Abstract
Deciphering the processes through which cancer cells overcome stress, escape a repressive microenvironment and metastasize remains a challenge. Autophagy has been demonstrated to regulate cancer metastasis and C-terminal binding protein (CtBP) has been previously implicated in promoting metastasis in breast cancer. Here we identify the formation of a complex between CtBP and tripartite-motif-containing protein 28 (TRIM28) in the nucleus. Interestingly, this complex regulates the stability of both proteins, as the removal of either partner leads to degradation of the other. Furthermore, the stability of this complex in the nucleus inhibits autophagy through two independent mechanisms. Firstly, the formation of the complex sequesters TRIM28 in the nucleus, preventing its involvement in and its degradation through autophagy. Secondly, this complex participates in the suppression of PTEN expression and leads to inhibition of Unc-51-like kinase 1-mediated autophagy through activation of the protein kinase B–mammalian target of rapamycin pathway. Using mammary gland-specific CtBP-knockout mice, we demonstrate that repression of autophagy by the CtBP–TRIM28 complex modulates luminal duct formation. In breast cancer models, CtBP–TRIM28-dependent inhibition of cellular autophagy also promotes malignant metastasis. Therefore, our study reveals similarities between the mechanisms driving tumor progression and those involved in normal mammary gland development, potentially helping to pave the way toward targeted intervention in breast cancer metastasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All sequencing data created in this study are available in Supplementary Table 2. RNA-seq data were deposited to the Gene Expression Omnibus (GSE287092). All other relevant data supporting the key findings of this study are available within the article or from the corresponding authors upon reasonable request. The biological material used in this study is listed in Supplementary Tables 3–5. Source data are provided with this paper.
References
Vega-Rubin-de-Celis, S. et al. Increased autophagy blocks HER2-mediated breast tumorigenesis. Proc. Natl Acad. Sci. USA 115, 4176–4181 (2018).
Cicchini, M. et al. Autophagy regulator BECN1 suppresses mammary tumorigenesis driven by Wnt1 activation and following parity. Autophagy 10, 2036–2052 (2014).
Liang, X. H. et al. Induction of autophagy and inhibition of tumorigenesis by Beclin 1. Nature 402, 672–676 (1999).
Qu, X. et al. Promotion of tumorigenesis by heterozygous disruption of the Beclin 1 autophagy gene. J. Clin. Invest. 112, 1809–1820 (2003).
Yue, Z., Jin, S., Yang, C., Levine, A. J. & Heintz, N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl Acad. Sci. USA 100, 15077–15082 (2003).
Yang, S. et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 25, 717–729 (2011).
Guo, J. Y. et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 25, 460–470 (2011).
Lock, R. et al. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Mol. Biol. Cell 22, 165–178 (2011).
Kenific, C. M. & Debnath, J. Cellular and metabolic functions for autophagy in cancer cells. Trends Cell Biol. 25, 37–45 (2015).
Galluzzi, L. et al. Autophagy in malignant transformation and cancer progression. EMBO J. 34, 856–880 (2015).
Caswell, P. T. et al. Rab-coupling protein coordinates recycling of α5β1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J. Cell Biol. 183, 143–155 (2008).
Tuloup-Minguez, V. et al. Autophagy modulates cell migration and β1 integrin membrane recycling. Cell Cycle 12, 3317–3328 (2013).
Dower, C. M., Bhat, N., Wang, E. W. & Wang, H. G. Selective reversible inhibition of autophagy in hypoxic breast cancer cells promotes pulmonary metastasis. Cancer Res. 77, 646–657 (2017).
Bressan, C. et al. The dynamic interplay between ATP/ADP levels and autophagy sustain neuronal migration in vivo. eLife 9, e56006 (2020).
Sharifi, M. N. et al. Autophagy promotes focal adhesion disassembly and cell motility of metastatic tumor cells through the direct interaction of paxillin with LC3. Cell Rep. 15, 1660–1672 (2016).
Kenific, C. M. et al. NBR1 enables autophagy-dependent focal adhesion turnover. J. Cell Biol. 212, 577–590 (2016).
Li, Z. L. et al. Autophagy deficiency promotes triple-negative breast cancer resistance to T cell-mediated cytotoxicity by blocking tenascin-C degradation. Nat. Commun. 11, 3806 (2020).
Deng, R. et al. MAPK1/3 kinase-dependent ULK1 degradation attenuates mitophagy and promotes breast cancer bone metastasis. Autophagy 17, 3011–3029 (2021).
Warri, A. et al. Autophagy and unfolded protein response (UPR) regulate mammary gland involution by restraining apoptosis-driven irreversible changes. Cell Death Discov. 4, 40 (2018).
