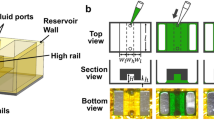
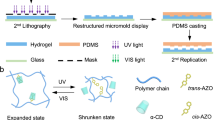
Abstract
Perfusable hydrogels have garnered substantial attention in recent years for the fabrication of microphysiological systems. However, current methodologies to fabricate microchannels in hydrogel platforms involve sophisticated equipment and techniques, which hinder progress of the field. In this protocol, we present a cost-effective, simple, versatile and ultrafast method to create perfusable microchannels of complex shapes in photopolymerizable hydrogels. Our method uses one-step UV photocross-linking and a photomask printed on inexpensive transparent films, to photopattern both synthetic (PEG-norbornene) and natural (hyaluronic acid-norbornene) hydrogels in just 0.8 s. Moreover, these perfusable hydrogels are fully integrated into a custom-made microfluidic device that allows continuous fluid perfusion when connected to an external pump system. This methodology can be easily reproduced by professionals with basic laboratory skills and a fundamental knowledge of polymers and materials science. In this protocol, we demonstrate the functionality of our photopatterned hydrogels by seeding human endothelial cells into the microchannels, culturing them under dynamic conditions for 7 d, and exposing them to inflammatory stimuli to elicit cellular responses. This highlights the versatility of our platform in fabricating microphysiological systems and different microenvironments. The fabrication of perfusable channels within the hydrogels, including the fabrication of the microfluidic devices, requires ~3 d. The development of the cell-seeded microphysiological system, including the stimulation of cells, takes ~7 d. In conclusion, our approach provides a straightforward and widely applicable solution to simplify and reduce the cost of biofabrication techniques for developing functional in vitro models using perfusable three-dimensional hydrogels.
Key points
-
This protocol describes the fabrication of perfusable microchannels of complex shapes by photopatterning hydrogels. It uses photomask transparency films to photopattern hydrogels and includes representative examples to recreate different physiological microenvironments.
-
This is performed by a one-step UV light-triggered cross-linking using a photomask printed on inexpensive transparent films. Compared with existing methods for fabricating perfusable hydrogels, this makes it a cost-effective, facile, versatile and ultrafast approach.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The main data discussed in this protocol are available in the supporting primary research paper4.
References
Menon, N. V., Tay, H. M., Wee, S. N., Li, K. H. H. & Hou, H. W. Micro-engineered perfusable 3D vasculatures for cardiovascular diseases. Lab Chip 17, 2960–2968 (2017).
Liu, Z. et al. Soft ionic devices by perfusable all-hydrogel microfluidics. J. Mater. Chem. C. 8, 2320–2325 (2020).
Liu, H. et al. Advances in hydrogels in organoids and organs-on-a-chip. Adv. Mater. 31, 1902042 (2019).
Mora-Boza, A. et al. Facile photopatterning of perfusable microchannels in synthetic hydrogels to recreate microphysiological environments. Adv. Mater. 35, e2306765 (2023).
Nikolaev, M. et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 585, 574–578 (2020).
Khan, O. F. & Sefton, M. V. Patterning collagen/poloxamine-methacrylate hydrogels for tissue-engineering-inspired microfluidic and laser lithography applications. J. Biomater. Sci. Polym. Ed. 22, 2499–2514 (2011).
Lin, C. C., Ki, C. S. & Shih, H. Thiol-norbornene photo-click hydrogels for tissue engineering applications. J. Appl. Polym. Sci. https://doi.org/10.1002/app.41563 (2015).
Gumuscu, B., Bomer, J. G., van den Berg, A. & Eijkel, J. C. Photopatterning of hydrogel microarrays in closed microchips. Biomacromolecules 16, 3802–3810 (2015).
Jiang, Z., Xia, B., McBride, R. & Oakey, J. A microfluidic-based cell encapsulation platform to achieve high long-term cell viability in photopolymerized PEGNB hydrogel microspheres. J. Mater. Chem. B 5, 173–180 (2017).
Ryma, M. et al. A print-and-fuse strategy for sacrificial filaments enables biomimetically structured perfusable microvascular networks with functional endothelium inside 3D hydrogels. Adv. Mater. 34, e2200653 (2022).
Xie, R., Zheng, W., Guan, L., Ai, Y. & Liang, Q. Engineering of hydrogel materials with perfusable microchannels for building vascularized tissues. Small 16, e1902838 (2020).
Davoodi, E. et al. Template-enabled biofabrication of thick 3D tissues with patterned perfusable macrochannels. Adv. Healthc. Mater. 11, e2102123 (2022).
Xue, D. et al. Projection-based 3D printing of cell patterning scaffolds with multiscale channels. ACS Appl. Mater. Interfaces 10, 19428–19435 (2018).
Elomaa, L., Lindner, M., Leben, R., Niesner, R. & Weinhart, M. In vitro vascularization of hydrogel-based tissue constructs via a combined approach of cell sheet engineering and dynamic perfusion cell culture. Biofabrication https://doi.org/10.1088/1758-5090/ac9433 (2022).
Soliman, B. G. et al. Development and characterization of gelatin-norbornene bioink to understand the interplay between physical architecture and micro-capillary formation in biofabricated vascularized constructs. Adv. Healthc. Mater. 11, e2101873 (2022).
