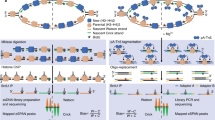
Abstract
Seamless site-directed mutagenesis is an important technique for studying protein functions, tuning enzyme catalytic activities and modifying genetic elements in multiple rounds because it can insert, delete or substitute nucleotides, DNA segments or even entire genes at the target site without introducing any unwanted change. To facilitate seamless site-directed mutagenesis in large plasmids and bacterial artificial chromosomes (BACs) with repetitive sequences, we recently developed the RedEx strategy. Compared with previous methods, our approach achieves the recovery of correct recombinants with high accuracy by circumventing unwanted recombination between repetitive sequences. RedEx readily yields more than 80% accuracy in seamless DNA insertion and deletion in large multimodular polyketide synthase gene clusters, which are among the most difficult targets due to the large number of repetitive DNA sequences in modules encoding almost identical enzymes. Here we present the RedEx method by describing in detail the seamless site-directed mutagenesis in a BAC vector. Overall, the process includes three parts: (1) insertion of the RedEx cassette containing the desired mutation together with selection–counterselection markers flanked by unique restriction sites and 20-bp overlapping sequences into the target site by recombineering, (2) removal of the selection–counterselection markers in the BAC by restriction digestion and (3) circularization of the linear BAC by exonuclease-mediated in vitro DNA annealing. This protocol can be performed within 3 weeks and will enable researchers with DNA cloning experience to master seamless site-directed mutagenesis to accelerate their research.
Key points
-
By combining recombineering, ccdB counterselection and exonuclease-mediated in vitro annealing, RedEx achieves seamless mutagenesis of large DNA molecules, including plasmids, fosmids and BACs.
-
Compared with CRISPR-based approaches, this method allows the efficient editing of highly repetitive, multimodular gene clusters and represents a powerful tool for modifying biosynthesis pathways and generating new natural products.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data supporting the findings of this study are available in the supporting primary research paper12.
References
Atanasov, A. G., Zotchev, S. B., Dirsch, V. M., the International Natural Product Sciences Taskforce & Supuran, C. T. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 20, 200–216 (2021).
Smanski, M. J. et al. Synthetic biology to access and expand nature’s chemical diversity. Nat. Rev. Microbiol. 14, 135–149 (2016).
Rutledge, P. J. & Challis, G. L. Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol. 13, 509–523 (2015).
Dutta, S. et al. Structure of a modular polyketide synthase. Nature 510, 512–517 (2014).
Bagde, S. R., Mathews, I. I., Fromme, J. C. & Kim, C. Y. Modular polyketide synthase contains two reaction chambers that operate asynchronously. Science 374, 723–729 (2021).
Grininger, M. Enzymology of assembly line synthesis by modular polyketide synthases. Nat. Chem. Biol. 19, 401–415 (2023).
Winn, M., Fyans, J. K., Zhuo, Y. & Micklefield, J. Recent advances in engineering nonribosomal peptide assembly lines. Nat. Prod. Rep. 33, 317–347 (2016).
Tao, X. B. et al. ClusterCAD 2.0: an updated computational platform for chimeric type I polyketide synthase and nonribosomal peptide synthetase design. Nucleic Acids Res. 51, D532–D538 (2023).
Englund, E. et al. Expanding extender substrate selection for unnatural polyketide biosynthesis by acyltransferase domain exchange within a modular polyketide synthase. J. Am. Chem. Soc. 145, 8822–8832 (2023).
Wlodek, A. et al. Diversity oriented biosynthesis via accelerated evolution of modular gene clusters. Nat. Commun. 8, 1206 (2017).
Ji, C. H. et al. Top-down synthetic biology approach for titer improvement of clinically important antibiotic daptomycin in Streptomyces roseosporus. Metab. Eng. 69, 40–49 (2022).
Song, C. et al. RedEx: a method for seamless DNA insertion and deletion in large multimodular polyketide synthase gene clusters. Nucleic Acids Res. 48, e130 (2020).
Zhang, Y., Buchholz, F., Muyrers, J. P. P. & Stewart, A. F. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet. 20, 123–128 (1998).
Zhang, Y., Muyrers, J. P. P., Testa, G. & Stewart, A. F. DNA cloning by homologous recombination in Escherichia coli. Nat. Biotechnol. 18, 1314–1317 (2000).
Sarov, M. et al. A recombineering pipeline for functional genomics applied to Caenorhabditis elegans. Nat. Methods 3, 839–844 (2006).
Bird, A. W. et al. High-efficiency counterselection recombineering for site-directed mutagenesis in bacterial artificial chromosomes. Nat. Methods 9, 103–109 (2012).