Elswood, J. et al. Autophagy regulates functional differentiation of mammary epithelial cells. Autophagy 17, 420–438 (2021).
Chinnadurai, G. Transcriptional regulation by C-terminal binding proteins. Int. J. Biochem. Cell Biol. 39, 1593–1607 (2007).
Dcona, M. M., Morris, B. L., Ellis, K. C. & Grossman, S. R. CtBP—an emerging oncogene and novel small molecule drug target: advances in the understanding of its oncogenic action and identification of therapeutic inhibitors. Cancer Biol. Ther. 18, 379–391 (2017).
Nardini, M. et al. The C-terminal domain of the transcriptional corepressor CtBP is intrinsically unstructured. Protein Sci. 15, 1042–1050 (2006).
Raicu, A. M. et al. The cynosure of CtBP: evolution of a bilaterian transcriptional corepressor. Mol. Biol. Evol. 40, msad003 (2023).
Chinnadurai, G. CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol. Cell 9, 213–224 (2002).
Cohen, M. J. et al. Dissection of the C-terminal region of E1A redefines the roles of CtBP and other cellular targets in oncogenic transformation. J. Virol. 87, 10348–10355 (2013).
Chawla, A. T. et al. CtBP—a targetable dependency for tumor-initiating cell activity and metastasis in pancreatic adenocarcinoma. Oncogenesis 8, 55 (2019).
de Barrios, O. et al. ZEB1-induced tumourigenesis requires senescence inhibition via activation of DKK1/mutant p53/Mdm2/CtBP and repression of macroH2A1. Gut 66, 666–682 (2017).
Zhao, Z. et al. CtBP promotes metastasis of breast cancer through repressing cholesterol and activating TGF-β signaling. Oncogene 38, 2076–2091 (2019).
Ichikawa, K. et al. MCRIP1, an ERK substrate, mediates ERK-induced gene silencing during epithelial-mesenchymal transition by regulating the co-repressor CtBP. Mol. Cell 58, 35–46 (2015).
Birts, C. N. et al. p53 is regulated by aerobic glycolysis in cancer cells by the CtBP family of NADH-dependent transcriptional regulators. Sci. Signal 13, eaau9529 (2020).
Zhang, Q. et al. Retraction for Zhang et al., Metabolic regulation of SIRT1 transcription via a HIC1:CtBP corepressor complex. Proc. Natl Acad. Sci. USA 112, E819 (2015).
Garriga-Canut, M. et al. 2-Deoxy-d-glucose reduces epilepsy progression by NRSF–CtBP-dependent metabolic regulation of chromatin structure. Nat. Neurosci. 9, 1382–1387 (2006).
Wang, L., Zhou, H., Wang, Y., Cui, G. & Di, L. J. CtBP maintains cancer cell growth and metabolic homeostasis via regulating SIRT4. Cell Death Dis. 6, e1620 (2015).
Wang, L. et al. Molecular link between glucose and glutamine consumption in cancer cells mediated by CtBP and SIRT4. Oncogenesis 7, 26 (2018).
Hao, D. et al. Non-classical estrogen signaling in ovarian cancer improves chemo-sensitivity and patients outcome. Theranostics 9, 3952–3965 (2019).
Li, J. et al. Metabolic modulation of CtBP dimeric status impacts the repression of DNA damage repair genes and the platinum sensitivity of ovarian cancer. Int J. Biol. Sci. 19, 2081–2096 (2023).
Addison, J. B. et al. KAP1 promotes proliferation and metastatic progression of breast cancer cells. Cancer Res. 75, 344–355 (2015).
Czerwinska, P., Mazurek, S. & Wiznerowicz, M. The complexity of TRIM28 contribution to cancer. J. Biomed. Sci. 24, 63 (2017).
Ivanov, A. V. et al. PHD domain-mediated E3 ligase activity directs intramolecular sumoylation of an adjacent bromodomain required for gene silencing. Mol. Cell 28, 823–837 (2007).
Lionnard, L. et al. TRIM17 and TRIM28 antagonistically regulate the ubiquitination and anti-apoptotic activity of BCL2A1. Cell Death Differ. 26, 902–917 (2019).
Pineda, C. T. et al. Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell 160, 715–728 (2015).
Wang, C. et al. MDM2 interaction with nuclear corepressor KAP1 contributes to p53 inactivation. EMBO J. 24, 3279–3290 (2005).
Doyle, J. M., Gao, J., Wang, J., Yang, M. & Potts, P. R. MAGE–RING protein complexes comprise a family of E3 ubiquitin ligases. Mol. Cell 39, 963–974 (2010).
