well-resolved clamp and stalk domains are indicated. d, Angular distribution plot for the Pol II-Alu-LA reconstruction. e, Fourier shell correlation plot for the Pol II-Alu-LA reconstruction and the Pol II-Alu-LA phase-randomized masked maps. f, Locally-filtered, non-B-factor-sharpened Pol II-Alu-LA reconstruction colored by estimated local resolution.
Extended Data Fig. 3 Cryo-EM analysis of Pol II-Alu-RA.
a, Cryo-EM micrograph representative field of view, -1.6 μm defocus. One of a total of 29,262 micrographs is shown. b, Representative 2D classes. c, Processing tree outlining the steps taken to generate the Pol II-Alu-RA reconstruction. Classes with well-resolved clamp and stalk domains are indicated. d, Angular distribution plot for the Pol II-Alu-RA reconstruction. e, Fourier shell correlation plot for the Pol II-Alu-RA reconstruction, the Pol II-Alu-RA phase-randomized masked maps, and the model-versus-map correlation for the Pol II-Alu-RA reconstruction and the Pol II core model. f, Locally-filtered, non-B-factor-sharpened Pol II-Alu-RA reconstruction colored by estimated local resolution. g, Comparison of the fork loop 2 conformation in the Pol II-Alu-RA structure and a transcription elongation complex structure (PDB ID 5OIK)27. Coloring is as follows: Pol II-Alu-RA: RBP2 tan, fork loop 2 yellow; EC: RPB2 beige, fork loop 2 light yellow, template DNA blue, nontemplate DNA cyan, RNA red.
Extended Data Fig. 4 Cryo-EM analysis of Pol II-minimal Alu-RA.
a, Cryo-EM micrograph representative field of view, -1.7 μm defocus. One of a total of 7,588 micrographs is shown. b, Representative 2D classes. c, Processing tree outlining the steps taken to generate the Pol II-minimal Alu-RA reconstructions. d, Angular distribution plot for the Pol II-minimal Alu-RA reconstructions. e, Fourier shell correlation plot for the Pol II-minimal Alu-RA reconstructions, the Pol II-minimal Alu-RA phase-randomized masked maps, and the model-versus-map correlation for the Pol II-minimal Alu-RA alternative conformation reconstruction and the model. f, Locally-filtered Pol II-minimal Alu-RA reconstructions colored by estimated local resolution. The Pol II-minimal Alu-RA canonical conformation reconstruction was not B-factor sharpened. g, Top-front and side views of 8-Å-filtered Pol II-minimal Alu-RA canonical and alternative conformation densities compared to the path of nucleic acids in a transcription elongation complex (PDB ID 5OIK)27. Minimal Alu-RA density is shown in semi-transparent gray. Compare to Fig. 1b,c. h, Schematics showing the secondary structures of the canonical and alternative conformations of minimal Alu-RA. Guanosine, green; cytidine, cyan; adenosine, blue; uridine, orange. i, Putative assignment of the regions of Alu-RA that bind in the DNA-RNA hybrid (putative lower stem) and the downstream DNA-binding (putative upper stem) regions of the Pol II cleft. Alu-RA difference density (purple), upper and lower stems (gray bars).
Extended Data Fig. 5 Comparison of Alu RNA and nucleic acids within other Pol II complexes.
a, Comparison of the paths of the nucleic acids in the Pol II-minimal Alu-RA structure and the yeast Pol II-Fc aptamer structure (PDB ID 2B63)10 after alignment on RPB2. b, Comparison of the paths of Fc aptamer and minimal Alu-RA and nucleic acids in a transcription elongation complex (PDB ID 5OIK)27. c, Top-front and side views of 8-Å-filtered Alu-LA and Alu-RA densities compared to the path of nucleic acids in a transcription initiation open promoter complex (PDB ID 5IYB)25.
Extended Data Fig. 6 Preparation and activity of complexes with TFIIF and TFIIF mutants.
a, Schematic detailing the TFIIF variants used in these studies. b, SDS-PAGE analysis of equimolar amounts of each TFIIF variant. c, Transcription assays show that the TFIIF variants employed in this study have differential ability to stimulate Pol II activity, as previously described47,48. Left, Urea PAGE analysis and phosphorimaging; right, quantification. Data are presented as mean values +/- standard deviation, with individual data points shown as black circles. TFIIF color code as in Fig. 2. d, Transcription assays showing the inhibitory effect of a single-stranded RNA. Only the RNA products well separated from a DNA template-independent RNA product were quantified. Left, Urea PAGE analysis and phosphorimaging; right, quantification. Data are presented as mean values +/- standard deviation, with individual data points shown as black circles. e, Urea-PAGE analysis of transcription assay quantified in Fig. 2c. f, Electrophoretic mobility shift assay (EMSA) showing the binding of wild-type TFIIF and the indicated TFIIF mutants to Alu-LA and Alu-RA. g, Quantification of the fraction of bound RNA monitored by the disappearance of the free RNA band. Results represent analysis of three independently prepared reactions. Data are plotted as mean values +/- standard deviation.
Supplementary information
Supplementary Table 1
Supplementary table containing the oligonucleotide sequences used in this study.
Source data
Source Data Fig. 2
Source anisotropy values plotted in Fig. 2a and numerical data for transcription assays in Fig. 2c.
Source Data Fig. 2
Uncropped gels analyzed in Fig. 2c. Cropped versions are shown in Extended Data Fig. 6e.
Source Data Extended Data Fig. 1
Uncropped gels shown in Extended Data Fig. 1a,b.
Source Data Extended Data Fig. 1
Mass photometry data analyzed in Extended Data Fig. 1c.
Source Data Extended Data Fig. 6
Uncropped gels shown in Extended Data Fig. 6.
Source Data Extended Data Fig. 6
Numerical data for transcription and binding quantifications.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tlučková, K., Kaczmarek, B., Salmazo, A. et al. Mechanism of mammalian transcriptional repression by noncoding RNA. Nat Struct Mol Biol 32, 607–612 (2025). https://doi.org/10.1038/s41594-024-01448-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41594-024-01448-7