Abstract
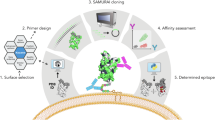
Antibodies play an important role in the immune system by binding to molecules called antigens at their respective epitopes. These interfaces or epitopes are structural entities determined by the interactions between an antibody and an antigen, making them ideal systems to analyze by using docking programs. Since the advent of high-throughput antibody sequencing, the ability to perform epitope mapping using only the sequence of the antibody has become a high priority. ClusPro, a leading protein–protein docking server, together with its template-based modeling version, ClusPro-TBM, have been re-purposed to map epitopes for specific antibody–antigen interactions by using the Antibody Epitope Mapping server (AbEMap). ClusPro-AbEMap offers three different modes for users depending on the information available on the antibody as follows: (i) X-ray structure, (ii) computational/predicted model of the structure or (iii) only the amino acid sequence. The AbEMap server presents a likelihood score for each antigen residue of being part of the epitope. We provide detailed information on the server’s capabilities for the three options and discuss how to obtain the best results. In light of the recent introduction of AlphaFold2 (AF2), we also show how one of the modes allows users to use their AF2-generated antibody models as input. The protocol describes the relative advantages of the server compared to other epitope-mapping tools, its limitations and potential areas of improvement. The server may take 45–90 min depending on the size of the proteins.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
Data availability
Data for all cases tested in the benchmark set used can be found in the following figshare link: https://doi.org/10.6084/m9.figshare.c.6295842.v1. Detailed results and direct links for the server results are shown in Anticipated results.
Code availability
AbeMap is available as a server at https://abemap.cluspro.org/ free of charge for non-commercial applications. The server can be used without registration, but in that case, the results will be publicly accessible. The advantage of registering is that the job does not show up on the website, but this option is available only to users with educational or governmental email addresses. The server provides options to view the results online, but protein visualization tools allow for more convenient analyses. We use and recommend PyMOL, which was used to demonstrate the analysis of results in this protocol.
References
Montgomery, R. A., Cozzi, E., West, L. J. & Warren, D. S. Humoral immunity and antibody-mediated rejection in solid organ transplantation. Semin. Immunol. 23, 224–234 (2011).
Sela-Culang, I., Kunik, V. & Ofran, Y. The structural basis of antibody-antigen recognition. Front. Immunol. 4, 302 (2013).
Danilov, S. M. et al. Fine epitope mapping of monoclonal antibody 5F1 reveals anticatalytic activity toward the N domain of human angiotensin-converting enzyme. Biochemistry 46, 9019–9031 (2007).
Sela-Culang, I. et al. Using a combined computational-experimental approach to predict antibody-specific B cell epitopes. Structure 22, 646–657 (2014).
Ehrhardt, S. A. et al. Polyclonal and convergent antibody response to Ebola virus vaccine rVSV-ZEBOV. Nat. Med. 25, 1589–1600 (2019).
Goldstein, L. D. et al. Massively parallel single-cell B-cell receptor sequencing enables rapid discovery of diverse antigen-reactive antibodies. Commun. Biol. 2, 304 (2019).
Horns, F., Dekker, C. L. & Quake, S. R. Memory B cell activation, broad anti-influenza antibodies, and bystander activation revealed by single-cell transcriptomics. Cell Rep. 30, 905–913.e6 (2020).
Kozlova, E. E. G. et al. Computational B-cell epitope identification and production of neutralizing murine antibodies against Atroxlysin-I. Sci. Rep. 8, 14904 (2018).
Hua, C. K. et al. Computationally-driven identification of antibody epitopes. Elife 6, e29023 (2017).
Qi, T. et al. SEPPA 2.0—more refined server to predict spatial epitope considering species of immune host and subcellular localization of protein antigen. Nucleic Acids Res. 42, W59–W63 (2014).
Sun, J. et al. SEPPA: a computational server for spatial epitope prediction of protein antigens. Nucleic Acids Res. 37, W612–W616 (2009).
Zhou, C. et al. SEPPA 3.0-enhanced spatial epitope prediction enabling glycoprotein antigens. Nucleic Acids Res. 47, W388–W394 (2019).
Sweredoski, M. J. & Baldi, P. PEPITO: improved discontinuous B-cell epitope prediction using multiple distance thresholds and half sphere exposure. Bioinformatics 24, 1459–1460 (2008).