Zwick, R. K. et al. Adipocyte hypertrophy and lipid dynamics underlie mammary gland remodeling after lactation. Nat. Commun. 9, 3592 (2018).
Di, L. J. et al. Genome-wide profiles of CtBP link metabolism with genome stability and epithelial reprogramming in breast cancer. Nat. Commun. 4, 1449 (2013).
Yang, Y. et al. Acetylated Hsp70 and KAP1-mediated Vps34 SUMOylation is required for autophagosome creation in autophagy. Proc. Natl Acad. Sci. USA 110, 6841–6846 (2013).
Wang, L., Klionsky, D. J. & Shen, H.-M. The emerging mechanisms and functions of microautophagy. Nat. Rev. Mol. Cell Biol. 24, 186–203 (2023).
Dice, J. F. Chaperone-mediated autophagy. Autophagy 3, 295–299 (2007).
Devkota, S. et al. Functional characterization of EI24-induced autophagy in the degradation of RING-domain E3 ligases. Autophagy 12, 2038–2053 (2016).
Zheng, Q. et al. Calcium transients on the ER surface trigger liquid-liquid phase separation of FIP200 to specify autophagosome initiation sites. Cell 185, 4082–4098 (2022).
Rabinowitz, J. D. & White, E. J. S. Autophagy and metabolism. Science 330, 1344–1348 (2010).
Nicklin, P. et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136, 521–534 (2009).
Qin, Y. et al. TRIM28 SUMOylates and stabilizes NLRP3 to facilitate inflammasome activation. Nat. Commun. 12, 4794 (2021).
Zeng, L. et al. Structural insights into human KAP1 PHD finger–bromodomain and its role in gene silencing. Nat. Struct. Mol. Biol. 15, 626–633 (2008).
Zhao, Q. et al. GPS-SUMO: a tool for the prediction of sumoylation sites and SUMO-interaction motifs. Nucleic Acids Res. 42, W325–W330 (2014).
Beauclair, G., Bridier-Nahmias, A., Zagury, J. F., Saïb, A. & Zamborlini, A. JASSA: a comprehensive tool for prediction of SUMOylation sites and SIMs. Bioinformatics 31, 3483–3491 (2015).
Drag, M. & Salvesen, G. S. DeSUMOylating enzymes—SENPs. IUBMB Life 60, 734–742 (2008).
Bellesis, A. G., Jecrois, A. M., Hayes, J. A., Schiffer, C. A. & Royer, W. E. Jr Assembly of human C-terminal binding protein (CtBP) into tetramers. J. Biol. Chem. 293, 9101–9112 (2018).
Di, L. J., Fernandez, A. G., De Siervi, A., Longo, D. L. & Gardner, K. Transcriptional regulation of BRCA1 expression by a metabolic switch. Nat. Struct. Mol. Biol. 17, 1406–1413 (2010).
Desgrosellier, J. S. & Cheresh, D. A. Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 10, 9–22 (2010).
Hildebrand, J. D. & Soriano, P. Overlapping and unique roles for C-terminal binding protein 1 (CtBP1) and CtBP2 during mouse development. Mol. Cell. Biol. 22, 5296–5307 (2002).
Xie, Z. et al. Smad6 promotes neuronal differentiation in the intermediate zone of the dorsal neural tube by inhibition of the Wnt/β-catenin pathway. Proc. Natl Acad. Sci. USA 108, 12119–12124 (2011).
Schaeper, U., Subramanian, T., Lim, L., Boyd, J. M. & Chinnadurai, G. Interaction between a cellular protein that binds to the C-terminal region of adenovirus E1A (CtBP) and a novel cellular protein is disrupted by E1A through a conserved PLDLS motif. J. Biol. Chem. 273, 8549–8552 (1998).
Vo, N., Fjeld, C. & Goodman, R. H. Acetylation of nuclear hormone receptor-interacting protein RIP140 regulates binding of the transcriptional corepressor CtBP. Mol. Cell. Biol. 21, 6181–6188 (2001).
Shi, Y. et al. Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature 422, 735–738 (2003).
Kuppuswamy, M. et al. Role of the PLDLS-binding cleft region of CtBP1 in recruitment of core and auxiliary components of the corepressor complex. Mol. Cell. Biol. 28, 269–281 (2008).
Phelps, R. A. et al. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell 137, 623–634 (2009).
Birts, C. N. et al. Expression of CtBP family protein isoforms in breast cancer and their role in chemoresistance. Biol. Cell 103, 1–19 (2010).
Cai, J. et al. CK1α suppresses lung tumour growth by stabilizing PTEN and inducing autophagy. Nat. Cell Biol. 20, 465–478 (2018).