Wang, H. et al. Improved seamless mutagenesis by recombineering using ccdB for counterselection. Nucleic Acids Res. 42, e37 (2014).
Wang, H. et al. RecET direct cloning and Redαβ recombineering of biosynthetic gene clusters, large operons or single genes for heterologous expression. Nat. Protoc. 11, 1175–1190 (2016).
Wang, H. H. et al. Programming cells by multiplex genome engineering and accelerated evolution. Nature 460, 894–898 (2009).
Isaacs, F. J. et al. Precise manipulation of chromosomes in vivo enables genome-wide codon replacement. Science 333, 348–353 (2011).
Gallagher, R. R., Li, Z., Lewis, A. O. & Isaacs, F. J. Rapid editing and evolution of bacterial genomes using libraries of synthetic DNA. Nat. Protoc. 9, 2301–2316 (2014).
Fredens, J. et al. Total synthesis of Escherichia coli with a recoded genome. Nature 569, 514–518 (2019).
Sharan, S. K., Thomason, L. C., Kuznetsov, S. G. & Court, D. L. Recombineering: a homologous recombination-based method of genetic engineering. Nat. Protoc. 4, 206–223 (2009).
Li, R., Li, A., Zhang, Y. & Fu, J. The emerging role of recombineering in microbiology. Eng. Microbiol. 3, 100097 (2023).
Zheng, L., Baumann, U. & Reymond, J. L. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 32, e115 (2004).
Muyrers, J. P. P. et al. Point mutation of bacterial artificial chromosomes by ET recombination. EMBO Rep. 1, 239–243 (2000).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Wang, H. et al. ExoCET: exonuclease in vitro assembly combined with RecET recombination for highly efficient direct DNA cloning from complex genomes. Nucleic Acids Res. 46, e28 (2018).
Wong, Q. N. et al. Efficient and seamless DNA recombineering using a thymidylate synthase A selection system in Escherichia coli. Nucleic Acids Res. 33, e59 (2005).
Warming, S., Costantino, N., Court, D. L., Jenkins, N. A. & Copeland, N. G. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33, e36 (2005).
DeVito, J. A. Recombineering with tolC as a selectable/counter-selectable marker: remodeling the rRNA operons of Escherichia coli. Nucleic Acids Res. 36, e4 (2008).
Kudo, K. et al. In vitro Cas9-assisted editing of modular polyketide synthase genes to produce desired natural product derivatives. Nat. Commun. 11, 4022 (2020).
Shao, Z. & Zhao, H. Manipulating natural product biosynthetic pathways via DNA assembler. Curr. Protoc. Chem. Biol. 6, 65–100 (2014).
Luo, Y., Zhang, L., Barton, K. W. & Zhao, H. Systematic identification of a panel of strong constitutive promoters from Streptomyces albus. ACS Synth. Biol. 4, 1001–1010 (2015).
Engler, C., Kandzia, R. & Marillonnet, S. A one pot, one step, precision cloning method with high throughput capability. PLoS ONE 3, e3647 (2008).
Scior, A., Preissler, S., Koch, M. & Deuerling, E. Directed PCR-free engineering of highly repetitive DNA sequences. BMC Biotechnol. 11, 87 (2011).
Iacovelli, R., Zwahlen, R. D., Bovenberg, R. A. L. & Driessen, A. J. M. Biochemical characterization of the Nocardia lactamdurans ACV synthetase. PLoS ONE 15, e0231290 (2020).
Bian, X., Plaza, A., Yan, F., Zhang, Y. & Müller, R. Rational and efficient site-directed mutagenesis of adenylation domain alters relative yields of luminmide derivatives in vivo. Biotechnol. Bioeng. 112, 1343–1353 (2015).
Zhong, L. et al. Engineering and elucidation of the lipoinitiation process in nonribosomal peptide biosynthesis. Nat. Commun. 12, 296 (2021).
Soeriyadi, A. H. et al. Tailoring enzyme stringency masks the multispecificity of a lyngbyatoxin (indolactam alkaloid) nonribosomal peptide synthetase. ChemBioChem 23, e202100574 (2022).
Konigs, V. et al. SRSF7 maintains its homeostasis through the expression of split-ORFs and nuclear body assembly. Nat. Struct. Mol. Biol. 27, 260–273 (2020).
Baker, O. et al. The contribution of homology arms to nuclease-assisted genome engineering. Nucleic Acids Res. 45, 8105–8115 (2017).
Medina-Ruiz, L. et al. Analysis of combinatorial chemokine receptor expression dynamics using multi-receptor reporter mice. eLife 11, e72418 (2022).
Blanch-Asensio, A. et al. STRAIGHT-IN enables high-throughput targeting of large DNA payloads in human pluripotent stem cells. Cell Rep. Methods 2, 100300 (2022).