Rubinstein, N. D., Mayrose, I., Martz, E. & Pupko, T. Epitopia: a web-server for predicting B-cell epitopes. BMC Bioinforma. 10, 287 (2009).
Kulkarni-Kale, U., Bhosle, S. & Kolaskar, A. S. CEP: a conformational epitope prediction server. Nucleic Acids Res. 33, W168–W171 (2005).
Hopp, T. P. & Woods, K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl Acad. Sci. USA 78, 3824–3828 (1981).
Jespersen, M. C., Peters, B., Nielsen, M. & Marcatili, P. BepiPred-2.0: improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res. 45, W24–W29 (2017).
Potocnakova, L., Bhide, M. & Pulzova, L. B. An introduction to B-cell epitope mapping and in silico epitope prediction. J. Immunol. Res. 2016, 6760830 (2016).
Holmes, M. A., Buss, T. N. & Foote, J. Conformational correction mechanisms aiding antigen recognition by a humanized antibody. J. Exp. Med. 187, 479–485 (1998).
Li, Y., Li, H., Smith-Gill, S. J. & Mariuzza, R. A. Three-dimensional structures of the free and antigen-bound Fab from monoclonal antilysozyme antibody HyHEL-63. Biochemistry 39, 6296–6309 (2000).
Stanfield, R. L., Dooley, H., Verdino, P., Flajnik, M. F. & Wilson, I. A. Maturation of shark single-domain (IgNAR) antibodies: evidence for induced-fit binding. J. Mol. Biol. 367, 358–372 (2007).
Braden, B. C. et al. Three-dimensional structures of the free and the antigen-complexed Fab from monoclonal anti-lysozyme antibody D44.1. J. Mol. Biol. 243, 767–781 (1994).
Halperin, I., Ma, B., Wolfson, H. & Nussinov, R. Principles of docking: an overview of search algorithms and a guide to scoring functions. Proteins 47, 409–443 (2002).
Comeau, S. R., Gatchell, D. W., Vajda, S. & Camacho, C. J. ClusPro: a fully automated algorithm for protein-protein docking. Nucleic Acids Res. 32, W96–W99 (2004).
Comeau, S. R., Gatchell, D. W., Vajda, S. & Camacho, C. J. ClusPro: an automated docking and discrimination method for the prediction of protein complexes. Bioinformatics 20, 45–50 (2004).
Kozakov, D. et al. The ClusPro web server for protein-protein docking. Nat. Protoc. 12, 255–278 (2017).
Kozakov, D., Brenke, R., Comeau, S. R. & Vajda, S. PIPER: an FFT-based protein docking program with pairwise potentials. Proteins 65, 392–406 (2006).
Brenke, R. et al. Application of asymmetric statistical potentials to antibody-protein docking. Bioinformatics 28, 2608–2614 (2012).
Guest, J. D. et al. An expanded benchmark for antibody-antigen docking and affinity prediction reveals insights into antibody recognition determinants. Structure 29, 606–621.e5 (2021).
Krawczyk, K., Liu, X., Baker, T., Shi, J. & Deane, C. M. Improving B-cell epitope prediction and its application to global antibody-antigen docking. Bioinformatics 30, 2288–2294 (2014).
Krawczyk, K., Baker, T., Shi, J. & Deane, C. M. Antibody i-Patch prediction of the antibody binding site improves rigid local antibody-antigen docking. Protein Eng. Des. Sel. 26, 621–629 (2013).
Sikora, M. et al. Computational epitope map of SARS-CoV-2 spike protein. PLoS Comput. Biol. 17, e1008790 (2021).
Marks, C. & Deane, C. M. How repertoire data are changing antibody science. J. Biol. Chem. 295, 9823–9837 (2020).
Vajda, S., Porter, K. A. & Kozakov, D. Progress toward improved understanding of antibody maturation. Curr. Opin. Struct. Biol. 67, 226–231 (2021).
Porter, K. A. et al. Template-based modeling by ClusPro in CASP13 and the potential for using co-evolutionary information in docking. Proteins 87, 1241–1248 (2019).
Padhorny, D. et al. Protein-protein docking by fast generalized Fourier transforms on 5D rotational manifolds. Proc. Natl Acad. Sci. USA 113, E4286–E4293 (2016).
Ngan, C. H. et al. FTSite: high accuracy detection of ligand binding sites on unbound protein structures. Bioinformatics 28, 286–287 (2012).
