Abstract
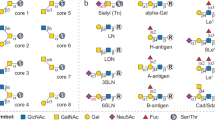
O-GalNAc glycans, also known as mucin-type O-glycans, are primary constituents of mucins on various mucosal sites of the body and also ubiquitously expressed on cell surface and secreted proteins. They have crucial roles in a wide range of physiological and pathological processes, including tumor growth and progression. In addition, altered expression of O-GalNAc glycans is frequently observed during different disease states. Research dedicated to unraveling the structure–function relationships of O-GalNAc glycans has led to the discovery of disease biomarkers and diagnostic tools and the development of O-glycopeptide-based cancer vaccines. Many of these efforts require amino acid-linked O-GalNAc core structures as building blocks to assemble complex O-glycans and glycopeptides. There are eight core structures (cores one to eight), from which all mucin-type O-glycans are derived. In this protocol, we describe the first divergent synthesis of all eight cores from a versatile precursor in practical scales. The protocol involves (i) chemical synthesis of the orthogonally protected precursor (3 days) from commercially available materials, (ii) chemical synthesis of five unique glycosyl donors (1–2 days for each donor) and (iii) selective deprotection of the precursor and assembly of the eight cores (2–4 days for each core). The procedure can be adopted to prepare O-GalNAc cores linked to serine, threonine and tyrosine, which can then be utilized directly for solid-phase glycopeptide synthesis or chemoenzymatic synthesis of complex O-glycans. The procedure empowers researchers with fundamental organic chemistry skills to prepare gram scales of any desired O-GalNAc core(s) or all eight cores concurrently.
Key points
-
O-GalNAc glycans are primary constituents of mucins and are ubiquitously expressed on cell surface and secreted proteins. Synthetic versions are used to study their role in normal and pathological physiology. Most O-GalNAc glycans can be synthesized from eight core structures.
-
In this protocol, the eight amino acid-linked O-GalNAc core structures are synthesized from a single precursor and five glycosyl donors. The precursor is Tn antigen with distinct orthogonal protecting groups.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data Availability
All relavent data (NMR and MS results) are available in ‘Analytical Data’ and Supplementary Information.
References
Corfield, A. P. Mucins: a biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta 1850, 236–252 (2015).
Linden, S. K., Sutton, P., Karlsson, N. G., Korolik, V. & McGuckin, M. A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 1, 183–197 (2008).
Hansson, G. C. Mucins and the microbiome. Annu. Rev. Biochem. 89, 769–793 (2020).
Steentoft, C. et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 32, 1478–1488 (2013).
Brockhausen, I., Wandall, H. H., Hagen, K. G. T. & Stanley, P. in Essentials of Glycobiology 4th edn (eds Varki A. et al.) 117–128 (Cold Spring Harbor, 2022).
Bagdonaite, I., Pallesen, E. M. H., Nielsen, M. I., Bennett, E. P. & Wandall, H. H. Mucin-type O-GalNAc glycosylation in health and disease. Adv. Exp. Med. Biol. 1325, 25–60 (2021).
Reily, C., Stewart, T. J., Renfrow, M. B. & Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 15, 346–366 (2019).
Dube, D. H. & Bertozzi, C. R. Glycans in cancer and inflammation—potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 4, 477–488 (2005).
Kufe, D. W. Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer 9, 874–885 (2009).
Martinez-Saez, N., Peregrina, J. M. & Corzana, F. Principles of mucin structure: implications for the rational design of cancer vaccines derived from MUC1-glycopeptides. Chem. Soc. Rev. 46, 7154–7175 (2017).
Gaidzik, N., Westerlind, U. & Kunz, H. The development of synthetic antitumour vaccines from mucin glycopeptide antigens. Chem. Soc. Rev. 42, 4421–4442 (2013).
Borgert, A. et al. Deciphering structural elements of mucin glycoprotein recognition. ACS Chem. Biol. 7, 1031–1039 (2012).
Behren, S. et al. Fucose binding motifs on mucin core glycopeptides impact bacterial lectin recognition. Angew. Chem. Int. Ed. 62, e202302437 (2023).