Nikolatou, K. et al. PTEN deficiency exposes a requirement for an ARF GTPase module for integrin-dependent invasion in ovarian cancer. EMBO J. 42, e113987 (2023).
Hsu, V. W., Bai, M. & Li, J. Getting active: protein sorting in endocytic recycling. Nat. Rev. Mol. Cell Biol. 13, 323–328 (2012).
Bottcher, R. T. et al. Sorting nexin 17 prevents lysosomal degradation of β1 integrins by binding to the β1-integrin tail. Nat. Cell Biol. 14, 584–592 (2012).
Margadant, C., Kreft, M., de Groot, D. J., Norman, J. C. & Sonnenberg, A. Distinct roles of talin and kindlin in regulating integrin α5β1 function and trafficking. Curr. Biol. 22, 1554–1563 (2012).
Hyttinen, J. M., Niittykoski, M., Salminen, A. & Kaarniranta, K. Maturation of autophagosomes and endosomes: a key role for Rab7. Biochim. Biophys. Acta 1833, 503–510 (2013).
Remy, I. & Michnick, S. W. A highly sensitive protein–protein interaction assay based on Gaussia luciferase. Nat. Methods 3, 977–979 (2006).
Jarvius, M. et al. In situ detection of phosphorylated platelet-derived growth factor receptor β using a generalized proximity ligation method. Mol. Cell Proteom. 6, 1500–1509 (2007).
Söderberg, O. et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000 (2006).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Plante, I., Stewart, M. K. & Laird, D. W. Evaluation of mammary gland development and function in mouse models. J. Vis. Exp. 53, 2828 (2011).
Colleluori, G., Perugini, J., Barbatelli, G. & Cinti, S. Mammary gland adipocytes in lactation cycle, obesity and breast cancer. Rev. Endocr. Metab. Disord. 22, 241–255 (2021).
McGinley, J. N. & Thompson, H. J. Quantitative assessment of mammary gland density in rodents using digital image analysis. Biol. Proced. Online 13, 4 (2011).
Wawryk-Gawda, E. et al. CB2R agonist prevents nicotine induced lung fibrosis. Exp. Lung Res. 44, 344–351 (2018).
Fort, L. et al. Fam49/CYRI interacts with Rac1 and locally suppresses protrusions. Nat. Cell Biol. 20, 1159–1171 (2018).
Godet, I. et al. Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis. Nat. Commun. 10, 4862 (2019).
Niu, F., Sun, K., Wei, W., Yu, C. & Wei, Z. F-actin disassembly factor MICAL1 binding to myosin Va mediates cargo unloading during cytokinesis. Sci. Adv. 6, eabb1307 (2020).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2022YFE0205800 to S.L.), the Science and Technology Development Fund of Macao SAR (FDCT/0014/2018/A1, FDCT/0117/2018/A3, FDCT/0048/2022/A1 and FDCT/0054/2022/AMJ to L.-j.D.), the Multiyear Research Grant from the University of Macau (MYRG2022-00173-FHS to L.-j.D.), the National Natural Science Foundation of China (81772980 to L.-j.D.) and the Science and Technology Development Fund, Macau SAR under the Funding Scheme for Postdoctoral Researchers of Higher Education Institutions 2021 (0037/2021/APD to H.L.). We also thank the Animal Research Core, Biological Imaging and Stem Cell Core, Genomics, Bioinformatics and Single-Cell Analysis Core and Proteomics, Metabolomics and Drug Development Core for their supportive services.
Author information
Authors and Affiliations
Contributions
L.T. conceptualized the research, performed the experiments and data analysis, and drafted the paper. D.Z. and Y.H. analyzed the data. P.T., Jiajia Li and P.L. performed the experiments and data analysis. Z.T., H.-x.W., S.L., H.L., K.M., M.D., L.Z. and Junyi Li provided technical assistance and contributed reagents. H.-M.S. and C.D. contributed reagents and prepared the paper. L.W. and L.-j.D. conceptualized the research, supervised the project and drafted the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Jessie Yanxiang Guo, Nobuo Noda and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Dimitris Typas, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 CtBP represses autophagy in epithelial and tumor cells.
