Abstract
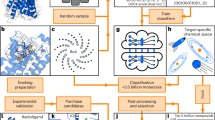
With the recent explosion of chemical libraries beyond a billion molecules, more efficient virtual screening approaches are needed. The Deep Docking (DD) platform enables up to 100-fold acceleration of structure-based virtual screening by docking only a subset of a chemical library, iteratively synchronized with a ligand-based prediction of the remaining docking scores. This method results in hundreds- to thousands-fold virtual hit enrichment (without significant loss of potential drug candidates) and hence enables the screening of billion molecule–sized chemical libraries without using extraordinary computational resources. Herein, we present and discuss the generalized DD protocol that has been proven successful in various computer-aided drug discovery (CADD) campaigns and can be applied in conjunction with any conventional docking program. The protocol encompasses eight consecutive stages: molecular library preparation, receptor preparation, random sampling of a library, ligand preparation, molecular docking, model training, model inference and the residual docking. The standard DD workflow enables iterative application of stages 3–7 with continuous augmentation of the training set, and the number of such iterations can be adjusted by the user. A predefined recall value allows for control of the percentage of top-scoring molecules that are retained by DD and can be adjusted to control the library size reduction. The procedure takes 1–2 weeks (depending on the available resources) and can be completely automated on computing clusters managed by job schedulers. This open-source protocol, at https://github.com/jamesgleave/DD_protocol, can be readily deployed by CADD researchers and can significantly accelerate the effective exploration of ultra-large portions of a chemical space.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The prepared version of ZINC20 can be freely obtained from https://files.docking.org/zinc20-ML/. The example iteration is freely available from the Federated Research Data Repository (https://doi.org/10.20383/102.0489). Source data for Figs. 2 and 5 are freely available from the Federated Research Data Repository (https://doi.org/10.20383/102.0489).
Code availability
The DD code is freely available at https://github.com/jamesgleave/DD_protocol.
Change history
27 March 2024
In Step 34, the text “Run the procedure from Steps 16–31” originally read “14–31”. This has now been amended in the HTML and PDF versions of the article.
References
Lyu, J. et al. Ultra-large library docking for discovering new chemotypes. Nature 566, 224–229 (2019).
Gorgulla, C. et al. An open-source drug discovery platform enables ultra-large virtual screens. Nature 580, 663–668 (2020).
Stein, R. M. et al. Virtual discovery of melatonin receptor ligands to modulate circadian rhythms. Nature 579, 609–614 (2020).
Grygorenko, O. O. et al. Generating multibillion chemical space of readily accessible screening compounds. iScience 23, 101681 (2020).
Acharya, A. et al. Supercomputer-based ensemble docking drug discovery pipeline with application to Covid-19. J. Chem. Inf. Model. 60, 5832–5852 (2020).
Shoichet, B. K. Virtual screening of chemical libraries. Nature 432, 862–865 (2004).
Cherkasov, A., Ban, F., Li, Y., Fallahi, M. & Hammond, G. L. Progressive docking: a hybrid QSAR/docking approach for accelerating in silico high throughput screening. J. Med. Chem. 49, 7466–7478 (2006).
Svensson, F., Norinder, U. & Bender, A. Improving screening efficiency through iterative screening using docking and conformal prediction. J. Chem. Inf. Model. 57, 439–444 (2017).
Ahmed, L. et al. Efficient iterative virtual screening with Apache Spark and conformal prediction. J. Cheminform. 10, 8 (2018).
Gentile, F. et al. Deep Docking: a deep learning platform for augmentation of structure based drug discovery. ACS Cent. Sci. 6, 939–949 (2020).
Sterling, T. & Irwin, J. J. ZINC 15—ligand discovery for everyone. J. Chem. Inf. Model. 55, 2324–2337 (2015).
McGann, M. FRED pose prediction and virtual screening accuracy. J. Chem. Inf. Model. 51, 578–596 (2011).
Friesner, R. A. et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 47, 1739–1749 (2004).
Ton, A.-T., Gentile, F., Hsing, M., Ban, F. & Cherkasov, A. Rapid identification of potential inhibitors of SARS-CoV-2 main protease by deep docking of 1.3 billion compounds. Mol. Inf. 39, e2000028 (2020).
Muratov, E. N. et al. A critical overview of computational approaches employed for COVID-19 drug discovery. Chem. Soc. Rev. 50, 9121–9151 (2021).
Gentile, F., Ton, A.-T., Mslati, H., Ban, F. & Cherkasov, A. Discovery of SARS-CoV-2 main protease inhibitors through Deep Docking of 1.36 billion compounds. in 26th Congress of the European Society of Biomechanics (European Society of Biomechanics, 2021).