Marcaurelle, L. A. & Bertozzi, C. R. Recent advances in the chemical synthesis of mucin-like glycoproteins. Glycobiology 12, 69R–77R (2002).
Bello, C. et al. Chemoenzymatic synthesis of glycopeptides to explore the role of mucin 1 glycosylation in cell adhesion. Chembiochem 24, e202200741 (2023).
Pett, C. et al. Microarray analysis of antibodies induced with synthetic antitumor vaccines: specificity against diverse mucin core structures. Chemistry 23, 3875–3884 (2017).
Pedersen, J. W. et al. Seromic profiling of colorectal cancer patients with novel glycopeptide microarray. Int. J. Cancer 128, 1860–1871 (2011).
Naito, S. et al. Generation of novel anti-MUC1 monoclonal antibodies with designed carbohydrate specificities using MUC1 glycopeptide library. ACS Omega 2, 7493–7505 (2017).
Wandall, H. H. et al. Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res. 70, 1306–1313 (2010).
Takagi, J. et al. Mucin O-glycans are natural inhibitors of Candida albicans pathogenicity. Nat. Chem. Biol. 18, 762–773 (2022).
Liu, D. et al. O-Glycosylation induces amyloid-beta to form new fibril polymorphs vulnerable for degradation. J. Am. Chem. Soc. 143, 20216–20223 (2021).
Jank, T. et al. Tyrosine glycosylation of Rho by Yersinia toxin impairs blastomere cell behaviour in zebrafish embryos. Nat. Commun. 6, 7807 (2015).
Halim, A. et al. Site-specific characterization of threonine, serine, and tyrosine glycosylations of amyloid precursor protein/amyloid beta-peptides in human cerebrospinal fluid. Proc. Natl Acad. Sci. USA 108, 11848–11853 (2011).
Steentoft, C. et al. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat. Methods 8, 977–982 (2011).
Hounsell, E. F. et al. Structural analysis of the O-glycosidically linked core-region oligosaccharides of human meconium glycoproteins which express oncofoetal antigens. Eur. J. Biochem. 148, 367–377 (1985).
Jin, C. et al. Structural diversity of human gastric mucin glycans. Mol. Cell. Proteom. 16, 743–758 (2017).
Kurosaka, A. et al. Structures of the major oligosaccharides from a human rectal adenocarcinoma glycoprotein. J. Biol. Chem. 258, 11594–11598 (1983).
Capon, C. et al. Structures of O-glycosidically linked oligosaccharides isolated from human meconium glycoproteins. Eur. J. Biochem. 182, 139–152 (1989).
Madunic, K. et al. Specific (sialyl-)Lewis core 2 O-glycans differentiate colorectal cancer from healthy colon epithelium. Theranostics 12, 4498–4512 (2022).
Sumi, T. et al. Structural characterization of the trisaccharide chain from the skin mucus glycoprotein of the rainbow trout, Salmo gairdneri. Fish. Physiol. Biochem. 25, 11–17 (2001).
Jin, C. et al. Atlantic salmon carries a range of novel O-glycan structures differentially localized on skin and intestinal mucins. J. Proteome Res. 14, 3239–3251 (2015).
Padra, J. T. et al. Aeromonas salmonicida growth in response to atlantic salmon mucins differs between epithelial sites, is governed by sialylated and N-acetylhexosamine-containing O-glycans, and is affected by Ca(2). Infect. Immun. 85, e00189-17 (2017).
Thomsson, K. A. et al. Mucin O-glycosylation and pathogen binding ability differ between rainbow trout epithelial sites. Fish Shellfish Immunol. 131, 349–357 (2022).
Chai, W. G. et al. Neutral oligosaccharides of bovine submaxillary mucin. A combined mass spectrometry and 1H-NMR study. Eur. J. Biochem. 203, 257–268 (1992).
van Halbeek, H. et al. Structures of monosialyl oligosaccharides isolated from the respiratory mucins of a non-secretor (O, Lea+b−) patient suffering from chronic bronchitis. Characterization of a novel type of mucin carbohydrate core structure. Glycobiology 4, 203–219 (1994).