(a) CtBP2flox/flox and MMTV-Cre mice genotyping. MMTV-Cre and CtBP2flox/flox mice were used to breed for generating MG specific CtBP2-/- mice. Genotypes verified by 2% DNA agarose gel electrophoresis (left). Top (right): CtBP2 gene locus, loxP positions & primers. Bottom (right): breeding scheme made By Figdraw (https://www.figdraw.com/#/) (mouse element NO. FD20024642; FD20026219; FD20026217). (b) Quantitation of CtBP2 in MG sections. IHC assay quantitation was mean staining intensity of cells in individual MG (n = 15), each MG containing at least 30 cells derived from 3 mice. The H-socre was quantitated by pathologists based on individual MG (n = 15) from 3 mice. (c) Whole-mount staining of MG isolated from wild type virgin mice & lactating mice. Scale bars=50 µm. (d) H-score quantitation of Cleaved-caspase 3, Ki67 and P62 in MG sections by pathologists based on individual MG (n = 15) from 3 mice. (e) WB of CtBP in MDA-MB-231 cells with stable CtBP KD. (f, g) Tumors from nude mice after subcutaneous injection of MDA-MB-231 cells with CtBP KD. Photographed (f), measured for volume and weight (g) (n = 4). (h) Nude mice were intravenously (iv) injected with MDA-MB-231 cells (CtBP KD and luciferase overexpression). Lung metastasis images and quantitated luminescence intensity (n = 4 mice per condition). (i) Images of lungs and H&E staining of the metastatic tumors. Black arrows and ‘T’ indicate tumors. Scale bar: 500 μm. Tumor area was quantitated by image J (n = 4). Scale bars: 500 µm. (j) Sections of MDA-MB-231 engrafted tumors with CtBP KD were stained by indicated antibodies. Scale bar=50 μm. (k) WB of CtBP in xenografted tumors. (l, m) The mRNA level changes of RB1CC1 (l) and ULK1 (m) in MDA-MB-231 cells (n = 3). (n, o) P62 and LC3B expression in clinical breast tissue (normal: n = 572; tumor: n = 1104) (n) or different tumor stages (stage I: n = 202; stage II: n = 693; stage III/IV/V: n = 309; stage I: n = 202) (o) were retrieved from TCGA (ID: TCGA.BRCA.sampleMap/HiSeqV2) and GTEx (gene_reads_2017-06-05_v8_breast_mammary_tissue) databases. Data are shown as the mean with s.d. (b,d,g,h,i,l-o); Statistical analysis was performed using a two-tailed, unpaired Student’s t-test (b,d,g,h,i,l-o); n.s. P > 0.05, *P < 0.05, **P < 0.01 and ***P < 0.001.
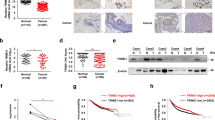
Extended Data Fig. 2 TRIM28 shows positive correlation with CtBP.
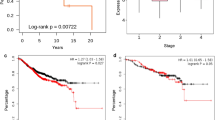
(a) The mRNA levels of CtBP1, CtBP2 and TRIM28 in clinical breast normal (n = 98), and stage I (n = 202), stage II (n = 787), stage III/IV (n = 310) tumor tissues was analyzed based on TCGA-BRCA dataset. (b) The transcriptional Pearson correlation between CtBP and TRIM28 was analyzed based on TCGA-BRCA, CCLE-BRCA and GTEx-Breast datasets. Statistical analysis was performed using a two-tailed, Pearson correlation coefficient test. (c, d) WB of protein level of TRIM28 and CtBP in different types of breast cancer cells (c). The quantification was performed by image J software. The Pearson correlation between TRIM28 and CtBP was plotted after normalized to GAPDH, n = 12 (d). Statistical analysis was performed using a two-tailed, Pearson correlation coefficient test, r = 0.8246, p = 0.0010. (e) PLA assay detecting CtBP and TRIM28 interaction on tissue array including clinical breast normal or cancer samples. PLA signal intensity was quantitated and plotted between normal and different stages of cancer tissues (Normal, n = 25; Stage I&II, n = 129; Stage III&IV, n = 87). (f) Kaplan-Meier survival analysis of overall survival (OS) (TCGA.BRCA.sampleMap/HiSeqV2) based on TRIM28 and CtBP expression in the breast cancer patients from TCGA datasets. Patients were classified as TRIM28highCtBP2high (n = 125) and TRIM28lowCtBP2low (n = 547). Statistical analysis was performed using Log-rank based on Mantel-Cox test, p = 0.0135. Data are shown as the mean with s.d. (a,e); n = 3 biologically independent replicates (c). Statistical analysis was performed using a two-tailed, unpaired Student’s t-test (a,e); n.s. P > 0.05, *P < 0.05, **P < 0.01 and ***P < 0.001.
Extended Data Fig. 3 CtBP represses autophagy via TRIM28 retention in nucleus.