Desta, I. T. et al. Mapping of antibody epitopes based on docking and homology modeling. Proteins 91, 171–182 (2023).
Jumper, J. et al. Applying and improving AlphaFold at CASP14. Proteins 89, 1711–1721 (2021).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Tunyasuvunakool, K. et al. Highly accurate protein structure prediction for the human proteome. Nature 596, 590–596 (2021).
Evans, R. et al. Protein complex prediction with AlphaFold-Multimer. Preprint at https://www.biorxiv.org/content/10.1101/2021.10.04.463034v2 (2021).
Ghani, U. et al. Improved docking of protein models by a combination of Alphafold2 and ClusPro. Preprint at https://www.biorxiv.org/content/10.1101/2021.09.07.459290v1 (2021).
Ko, J. & Lee, J. Can AlphaFold2 predict protein-peptide complex structures accurately? Preprint at https://www.biorxiv.org/content/10.1101/2021.07.27.453972v1.full (2021).
Mirdita, M., Ovchinnikov, S. & Steinegger, M. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Desta, I. T., Porter, K. A., Xia, B., Kozakov, D. & Vajda, S. Performance and its limits in rigid body protein-protein docking. Structure 28, 1071–1081.e3 (2020).
Webb, B. & Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Prot. Sci. 86, 2.9.1–2.9.37 (2016).
Katchalski-Katzir, E. et al. Molecular surface recognition: determination of geometric fit between proteins and their ligands by correlation techniques. Proc. Natl Acad. Sci. USA 89, 2195–2199 (1992).
Lindemann, S. R., Yershova, A. & LaValle, S. M. Incremental grid sampling strategies in robotics. In Algorithmic Foundations of Robotics VI (eds Erdmann, M., Overmars, M., Hsu, D., & van der Stappen, F.) 313–328 (Springer Berlin, Heidelberg, 2005).
Chuang, G. Y., Kozakov, D., Brenke, R., Comeau, S. R. & Vajda, S. DARS (Decoys As the Reference State) potentials for protein-protein docking. Biophys. J. 95, 4217–4227 (2008).
Lee, B. & Richards, F. M. The interpretation of protein structures: estimation of static accessibility. J. Mol. Biol. 55, 379–400 (1971).
Vreven, T. et al. Updates to the integrated protein-protein interaction benchmarks: docking benchmark version 5 and affinity benchmark version 2. J. Mol. Biol. 427, 3031–3041 (2015).
Fox, N. K., Brenner, S. E. & Chandonia, J. M. SCOPe: structural classification of proteins—extended, integrating SCOP and ASTRAL data and classification of new structures. Nucleic Acids Res. 42, D304–D309 (2014).
Akbar, R. et al. A compact vocabulary of paratope-epitope interactions enables predictability of antibody-antigen binding. Cell Rep. 34, 108856 (2021).
Salamanca Viloria, J., Allega, M. F., Lambrughi, M. & Papaleo, E. An optimal distance cutoff for contact-based Protein Structure Networks using side-chain centers of mass. Sci. Rep. 7, 2838 (2017).
Stave, J. W. & Lindpaintner, K. Antibody and antigen contact residues define epitope and paratope size and structure. J. Immunol. 191, 1428–1435 (2013).
Pittala, S. & Bailey-Kellogg, C. Learning context-aware structural representations to predict antigen and antibody binding interfaces. Bioinformatics 36, 3996–4003 (2020).
Sivasubramanian, A., Sircar, A., Chaudhury, S. & Gray, J. J. Toward high-resolution homology modeling of antibody Fv regions and application to antibody-antigen docking. Proteins 74, 497–514 (2009).
Padhorny, D. et al. ClusPro in rounds 38 to 45 of CAPRI: toward combining template-based methods with free docking. Proteins 88, 1082–1090 (2020).
Weitzner, B. D. et al. Modeling and docking of antibody structures with Rosetta. Nat. Protoc. 12, 401–416 (2017).
Lepore, R., Olimpieri, P. P., Messih, M. A. & Tramontano, A. PIGSPro: prediction of immunoGlobulin structures v2. Nucleic Acids Res. 45, W17–W23 (2017).
Klausen, M. S., Anderson, M. V., Jespersen, M. C., Nielsen, M. & Marcatili, P. LYRA, a webserver for lymphocyte receptor structural modeling. Nucleic Acids Res. 43, W349–W355 (2015).