Rossetti, G. G. et al. Identification of low micromolar SARS-CoV-2 Mpro inhibitors from hits identified by in silico screens. Preprint at bioRxiv https://doi.org/10.1101/2020.12.03.409441(2020).
Jastrzębski, S. et al. Emulating docking results using a deep neural network: a new perspective for virtual screening. J. Chem. Inf. Model. 60, 4246–4262 (2020).
Al Saadi, A. et al. IMPECCABLE: Integrated Modeling PipelinE for COVID Cure by Assessing Better LEads. in ACM International Conference Proceeding Series (Association for Computing Machinery, 2021); https://doi.org/10.1145/3472456.3473524
Berenger, F., Kumar, A., Zhang, K. Y. J. & Yamanishi, Y. Lean-docking: exploiting ligands’ predicted docking scores to accelerate molecular docking. J. Chem. Inf. Model. 61, 2341––2352 (2021).
Graff, D. E., Shakhnovich, E. I. & Coley, C. W. Accelerating high-throughput virtual screening through molecular pool-based active learning. Chem. Sci. 12, 7866–7881 (2021).
Yang, Y. et al. Efficient exploration of chemical space with docking and deep-learning. Preprint at https://chemrxiv.org/engage/chemrxiv/article-details/60c755bf842e65adc6db4393 (2021).
Sessions, Z. et al. Recent progress on cheminformatics approaches to epigenetic drug discovery. Drug Discov. Today 25, 2268–2276 (2020).
Coley, C. W. Defining and exploring chemical spaces. Trends Chem. 3, 133–145 (2021).
Irwin, J. J. et al. ZINC20—a free ultralarge-scale chemical database for ligand discovery. J. Chem. Inf. Model. 60, 6065–6073 (2020).
Enamine. REAL Database https://enamine.net/library-synthesis/real-compounds/real-database# (2021).
Enamine. REAL Space https://enamine.net/compound-collections/real-compounds/real-space-navigator (2021).
Hawkins, P. C. D., Skillman, A. G., Warren, G. L., Ellingson, B. A. & Stahl, M. T. Conformer generation with OMEGA: algorithm and validation using high quality structures from the Protein Databank and Cambridge Structural Database. J. Chem. Inf. Model. 50, 572–584 (2010).
The RDKit Documentation—The RDKit 2020.03.1 Documentation. https://www.rdkit.org/docs/ (2020).
QUACPAC 2.0.2.2. (OpenEye Scientific Software, 2019).
O’Boyle, N. M. et al. Open Babel: an open chemical toolbox. J. Cheminform. 3, 33 (2011).
Kochev, N. T., Paskaleva, V. H. & Jeliazkova, N. Ambit-Tautomer: an open source tool for tautomer generation. Mol. Inf. 32, 481–504 (2013).
Morgan, H. L. The generation of a unique machine description for chemical structures—a technique developed at Chemical Abstracts Service. J. Chem. Doc. 5, 107–113 (1965).
Rogers, D. & Hahn, M. Extended-connectivity fingerprints. J. Chem. Inf. Model. 50, 742–754 (2010).
Extended Connectivity Fingerprint ECFP https://docs.chemaxon.com/display/docs/extended-connectivity-fingerprint-ecfp.md (ChemAxon, 2021).
Maestro v9.3. (Schrödinger, 2019).
Molecular Operating Environment 2019 (Chemical Computing Group, 2019).
Moustakas, D. T. et al. Development and validation of a modular, extensible docking program: DOCK 5. J. Comput. Aided Mol. Des. 20, 601–619 (2006).
Shaffer, P. L., Jivan, A., Dollins, D. E., Claessens, F. & Gewirth, D. T. Structural basis of androgen receptor binding to selective androgen response elements. Proc. Natl Acad. Sci. USA. 101, 4758–4763 (2004).
Santos-Martins, D. et al. Accelerating AutoDock4 with GPUs and gradient-based local search. J. Chem. Theory Comput. 17, 1060–1073 (2021).
Alhossary, A., Handoko, S. D., Mu, Y. & Kwoh, C.-K. Fast, accurate, and reliable molecular docking with QuickVina 2. Bioinformatics 31, 2214–2216 (2015).
Abagyan, R., Totrov, M. & Kuznetsov, D. ICM—a new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. J. Comput. Chem. 15, 488–506 (1994).
Neves, M. A. C., Totrov, M. & Abagyan, R. Docking and scoring with ICM: the benchmarking results and strategies for improvement. J. Comput. Aided Mol. Des. 26, 675–686 (2012).