Botti, V. et al. Cellular differentiation state modulates the mRNA export activity of SR proteins. J. Cell Biol. 216, 1993–2009 (2017).
Bahlmann, N. A. et al. Properties of adenovirus vectors with increased affinity to DSG2 and the potential benefits of oncolytic approaches and gene therapy. Viruses 14, 1835 (2022).
Hidalgo, P. et al. Evidence that the adenovirus single-stranded DNA binding protein mediates the assembly of biomolecular condensates to form viral replication compartments. Viruses 13, 1778 (2021).
Berman, C. M. et al. An adaptable platform for directed evolution in human cells. J. Am. Chem. Soc. 140, 18093–18103 (2018).
Zhang, W. et al. An engineered virus library as a resource for the spectrum-wide exploration of virus and vector diversity. Cell Rep. 19, 1698–1709 (2017).
Zhang, Y. et al. Fiber2 and hexon genes are closely associated with the virulence of the emerging and highly pathogenic fowl adenovirus 4. Emerg. Microbes Infect. 7, 199 (2018).
Doerner, J. et al. Novel group C oncolytic adenoviruses carrying a miRNA inhibitor demonstrate enhanced oncolytic activity in vitro and in vivo. Mol. Cancer Ther. 21, 460–470 (2022).
Cong, L. et al. Multiplex genome engineering using CRISPR–Cas systems. Science 339, 819–823 (2013).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Wang, Z. J. et al. Engineered biosynthesis of complex disorazol polyketides in a streamlined Burkholderia thailandensis. ACS Synth. Biol. 12, 971–977 (2023).
Wang, Z. J. et al. Engineering of Burkholderia thailandensis strain E264 serves as a chassis for expression of complex specialized metabolites. Front. Microbiol. 13, 1073243 (2022).
Yi, J. et al. High-efficiency genetic engineering toolkit for virus based on lambda red-mediated recombination. Biotechnol. Lett. 45, 1327–1337 (2023).
Song, C. et al. Enhanced heterologous spinosad production from a 79-kb synthetic multi-operon assembly. ACS Synth. Biol. 8, 137–147 (2019).
Jiang, C. et al. Establishing an efficient salinomycin biosynthetic pathway in three heterologous Streptomyces hosts by constructing a 106-kb multioperon artificial gene cluster. Biotechnol. Bioeng. 118, 4668–4677 (2021).
Fu, J. et al. Full-length RecE enhances linear-linear homologous recombination and facilitates direct cloning for bioprospecting. Nat. Biotechnol. 30, 440–446 (2012).
Li, M. Z. & Elledge, S. J. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat. Methods 4, 251–256 (2007).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32122049); Natural Science Foundation of Shandong Province (ZR2022JQ11 and ZR2019ZD22); National Key Research and Development Program of China (2018YFA0900400, 2021YFC2101000 and 2019YFA0904000); the Fund for Distinguished Young Scholars of SDU; the Fundamental Research Funds of Shandong University (2023QNTD001); Qingdao Key Technology Research and Industrialization Demonstration Project (22-3-4-xxgg-1-nsh); and the 111 Project (B16030). The authors acknowledge J. Qu, J. Zhu and Z. Li of the Core Facilities for Life and Environmental Sciences, State Key laboratory of Microbial Technology of Shandong University for their technical assistance.
Author information
Authors and Affiliations
Contributions
J.L., A.F.S., J.F., Y.Z. and H.W. designed and supervised the project. J.L., C.S., Y.L., R.H., R.G., Q.C., C.J., X.L. and K.H. performed the experiments. J.L. and H.W. wrote the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Imre Berger, Hang Wu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Wang, H. et al. Nucleic Acids Res. 42, e37 (2014): https://doi.org/10.1093/nar/gkt1339
Song, C. et al. Nucleic Acids Res. 48, e130 (2020): https://doi.org/10.1093/nar/gkaa956
Zhang, Y. et al. Nat. Genet. 20, 123–128 (1998): https://doi.org/10.1038/2417
Muyrers, J. P. P. et al. EMBO Rep. 1, 239–243 (2000): https://doi.org/10.1093/embo-reports/kvd049
Bird, A. W. et al. Nat. Methods 9, 103–109 (2012): https://doi.org/10.1038/nmeth.1803
Supplementary information
Supplementary Information
Supplementary Notes 1–4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luan, J., Song, C., Liu, Y. et al. Seamless site-directed mutagenesis in complex cloned DNA sequences using the RedEx method. Nat Protoc 19, 3360–3388 (2024). https://doi.org/10.1038/s41596-024-01016-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-024-01016-9