(a) Quantitation of TRIM28 H-score in MG sections from CtBP2flox/flox or MG-specific CtBP2-/- mice by pathologists based on individual MG (n = 15) from 3 mice. (b) WB of TRIM28 in MDA-MB-231 and MCF-7 with CtBP KD (3 or 5days). (c) The mRNA level of TRIM28 within 7 days after CtBP1/2 KD. (d–g) WB of TRIM28, P62, LC3B, CtBP in MDA-MB-231 and MCF-7 with CtBP OE (3 days) (d), in MDA-MB-231 with CtBP KD extend to 9 days (e), in A549, SKOV3, HEPG2 cells with CtBP KD (48 h) (f), in MDA-MB-231 and MCF-7 with CtBP KD, followed by 10 μM MG132 treatment (8 h) (g). (h, i) Stable GFP-LC3B OE cells were treated with Rapamycin (24 h) or CtBP KD in combination with TRIM28 KD (48 h). GFP-LC3B puncta (green) and DAPI-stained nuclei (blue) imaged (h). Scale bars=10 µm. Quantitated puncta per cell using Image J (n = 9) (i). (j) WB of P62, GFP-LC3B, CtBP and TRIM28 in MDA-MB-231 (GFP-LC3B OE) cells with TRIM28 KD or CtBP KD for 48 h or treated by Rapamycin (24 h). (k, l) MDA-MB-231 cells with stable mCherry-GFP-LC3B OE were treated with HBSS (8 h) or with CtBP KD in combination with TRIM28 KD (48 h). mCherry (red), mCherry-GFP-LC3B(yellow) puncta and DAPI-stained nuclei (blue) imaged (k). Scale bars=10 µm. Quantitated puncta per cell using Image J (n = 13) (l). (m) Flag-TRIM28-FL or Flag-TRIM28-ΔNLS plasmid was transfected in HEK-293T cells(48 h). Flag tag (green) and DAPI-stained nuclei (blue) imaged. Scale bars=10 µm. (n, o) QRT-PCR for LAMP2A (n) or EI24 (o) with siRNA treatment (48 h) (n = 3). (p, q) MDA-MB-231 cells were treated with HBSS or Rapamycin in combination with CQ (8 h). TRIM28 (green) and EI24 (red) and DAPI-stained nuclei (blue) imaged (blue) (p). Scale bars=10 µm. Quantitated colocalized puncta per cell using Image J (n = 15) (q). (r) QRT-PCR for EI24 with EI24 siRNA treatment (48 h) in MDA-MB-231 cells (n = 3). Data are shown as the mean with s.d. (a,c,i,l,n,o,q,r); n = 3 biologically independent replicates (b,d,e-g,j). Statistical analysis was performed using a two-tailed, unpaired Student’s t-test (a,c,i,l,n,o,q,r); n.s. P > 0.05, *P < 0.05, **P < 0.01 and ***P < 0.001.
Extended Data Fig. 4 CtBP-TRIM28 activates autophagy by promoting AKT-mTOR signaling pathway.
(a) WB of P62, LC3B-II and LC3B-I in MDA-MB-231 cells transfected with TRIM28-FL or TRIM28-ΔNLS mutant for 72 h. (b–d) WB of P62, LC3B AMPKα1 or p-AMPKα1 in MDA-MB-231 and MCF-7 cells with TRIM28 KD (3 or 5 days) (b), in MDA-MB-231 with CtBP KD or TRIM28 KD (3 d) (c), in MDA-MB-231 with CtBP KD in combination with compound C treatment (24 h) (d). (e) Cytoplasmic Acetyl-coA (Ac-CoA) quantitation in MDA-MB-231 and MCF-7 cells with CtBP KD or TRIM28 KD (48 h) (n = 3). (f, g) WB of whole cell Acetylated-lysine (Ac-lys), Acetylated histone H3 (Ac-H3) or total-H3 in MDA-MB-231 with CtBP KD (48 h) (f), with CtBP KD or TRIM28 KD (48 h) (g). (h) Protein level Pearson correlation between CtBP1, CtBP2 or TRIM28 and P62, LC3B, BECN1, ATG14 and ATG16L in clinical breast samples (n = 222) retrieved from TCGA (ID: TCGA_Breast_BI_Proteome). Two-tailed, Pearson correlation coefficient test. (i) Kaplan-Meier DSS survival analysis of breast cancer patients from TCGA by P62, LC3B, BECN1, ATG14, ATG16L expression from TCGA datasets (TCGA_Breast_BI_Proteome). Patients were classified to high expression and low expression groups. Log-rank based on Mantel-Cox test. (j) Gene Ontology (GO) analysis of the up-regulated genes in MCF-7 cells with CtBP KD or TRIM28 KD by Metascape. (k) GSEA analysis of the differentially expressed genes (DEGs) in CtBP KD versus Ctrl in MCF-7 cells. Top hits include KEGG-autophagy (hsa04140) and Reactome-autophagy (R-HSA-9612973) datasets. (l, m) QRT-PCR validation of autophagy related gene (ATGs) expression in MCF-7 cells with CtBP KD, TRIM28 KD (l), CtBP OE or TRIM28 OE (m). (n) Quantification of t-AKT, p-AKT (Ser473), t-mTOR, p-mTOR, t-ULK1, p-ULK1(S758), t-S6K and p-S6K (Thr389) in MDA-MB-231 with CtBP KD in combination with PTEN KD by Image J (n = 3). Data are shown as the mean with s.d. (e,l-n); n = 3 biologically independent replicates (a-d,f,g). Statistical analysis was performed using a two-tailed, unpaired Student’s t-test (e,l-n); n.s. P > 0.05, *P < 0.05, **P < 0.01 and ***P < 0.001.