Giga Docking Guide—Orion Programming Guide. 1.0 documentation https://docs.eyesopen.com/orion-developer/2020-2-1/modules/large-scale-floes/docs/source/giga_docking_guide.html (OpenEye Software, 2020).
LeGrand, S. et al. GPU-accelerated drug discovery with docking on the Summit supercomputer: porting, optimization, and application to COVID-19 research. Preprint at https://arxiv.org/abs/2007.03678 (2020).
Bender, B. J. et al. A practical guide to large-scale docking. Nat. Protoc. 16, 4799–4832 (2021).
Jorgensen, W. L. The many roles of computation in drug discovery. Science 303, 1813–1818 (2004).
OEDOCKING v3.3.0.3 (OpenEye Scientific Software, 2021).
Abadi, M. et al. TensorFlow: a system for large-scale machine learning. Proceedings of the 12th USENIX Symposium on Operating Systems Design and Implementation, OSDI 2016 265–283 (The USENIX Association, 2016).
Pedregosa, F. et al. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2012).
Berman, H. M. The Protein Data Bank. Nucleic Acids Res 28, 235–242 (2000).
Morris, G. M. et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 19, 1639–1662 (1998).
Melo, F. Area under the ROC curve. in Encyclopedia of Systems Biology (eds. Dubitzky, W. et al.) 38–39 (Springer, 2013).
Hur, E. et al. Recognition and accommodation at the androgen receptor coactivator binding interface. PLoS Biol. 2, E274 (2004).
Melo, F. Receiver operating characteristic (ROC) curve. in Encyclopedia of Systems Biology (eds. Dubitzky, W. et al.) 1818–1823 (Springer, 2013).
Shen, Z. et al. Design of SARS-CoV-2 PLpro inhibitors for COVID-19 antiviral therapy leveraging binding cooperativity. J. Med. Chem. https://doi.org/10.1021/acs.jmedchem.1c01307 (2021).
Acknowledgements
F.G. is supported by fellowships from the Canadian Institutes for Health Research (MFE-171324), the Michael Smith Foundation for Health Research/VCHRI & VGH UBC Hospital Foundation (RT-2020-0408) and the Ermenegildo Zegna Foundation. F.B. is supported by a UBC Data Science Institute fellowship. We thank J. Irwin for his support in sharing the DD-prepared version of the ZINC20 library.
Author information
Authors and Affiliations
Contributions
F.G. and A.C. conceived the work. F.G. wrote the manuscript with the help of M.F., J.C.Y., J.G., A.-T.T. and F.B. F.G. developed the protocol, with the help of J.C.Y., J.G. and A.S. J.C.Y., J.G. and F.G. wrote the current version of the code. A.-T.T. and M.F. provided support with critical evaluation and tested user-friendliness of the protocol. A.S. contributed to discussing and revising the protocol. A.C. supervised experiments and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks John Karanicolas and Ying Yang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Gentile, F. et al. Chem. Sci. 12, 15960–15974 (2021): https://doi.org/10.1039/d1sc05579h
Gentile, F. et al. ACS Cent. Sci. 6, 939–949 (2020): https://doi.org/10.1021/acscentsci.0c00229
Ton, A.-T. et al. Mol. Inform. 39, e2000028 (2020): https://doi.org/10.1002/minf.202000028
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2, Supplementary Figs. 1 and 2 and Supplementary References.
Supplementary Table 3
evaluation.csv file obtained from evaluating different training sizes in one DD iteration, screening the ZINC20 library against the AR dimerization site (PDB ID: 1R4I ; ref. 40) using Glide SP for docking and a recall of 0.90. Validation and test sets comprised 700,000 molecules each.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gentile, F., Yaacoub, J.C., Gleave, J. et al. Artificial intelligence–enabled virtual screening of ultra-large chemical libraries with deep docking. Nat Protoc 17, 672–697 (2022). https://doi.org/10.1038/s41596-021-00659-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-021-00659-2
This article is cited by
-
Developments and challenges in hit progression within fragment-based drug discovery
Nature Communications (2026)
-
Active learning enables generation of molecules that advance the known Pareto front
npj Computational Materials (2026)
-
Quantum-machine-assisted drug discovery
npj Drug Discovery (2026)
-
SLICE (SMARTS and Logic In ChEmistry): fast generation of molecules using advanced chemical synthesis logic and modern coding style
Journal of Cheminformatics (2025)
-
UMAP-based clustering split for rigorous evaluation of AI models for virtual screening on cancer cell lines*
Journal of Cheminformatics (2025)