Wang, S. et al. Chemoenzymatic modular assembly of O-GalNAc glycans for functional glycomics. Nat. Commun. 12, 3573 (2021).
Xu, Z. et al. Diversity-oriented chemoenzymatic synthesis of sulfated and nonsulfated core 2 O-GalNAc glycans. J. Org. Chem. 86, 10819–10828 (2021).
Gadi, M. R. et al. Convergent chemoenzymatic synthesis of O-GalNAc rare cores 5, 7, 8 and their sialylated forms. Chem. Sci. 14, 1837–1843 (2023).
Pratt, M. R. & Bertozzi, C. R. Synthetic glycopeptides and glycoproteins as tools for biology. Chem. Soc. Rev. 34, 58–68 (2005).
Stergiou, N. et al. The development of vaccines from synthetic tumor-associated mucin glycopeptides and their glycosylation-dependent immune response. Chem. Rec. 21, 3313–3331 (2021).
Dziadek, S. & Kunz, H. Synthesis of tumor-associated glycopeptide antigens for the development of tumor-selective vaccines. Chem. Rec. 3, 308–321 (2004).
Maemura, M., Ohgaki, A., Nakahara, Y., Hojo, H. & Nakahara, Y. Solid-phase synthesis of core 8 O-glycan-linked MUC5AC glycopeptide. Biosci. Biotechnol. Biochem. 69, 1575–1583 (2005).
Komba, S., Meldal, M., Werdelin, O., Jensen, T. & Bock, K. Convenient synthesis of Thr and Ser carrying the tumor associated sialyl-(2-3)-T antigen as building blocks for solid-phase glycopeptide synthesis. J. Chem. Soc. 1, 415–420 (1999).
Pett, C., Schorlemer, M. & Westerlind, U. A unified strategy for the synthesis of mucin cores 1–4 saccharides and the assembled multivalent glycopeptides. Chem. Eur. J. 19, 17001–17010 (2013).
Keil, S., Claus, C., Dippold, W. & Kunz, H. Towards the development of antitumor vaccines: a synthetic conjugate of a tumor-associated MUC1 glycopeptide antigen and a tetanus toxin epitope. Angew. Chem. Int. Ed. 40, 366–369 (2001).
Liebe, B. & Kunz, H. Solid-phase synthesis of a tumor-associated sialyl-TN antigen glycopeptide with a partial sequence of the ‘tandem repeat’ of the MUC-1 mucin. Angew. Chem. Int. Ed. 36, 618–621 (1997).
Kunz, H. & Birnbach, S. Synthesis of O-glycopeptides of the tumor-associated TN- and T-antigen type and their binding to bovine serum albumin. Angew. Chem. Int. Ed. 25, 360–362 (1986).
Kuduk, S. D. et al. Synthetic and immunological studies on clustered modes of mucin-related Tn and TF O-linked antigens: the preparation of a glycopeptide-based vaccine for clinical trials against prostate cancer. J. Am. Chem. Soc. 120, 12474–12485 (1998).
Paulsen, H. & Hölck, J.-P. Synthese der glycopeptide O-b-d-galactopyranosyl-(1-3)-O-(2-acetamido-2-desoxy-a-d-galactopyranosyl)-(1-3)-l-serin und -l-threonin. Carbohydr. Res. 109, 89–107 (1982).
Liebe, B. & Kunz, H. Solid-phase synthesis of a sialyl-Tn-glycoundecapeptide of the MUC1 repeating unit. Helv. Chim. Acta 80, 1473–1482 (1997).
Cato, D., Buskas, T. & Boons, G. J. Highly efficient stereospecific preparation of Tn and TF building blocks using thioglycosyl donors and the Ph2SO/Tf2O promotor system. J. Carbohydr. Chem. 24, 503–516 (2005).
Shaik, A. A., Nishat, S. & Andreana, P. R. Stereoselective synthesis of natural and non-natural thomsen-nouveau antigens and hydrazide derivatives. Org. Lett. 17, 2582–2585 (2015).