Extended Data Fig. 5 TRIM28 protects CtBP from degradation by SUMOylating CtBP.
(a) WB of P62 and CtBP in MDA-MB-231 cells treated by HBSS with increased time in combination with CQ treatment. (b, c) WB of CtBP1 and CtBP2 in MDA-MB-231 and MCF-7 cells with TRIM28 KD (b) for 3 d or 5 d or OE (c) for 3 d (d) qRT-PCR quantitation of CtBP1 and CtBP2 in MCF-7 and MDA-MB-231 cells with siTRIM28 up to 7 days (n = 3). (e) WB of CtBP in MDA-MB-231 cells treated with HBSS for 8 h, followed by 10 μM MG132 treatment for 8 h. (f) WB of CtBP in MDA-MB-231 cells with TRIM28 overexpression for 48 h, followed by increased GA treatment for 24 h. (g) Gluc PCA assay of TRIM28 + CtBP1 or CtBP2 (TR28 + C1, TR28 + C2) in HEK-293T cells treated by different concentrations of 2-D08 (24 h) or GA for 12 h, each dot represents the luminescence intensity per 100 μl reaction system (n = 4). (h) WB of P62 and LC3B in MDA-MB-231 cells, followed by increased GA treatment for 24 h, LC3B-II/LC3B-I ratio is also shown. (i) Co-IP analysis of CtBP by TRIM28 in MDA-MB-231 cells with Flag-TRIM28 FL OE or Flag-TRIM28 (C651A) OE for 48 h followed by MG132 treatment for 8 h. (j) IP analysis of CtBP1 WT, CtBP1 (k428r), CtBP2 WT and CtBP2 (K434R) ubiquitination in MDA-MB-231 cells upon TRIM28 FL OE for 48 h in combination with MG132 treatment for 8 h. (k) QRT-PCR measurement of SENPs after being KD for 48 h (n = 3). (l) WB of CtBP1 and CtBP2 in MCF-10A cells with siSENPs in combination with siTRIM28. Data are shown as the mean with s.d. (d,g,k); n = 3 biologically independent replicates (a-c,e,f,h-j,l). Statistical analysis was performed using a two-tailed, unpaired Student’s t-test (d,g,k); n.s. P > 0.05, *P < 0.05, **P < 0.01 and ***P < 0.001.
Extended Data Fig. 6 PGDLV motif of TRIM28 interacts with C-terminal of CtBP.
(a) ITC titration and fitting curve measurement of interaction between TRIM28-C term and CtBP1 (left) or CtBP2 (right). (b) Schematic diagram showing CtBP2 protein major domains and the truncation mutants. (c) Gluc PCA assay of CtBP2 truncation mutants interaction with TRIM28 in HEK-293T, each dot represents the luminescence intensity per 100 μl reaction system (n = 5). (d) Structure prediction of interaction between TRIM28-C-terminal and CtBP2 (333-445) by Alphafold3 (AlphaFold Server - Google DeepMind). This structural image was generated by the PyMOL Molecular Graphics System (Version 3.0 Schrödinger, LLC). TRIM28-C-terminal is shown in cyan, PGDLV motif in magenta, CtBP2 (333-445) in green. The interaction details are enlarged and shown in right figure, yellow dash line means hydrogen bound. (e–i) Gluc PCA assay of CtBP2 and CtBP2 truncation mutants (1-445, 1-127, 127-333, 333-445) (e) and CtBP2 truncation mutants (1-333, 127-445) (f) interaction, each dot represents the luminescence intensity per 100 μl reaction system (n = 5), CtBP2 truncation mutants (333-358) (g) and (333-445) (h) interaction with TRIM28 or TRIM28 mutants (n = 5), and CtBP1 or CtBP2 interaction with CtBP2 dimerization defect mutants (i) in HEK-293T cells (n = 4). Data are shown as the mean with s.d. (c,e-i); Statistical analysis was performed using a two-tailed, unpaired Student’s t-test (c,e-i); n.s. P > 0.05, *P < 0.05, **P < 0.01 and ***P < 0.001.
