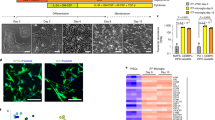
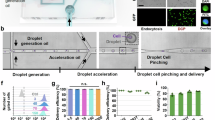
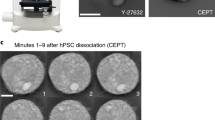
Abstract
Cell–cell interactions are essential for the function and contextual regulation of biological tissues. We present a platform for high-throughput microfluidics-supported genetic screening of functional regulators of cell–cell interactions. Systematic perturbation of encapsulated associated cells followed by sequencing (SPEAC-seq) combines genome-wide CRISPR libraries, cell coculture in droplets and microfluidic droplet sorting based on functional read-outs determined by fluorescent reporter circuits to enable the unbiased discovery of interaction regulators. This technique overcomes limitations of traditional methods for characterization of cell–cell communication, which require a priori knowledge of cellular interactions, are highly engineered and lack functional read-outs. As an example of this technique, we describe the investigation of neuroinflammatory intercellular communication between microglia and astrocytes, using genome-wide CRISPR–Cas9 inactivation libraries and fluorescent reporters of NF-κB activation. This approach enabled the discovery of thousands of microglial regulators of astrocyte NF-κB activation important for the control of central nervous system inflammation. Importantly, SPEAC-seq can be adapted to different cell types, screening modalities, cell functions and physiological contexts, only limited by the ability to fluorescently report cell functions and by droplet cultivation conditions. Performing genome-wide screening takes less than 2 weeks and requires microfluidics capabilities. Thus, SPEAC-seq enables the large-scale investigation of cell–cell interactions.
Key points
-
SPEAC-seq uses CRISPR screening, cell coculture in droplets and microfluidic sorting to enable forward genetic screens of regulators of cell–cell communication.
-
The procedure provides a detailed experimental workflow and discusses technical aspects. SPEAC-seq enables the defined pairing of interaction partners, is adaptable to different CRISPR screening modalities, is scalable for unbiased genome-wide discovery of functional regulators and can be adapted with few modifications to study a variety of cell types.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Zhou, X. et al. Circuit design features of a stable two-cell system. Cell 172, 744–757 e717 (2018).
Ramilowski, J. A. et al. A draft network of ligand-receptor-mediated multicellular signalling in human. Nat. Commun. 6, 7866 (2015).
Rieckmann, J. C. et al. Social network architecture of human immune cells unveiled by quantitative proteomics. Nat. Immunol. 18, 583–593 (2017).
Armingol, E., Officer, A., Harismendy, O. & Lewis, N. E. Deciphering cell-cell interactions and communication from gene expression. Nat. Rev. Genet. 22, 71–88 (2021).
Bock, C. et al. High-content CRISPR screening. Nat. Rev. Methods Primers https://doi.org/10.1038/s43586-022-00098-7 (2022).
Wheeler, M. A. et al. Droplet-based forward genetic screening of astrocyte-microglia cross-talk. Science 379, 1023–1030 (2023).
Efremova, M., Vento-Tormo, M., Teichmann, S. A. & Vento-Tormo, R. CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand–receptor complexes. Nat. Protoc. 15, 1484–1506 (2020).
Browaeys, R., Saelens, W. & Saeys, Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat. Methods 17, 159–162 (2020).
Jin, S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Jakobsson, J. E. T., Spjuth, O. & Lagerstrom, M. C. scConnect: a method for exploratory analysis of cell-cell communication based on single-cell RNA-sequencing data. Bioinformatics 37, 3501–3508 (2021).
Zhang, Y. et al. Cellinker: a platform of ligand-receptor interactions for intercellular communication analysis. Bioinformatics https://doi.org/10.1093/bioinformatics/btab036 (2021).
Ravi, V. M. et al. T-cell dysfunction in the glioblastoma microenvironment is mediated by myeloid cells releasing interleukin-10. Nat. Commun. 13, 925 (2022).
Pasqual, G. et al. Monitoring T cell-dendritic cell interactions in vivo by intercellular enzymatic labelling. Nature 553, 496–500 (2018).
Ge, Y. et al. Enzyme-mediated intercellular proximity labeling for detecting cell–cell interactions. J. Am. Chem. Soc. 141, 1833–1837 (2019).
Liu, Z. et al. Detecting tumor antigen-specific T cells via interaction-dependent fucosyl-biotinylation. Cell 183, 1117–1133 e1119 (2020).
Branon, T. C. et al. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 36, 880–887 (2018).
Qiu, S. et al. Use of intercellular proximity labeling to quantify and decipher cell–cell interactions directed by diversified molecular pairs. Sci. Adv. 8, eadd2337 (2022).
Ng, K. K. & Prescher, J. A. Generalized bioluminescent platform to observe and track cellular interactions. Bioconjug. Chem. 33, 1876–1884 (2022).
Nakandakari-Higa, S. et al. Universal recording of immune cell interactions in vivo. Nature 627, 399–399 (2024).
Muller, M. et al. Light-mediated discovery of surfaceome nanoscale organization and intercellular receptor interaction networks. Nat. Commun. 12, 7036 (2021).
Cho, K. F. et al. A light-gated transcriptional recorder for detecting cell-cell contacts. eLife https://doi.org/10.7554/eLife.70881 (2022).
Oslund, R. C. et al. Detection of cell–cell interactions via photocatalytic cell tagging. Nat. Chem. Biol. 18, 850–858 (2022).