Schritt, D. et al. Repertoire Builder: high-throughput structural modeling of B and T cell receptors. Mol. Syst. Des. Eng. 4, 761–768 (2019).
Karami, Y. et al. DaReUS-Loop: a web server to model multiple loops in homology models. Nucleic Acids Res. 47, W423–W428 (2019).
Dunbar, J. et al. SAbPred: a structure-based antibody prediction server. Nucleic Acids Res. 44, W474–W478 (2016).
Marks, C. & Deane, C. M. Antibody H3 structure prediction. Comput. Struct. Biotechnol. J. 15, 222–231 (2017).
Lensink, M. F. et al. Blind prediction of homo- and hetero-protein complexes: the CASP13-CAPRI experiment. Proteins 87, 1200–1221 (2019).
Ruffolo, J. A., Guerra, C., Mahajan, S. P., Sulam, J. & Gray, J. J. Geometric potentials from deep learning improve prediction of CDR H3 loop structures. Bioinformatics 36, i268–i275 (2020).
Jespersen, M. C., Mahajan, S., Peters, B., Nielsen, M. & Marcatili, P. Antibody specific B-Cell epitope predictions: leveraging information from antibody-antigen protein complexes. Front. Immunol. 10, 298 (2019).
Antonyuk, S. V. et al. Crystal structure of human prion protein bound to a therapeutic antibody. Proc. Natl Acad. Sci. USA 106, 2554–2558 (2009).
Maun, H. R. et al. Hedgehog pathway antagonist 5E1 binds hedgehog at the pseudo-active site. J. Biol. Chem. 285, 26570–26580 (2010).
Acknowledgements
This investigation was supported by grants DBI 1759277 and AF 1645512 from the National Science Foundation and R35GM118078, R21GM127952 and RM1135136 from the National Institute of General Medical Sciences.
Author information
Authors and Affiliations
Contributions
I.T.D., S.K., D.K., D.B. and Y.K. developed the methodology. I.T.D., S.K. and G.J. developed the server. I.T.D., U.G., M.A. and D.B. performed the experiments. I.T.D., S.K. and S.V. prepared the manuscript. D.K. and D.M.S. reviewed the paper.
Corresponding authors
Ethics declarations
Competing interests
The PIPER docking program, on which the ClusPro AbEMap server is based, has been licensed by Boston University to Acpharis Inc. Acpharis, in turn, offers commercial sublicenses of PIPER. D.K. and S.V. consult for Acpharis and own stock in the company, and D.B. is the acting CEO of the company. However, both the ClusPro and AbEMap servers are free for non-commercial use.
Peer review
Peer review information
Nature Protocols thanks Pritam Kumar Panda, Mohammad Mostafa Pourseif and Bruno Villoutreix for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Desta, I. T. et al. Proteins 91, 171–182 (2023): https://doi.org/10.1002/prot.26420
Porter, K. A. et al. Proteins 87, 1241–1248 (2019): https://doi.org/10.1002/prot.25808
Kozakov, D. et al. Nat. Protoc. 12, 255–278 (2017): https://doi.org/10.1038/nprot.2016.169
Padhorny, D. et al. Proc. Natl Acad. Sci. USA 113, E4286–E4293 (2016): https://doi.org/10.1073/pnas.1603929113
Ngan, C. H. et al. Bioinformatics 28, 286–287 (2012): https://doi.org/10.1093/bioinformatics/btr651
Key data used in this protocol
Desta, I. T. et al. Proteins 91, 171–182 (2023): https://doi.org/10.1002/prot.26420
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2 and Tables 1–3
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Desta, I.T., Kotelnikov, S., Jones, G. et al. The ClusPro AbEMap web server for the prediction of antibody epitopes. Nat Protoc 18, 1814–1840 (2023). https://doi.org/10.1038/s41596-023-00826-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-023-00826-7
This article is cited by
-
Computational and experimental discovery of peptide inhibitors targeting survivin for therapeutic potential in cancer
Scientific Reports (2025)
-
The evolutionary features and roles of single nucleotide variants and charged amino acid mutations in influenza outbreaks during NPI period
Scientific Reports (2024)
-
InflANNet: a neural network predictor for Influenza A CTL and HTL epitopes to aid robust vaccine design
Bulletin of the National Research Centre (2023)