Rio-Anneheim, S., Paulsen, H., Meldal, M. & Bock, K. Synthesis of the building blocks Na-Fmoc-O-[a-d-Ac3GalN3p-(1-3)-a-d-Ac2GalN3p]-Thr-OPfp and Na-Fmoc-O-[a-d-Ac3GalN3p-(1→6)-a-d-Ac2GalN3p]-Thr-OPfp and their application in the solid phase glycopeptide synthesis of core 5 and core 7 mucin O-glycopeptides. J. Chem. Soc. Perkin Trans. 1, 1071–1080 (1995).
Qiu, D. & Koganty, R. R. A novel glycosyl donor for the synthesis of cancer specific core 5 and sialyl core 5 as glycopeptide building blocks. Tetrahedron Lett. 38, 961–964 (1997).
Kakita, K. et al. A stereocontrolled construction of 2-azido-2-deoxy-1, 2-cis-α-galactosidic linkages utilizing 2-azido-4, 6-O-benzylidene-2-deoxygalactopyranosyl diphenyl phosphates: stereoselective synthesis of mucin core 5 and core 7 structures. Tetrahedron 68, 5005–5017 (2012).
Wakao, M. et al. Synthesis of mucin type core 3 and core 5 structures and their interaction analysis with sugar chips. Carbohydr. Res. 516, 108565 (2022).
Guo, Z. & Shao, N. Glycopeptide and glycoprotein synthesis involving unprotected carbohydrate building blocks. Med. Res. Rev. 25, 655–678 (2005).
Peng, W. et al. Recent H3N2 viruses have evolved specificity for extended, branched human-type receptors, conferring potential for increased avidity. Cell Host Microbe 21, 23–34 (2017).
Yu, H. et al. A multifunctional Pasteurella multocida sialyltransferase: a powerful tool for the synthesis of sialoside libraries. J. Am. Chem. Soc. 127, 17618–17619 (2005).
Lin, S. W., Yuan, T. M., Li, J. R. & Lin, C. H. Carboxyl terminus of Helicobacter pylori alpha1,3-fucosyltransferase determines the structure and stability. Biochemistry 45, 8108–8116 (2006).
Higashi, K. & Susaki, H. A novel glycosidation promoted by the combination of trimethylsilyl halide and zinc triflate. Chem. Pharm. Bull. 40, 2019–2022 (1992).
Orgueira, H. A. et al. Modular synthesis of heparin oligosaccharides. Chem. Eur. J. 9, 140–169 (2003).
Li, J. & Yu, B. A modular approach to the total synthesis of tunicamycins. Angew. Chem. Int. Ed. Engl. 54, 6618–6621 (2015).
Behrendt, R., White, P. & Offer, J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 22, 4–27 (2016).
Sugiarto, G. et al. A sialyltransferase mutant with decreased donor hydrolysis and reduced sialidase activities for directly sialylating LewisX. ACS Chem. Biol. 7, 1232–1240 (2012).
Acknowledgements
This work is supported by the National Institute of Health (R44GM123820 and U54HL142019 to L.L.). T.S. thanks the support of Center for Diagnostics & Therapeutics fellowship.
Author information
Authors and Affiliations
Contributions
M.R.G., J.H. and L.L. designed the research and developed the method. M.R.G., J.H., T.S. and S.F. performed the experiments. M.R.G., J.H. and L.L. drafted the manuscript, which was revised by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Akihiko Kameyama, Tiehai Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this process.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Wang, S. et al. Nat. Commun. 12, 3573 (2021): https://doi.org/10.1038/s41467-021-23428-x
Gadi, M. R. et al. Chem. Sci. 14, 1837–1843 (2023): https://doi.org/10.1039/D2SC06925C
Supplementary information
Supplementary Information
Supplementary Figs. 1–3, Table 1 and NMR data and spectra.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gadi, M.R., Han, J., Shen, T. et al. Divergent synthesis of amino acid-linked O-GalNAc glycan core structures. Nat Protoc 20, 480–517 (2025). https://doi.org/10.1038/s41596-024-01051-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41596-024-01051-6