Szklanny, A. A. et al. 3D bioprinting of engineered tissue flaps with hierarchical vessel networks (VesselNet) for direct host-to-implant perfusion. Adv. Mater. 33, 2102661 (2021).
Kolesky, D. B. et al. Bioprinting: 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 26, 2966–2966 (2014).
Hinton, T. J. et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 1, e1500758 (2015).
Valentin, T. M. et al. Stereolithographic printing of ionically-crosslinked alginate hydrogels for degradable biomaterials and microfluidics. Lab Chip 17, 3474–3488 (2017).
Im, H. et al. Fabrication of heterogeneous chemical patterns on stretchable hydrogels using single-photon lithography. Soft Matter 18, 4402–4413 (2022).
Zheng, Y. et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl Acad. Sci. USA 109, 9342–9347 (2012).
Grigoryan, B. et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364, 458–464 (2019).
Brady, S. R., Gohsman, S. B., Sepulveda, K. & Weaver, J. D. Engineering synthetic poly(ethylene) glycol-based hydrogels compatible with injection molding biofabrication. J. Biomed. Mater. Res. A 111, 814–824 (2023).
Davila, S., Cacheux, J. & Rodriguez, I. Microvessel-on-chip fabrication for the in vitro modeling of nanomedicine transport. ACS Omega 6, 25109–25115 (2021).
Wang, X. Y. et al. Engineering interconnected 3D vascular networks in hydrogels using molded sodium alginate lattice as the sacrificial template. Lab Chip 14, 2709–2716 (2014).
Tocchio, A. et al. Versatile fabrication of vascularizable scaffolds for large tissue engineering in bioreactor. Biomaterials 45, 124–131 (2015).
Qiu, Y. et al. Microvasculature-on-a-chip for the long-term study of endothelial barrier dysfunction and microvascular obstruction in disease. Nat. Biomed. Eng. 2, 453–463 (2018).
Lind, J. U. et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 16, 303–308 (2017).
Andrejecsk, J. W. & Hughes, C. C. Engineering perfused microvascular networks into microphysiological systems platforms. Curr. Opin. Biomed. Eng. 5, 74–81 (2018).
Rothbauer, M. et al. Recent advances in additive manufacturing and 3D bioprinting for organs-on-a-chip and microphysiological systems. Front. Bioeng. Biotechnol. 10, 837087 (2022).
Lim, K. S. et al. Fundamentals and applications of photo-cross-linking in bioprinting. Chem. Rev. 120, 10662–10694 (2020).
Duong, V. T. & Lin, C.-C. Digital light processing 3D bioprinting of gelatin-norbornene hydrogel for enhanced vascularization. Macromol. Biosci. 23, 2300213 (2023).
Yang, M. et al. Multi-material digital light processing (DLP) bioprinting of heterogeneous hydrogel constructs with perfusable networks. Adv. Funct. Mater. 34, 2316456 (2024).
Zhao, Z., Tian, X. & Song, X. Engineering materials with light: recent progress in digital light processing based 3D printing. J. Mater. Chem. C. 8, 13896–13917 (2020).
Shin, W. & Kim, H. J. 3D in vitro morphogenesis of human intestinal epithelium in a gut-on-a-chip or a hybrid chip with a cell culture insert. Nat. Protoc. 17, 910–939 (2022).
Cruz-Acuna, R. et al. PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery. Nat. Protoc. 13, 2102–2119 (2018).
McDonald, J. C. et al. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 21, 27–40 (2000).
Acknowledgements
The authors acknowledge financial support from the Wellcome Leap HOPE program awarded to A.S. and A.J.G. A.M.-B. acknowledges support from the European Research Executive Agency under Individual GLOBAL Marie Skłodowska-Curie Actions Fellowship (project no. 101028216 — SYNMAT FOR ORGANOIDS). A.M.-R. acknowledges support from the National Institute of Diabetes and Digestive and Kidney Diseases (F31 DK130581). N.D.C. and J.A.B. acknowledge the National Science Foundation through the Center for Engineering Mechanobiology STC (CMMI 15-48571). A.S. acknowledges the financial support from NIH/NCI (5R01CA238745-04).
Author information
Authors and Affiliations
Contributions
A.M.-B. designed the study, developed and optimized the protocol, performed the main experiments, interpreted the data and wrote the main manuscript. A.M.-R. performed the immunostaining experiments, imaged the samples and edited the manuscript. N.D.C. and J.A.B. provided the NorHA reagents and protocol, and edited the manuscript. E.O. contributed to the preparation of reagents, provided equipment information and edited the manuscript. A.S. and A.J.G. jointly conceived the study and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.M.-B., A.S. and A.J.G. are inventors on a patent application related to this work and owned by the Georgia Tech Research Corp. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Mora-Boza, A. et al. Adv. Mater. 35, 2306765 (2023): https://doi.org/10.1002/adma.202306765
Supporting information
Supplementary Data 1
Examples of perfusable channel designs.
Supplementary Data 2
Design of features for maskless instruments.
Supplementary Video 1
Fabrication steps.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mora-Boza, A., Mulero-Russe, A., Di Caprio, N. et al. Facile photopatterning of perfusable microchannels in hydrogels for microphysiological systems. Nat Protoc 20, 272–292 (2025). https://doi.org/10.1038/s41596-024-01041-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-024-01041-8