Ombrato, L. et al. Generation of neighbor-labeling cells to study intercellular interactions in vivo. Nat. Protoc. 16, 872–892 (2021).
Tang, R. et al. A versatile system to record cell-cell interactions. eLife https://doi.org/10.7554/eLife.61080 (2020).
Clark, I. C. et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 372, eabf1230 (2021).
Kinoshita, N. et al. Genetically encoded fluorescent indicator GRAPHIC delineates intercellular connections. iScience 15, 28–38 (2019).
Morsut, L. et al. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell 164, 780–791 (2016).
Zhang, S. et al. Monitoring of cell–cell communication and contact history in mammals. Science 378, eabo5503 (2022).
Giladi, A. et al. Dissecting cellular crosstalk by sequencing physically interacting cells. Nat. Biotechnol. 38, 629–637 (2020).
Dobson, C. S. et al. Antigen identification and high-throughput interaction mapping by reprogramming viral entry. Nat. Methods 19, 449–460 (2022).
Yu, B. et al. Engineered cell entry links receptor biology with single-cell genomics. Cell 185, 4904–4920 e4922 (2022).
Gee, M. H. et al. Antigen identification for orphan T cell receptors expressed on tumor-infiltrating lymphocytes. Cell 172, 549–563 e516 (2018).
Zilionis, R. et al. Single-cell barcoding and sequencing using droplet microfluidics. Nat. Protoc. 12, 44–73 (2017).
Segaliny, A. I. et al. Functional TCR T cell screening using single-cell droplet microfluidics. Lab Chip 18, 3733–3749 (2018).
Lorenz, H. et al. High-aspect-ratio, ultrathick, negative-tone near-UV photoresist and its applications for MEMS. Sens. Actuators A Phys. 64, 33–39 (1998).
Mazutis, L. et al. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 8, 870–891 (2013).
Joung, J. et al. Genome-scale CRISPR–Cas9 knockout and transcriptional activation screening. Nat. Protoc. 12, 828–863 (2017).
Macosko et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Shifrut, E. et al. Genome-wide CRISPR screens in primary human T cells reveal key regulators of immune function. Cell 175, 1958–1971 e1915 (2018).
Sart, S., Ronteix, G., Jain, S., Amselem, G. & Baroud, C. N. Cell culture in microfluidic droplets. Chem. Rev. 122, 7061–7096 (2022).
Abate, A. R., Chen, C. H., Agresti, J. J. & Weitz, D. A. Beating Poisson encapsulation statistics using close-packed ordering. Lab Chip 9, 2628–2631 (2009).
Sukovich, D. J., Kim, S. C., Ahmed, N. & Abate, A. R. Bulk double emulsification for flow cytometric analysis of microfluidic droplets. Analyst 142, 4618–4622 (2017).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR–Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Clark, I. C., Thakur, R. & Abate, A. R. Concentric electrodes improve microfluidic droplet sorting. Lab Chip 18, 710–713 (2018).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 15, 554 (2014).
Lee, H. G., Lee, J. H., Flausino, L. E. & Quintana, F. J. Neuroinflammation: an astrocyte perspective. Sci. Transl. Med. 15, eadi7828 (2023).
Everhart, M. B. et al. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J. Immunol. 176, 4995–5005 (2006).
Raval, N. et al. in Basic Fundamentals of Drug Delivery (Advances in Pharmaceutical Product Development and Research) (ed. Tekade, R. K.) 369–400 (Academic Press, 2019).
Acknowledgements
We thank all other Quintana lab members for helpful discussion related to this study. We thank the Harvard Medical School Microfabrication Core Facility for their assistance with microfabrication and access to photolithography equipment. RRID: Addgene_73632 was a gift from D. Root and J. Doench. RRID: Addgene_12260 was a gift from D. Trono. RRID: Addgene_8454 was a gift from B.Weinberg. This work was supported by grants NS102807, ES02530, ES029136, AI126880 from the National Institutes of Health; RG4111A1 and JF2161-A-5 from the NMSS; RSG-14-198-01-LIB from the American Cancer Society; and PA-1604-08459 from the International Progressive MS Alliance. M.A.W. was supported by National Institute of Neurological Disorders and Stroke, National Institute of Mental Health and National Cancer Institute (R01MH130458, R00NS114111, T32CA207201). I.C.C. was supported by K22AI152644 and DP2AI154435 from the National Institutes of Health. H.-G.L. was supported by a Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2021R1A6A3A14039088).
Author information
Authors and Affiliations
Contributions
C.F.A., M.A.W., I.C.C., M.L., H.-G.L., Z.L. and F.J.Q. designed SPEAC-seq, performed experiments or analyzed data presented in this protocol. C.F.A., M.A.W. and F.J.Q. wrote the manuscript with input from coauthors. F.J.Q. directed and supervised the work presented in this paper.
Corresponding author
Ethics declarations
Competing interests
M.A.W., I.C.C. and F.J.Q. have filed a patent on SPEAC-seq. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Naomi Habib and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Wheeler, M. A. et al. Science 379, 1023–1030 (2023): https://doi.org/10.1126/science.abq4822
Supplementary information
Supplementary Data 1
CAD design for microfluidic co-encapsulator device.
Supplementary Data 2
CAD design for microfluidic sorter device.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Faust Akl, C., Linnerbauer, M., Li, Z. et al. Droplet-based functional CRISPR screening of cell–cell interactions by SPEAC-seq. Nat Protoc 20, 440–461 (2025). https://doi.org/10.1038/s41596-024-01056-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-024-01056-1