Extended Data Fig. 7 CtBP-TRIM28 complex represses autophagy to promote metastasis.
(a) Pearson correlation between CtBP1, CtBP2 or TRIM28 protein levels and Macro-autophagy score (REACTOME, R-HSA-1632852). Data from CPTAC2 (PDC000120) (n = 149). Two-tailed, Pearson correlation coefficient test. (b) WB of TRIM28 in MDA-MB-231 with CtBP2, CtBP2 (G189A), TRIM28 or TRIM28 (V638S) overexpression. (c) Transwell assay of MDA-MB-231 cells overexpression indicated lenti-vectors were cultured in suspension for indicated time scale. Migration cells were stained with crystal violet after 24 h. (d) Flow cytometry analysis of apoptosis in MDA-MB-231 cells transduced with indicated lenti-vectors after culturing in suspension (4 days) (n = 3). (e) WB of TRIM28 in MDA-MB-231 with CtBP2, CtBP2 (G189A), TRIM28 or TRIM28 (V638S) overexpression. (f, g) WB of ITGA5, ITGB1 and P62 in MDA-MB-231 treated with HBSS (f) or CtBP KD (g) for indicated time scale. (h) WB of TRIM28 and CtBP2 in MDA-MB-231 with CtBP2 and TRIM28 overexpression. (i) WB of TRIM28, t-ULK1 and CtBP2 in MDA-MB-231 with stable CtBPKD, TRIM28 KD or ULK1 KD. (j) WB of ITGA5, ITGB1 and P62 in MDA-MB-231 with CtBP or TRIM28 overexpression after suspension culture (24 h or 48 h). (k) WB of RB1CC1, ULK1, TRIM28 and CtBP2 in MDA-MB-231 with stable RB1CC1 KD, ULK1 KD, TRIM28 KD or CtBP2 KD. (l) Lung metastasis of MDA-MB-231 cells with indicated shRNAs (n = 3) (left). Representative images of lungs from nude mice (black arrows indicate metastatic tumors), HE staining of lung tumor sections (dash line indicates metastatic tumor areas, scale bar: 500 μm) are shown at right. Middle, top is luminescence quantitation signal of lung metastatic tumors per mice (n = 3 mice per condition). Bottom is the quantitation of tumor area in lung sections (n = 3 sections per condition). Tumor area was quantitated by image J. (m) WB of TRIM28 and CtBP2 in MDA-MB-231 with stable TRIM28 and CtBP2 overexpression. Data are shown as the mean with s.d. (d,l); n = 3 biologically independent replicates (f,g,j). Statistical analysis was performed using a two-tailed, unpaired Student’s t-test (d,l); n.s. P > 0.05, *P < 0.05, **P < 0.01 and ***P < 0.001.
Supplementary information
Supplementary Table 1
The MS data for proteins interacting with CtBP1, CtBP2 or TRIM28 in MDA-MB-231 breast tumor cells.
Supplementary Table 2
Summary of RNA-seq data in MCF-7 breast tumor cells treated by knocking down CtBP1/2, TRIM28 or control.
Supplementary Table 3
Primer and siRNA list.
Supplementary Table 4
Cell line information.
Supplementary Table 5
Chemical and reagent information.
Source data
Source Data Fig. 1
Unprocessed WBs.
Source Data Fig. 2
Unprocessed WBs.
Source Data Fig. 3
Unprocessed WBs.
Source Data Fig. 4
Unprocessed WBs.
Source Data Fig. 5
Unprocessed WBs.
Source Data Fig. 6
Unprocessed WBs.
Source Data Extended Data Fig. 1
Unprocessed WBs.
Source Data Extended Data Fig. 2
Unprocessed WBs.
Source Data Extended Data Fig. 3
Unprocessed WBs.
Source Data Extended Data Fig. 4
Unprocessed WBs.
Source Data Extended Data Fig. 5
Unprocessed WBs.
Source Data Extended Data Fig. 7
Unprocessed WBs.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tai, L., Zhu, D., Tang, P. et al. Reciprocal stabilization of CtBP and TRIM28 represses autophagy to promote metastasis. Nat Struct Mol Biol 32, 1408–1423 (2025). https://doi.org/10.1038/s41594-025-01554-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41594-025-01554-0
This article is cited by
-
TRIM Protein Superfamily in Breast Cancer: Yin and Yang
Biochemical Genetics (2025)