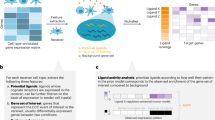
Abstract
Cell–cell communication is essential for tissue development, function and regeneration. The revolution of single-cell genomics technologies offers an unprecedented opportunity to uncover how cells communicate in vivo within their tissue niches and how disruption of these niches can lead to diseases and developmental abnormalities. CellPhoneDB is a bioinformatics toolkit designed to infer cell–cell communication by combining a curated repository of bona fide ligand-receptor interactions with methods to integrate these interactions with single-cell genomics data. Here we present a protocol for the latest version of CellPhoneDB (v5), offering several new features. First, the repository has been expanded by one-third with the addition of new interactions, including ~1,000 interactions mediated by nonpeptidic ligands such as steroidogenic hormones, neurotransmitters and small G-protein-coupled receptor (GPCR)-binding ligands. Second, we outline a new way of using the database that allows users to tailor queries to their experimental designs. Third, the update incorporates novel strategies to prioritize specific cell–cell interactions, leveraging information from other modalities such as tissue microenvironments derived from spatial transcriptomics technologies or transcription factor activities derived from a single-cell assay for transposase accessible chromatin assays. Finally, we describe the new CellPhoneDBViz module to interactively visualize and share results. Altogether, CellPhoneDB v5 enhances the precision of cell–cell communication inference, offering new insights into tissue biology in physiological microenvironments. This protocol typically takes ~15 min and requires basic knowledge of python.
Key points
-
Understanding cellular communication is essential for obtaining insights into normal tissue development and physiology and the dysregulation of these processes in disease states. CellPhoneDB is a popular bioinformatics toolkit for inferring cell–cell communication combining a curated database of interactions and single-cell expression data.
-
This protocol update describes how to use the newest implementation (CellPhoneDB v5), which expands the database to nonpeptide ligands and includes several strategies to prioritize and visualize interactions, incorporating information from epigenomics and spatial transcriptomics modalities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The CellPhoneDB database is available at GitHub via https://github.com/ventolab/CellphoneDB-data (ref. 94) and the processed data used in the case examples (Supplementary Notes 1–3) and to generate Fig. 3b–f are available at GitHub via https://github.com/ventolab/CellphoneDB/tree/master/NatureProtocols2024_case_studies (ref. 95). The raw data associated with the case studies are publicly available, with access detailed in the primary research articles13,14,15,17.
Code availability
The CellPhoneDB and CellphoneDBViz codes are available at GitHub via https://github.com/ventolab/CellphoneDB (ref. 96) and https://github.com/ventolab/CellphoneDBViz (ref. 97), respectively. Kplots are available at GitHub via https://github.com/zktuong/ktplots (ref. 98).
References
Vento-Tormo, R. et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 563, 347–353 (2018).
Efremova, M., Vento-Tormo, M., Teichmann, S. A. & Vento-Tormo, R. CellPhoneDB: inferring cell–cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat. Protoc. 15, 1484–1506 (2020).
Luo, H. et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat. Commun. 13, 6619 (2022).
Chen, Y.-P. et al. Single-cell transcriptomics reveals regulators underlying immune cell diversity and immune subtypes associated with prognosis in nasopharyngeal carcinoma. Cell Res. 30, 1024–1042 (2020).
Xu, J. et al. Delving into the heterogeneity of different breast cancer subtypes and the prognostic models utilizing scRNA-seq and bulk RNA-seq. Int. J. Mol. Sci. 23, 9936 (2022).
Zhang, Y. et al. CD39 inhibition and VISTA blockade may overcome radiotherapy resistance by targeting exhausted CD8+ T cells and immunosuppressive myeloid cells. Cell Rep. Med 4, 101151 (2023).
Hoo, R. et al. Acute response to pathogens in the early human placenta at single-cell resolution. Cell Syst. 15, 425–444.e9 (2024).
Davidson, S. et al. Single-cell RNA sequencing reveals a dynamic stromal niche that supports tumor growth. Cell Rep. 31, 107628 (2020).
Misselbeck, K. et al. A network-based approach to identify deregulated pathways and drug effects in metabolic syndrome. Nat. Commun. 10, 5215 (2019).
Zhao, Y. et al. Single-cell transcriptome analysis uncovers intratumoral heterogeneity and underlying mechanisms for drug resistance in hepatobiliary tumor organoids. Adv. Sci. 8, e2003897 (2021).
Qi, K. & Liu, R. Subpopulation cooperation renders drug resistance of hepatobiliary tumor organoids. Chin. J. Cancer Res. 34, 422–424 (2022).
Jansen, J. et al. Human pluripotent stem cell-derived kidney organoids for personalized congenital and idiopathic nephrotic syndrome modeling. Development 149, dev200198 (2022).
Garcia-Alonso, L. et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat. Genet. 53, 1698–1711 (2021).
Garcia-Alonso, L. et al. Single-cell roadmap of human gonadal development. Nature 607, 540–547 (2022).
Arutyunyan, A. et al. Spatial multiomics map of trophoblast development in early pregnancy. Nature 616, 143–151 (2023).
Kanemaru, K. et al. Spatially resolved multiomics of human cardiac niches. Nature 619, 801–810 (2023).
Marečková, M. et al. An integrated single-cell reference atlas of the human endometrium. Nat. Genet. 56, 1925–1937 (2024).
Jassal, B. et al. The reactome pathway knowledgebase. Nucleic Acids Res. 48, D498–D503 (2020).
UniProt Consortium. UniProt: the universal protein knowledgebase in 2023. Nucleic Acids Res. 51, D523–D531 (2023).
Derynck, R. & Budi, E. H. Specificity, versatility, and control of TGF-β family signaling. Sci. Signal. 12, eaav5183 (2019).
Brivanlou, A. H. & Darnell, J. E. Signal transduction and the control of gene expression. Science 295, 813–818 (2002).
Sriram, K. & Insel, P. A. G protein-coupled receptors as targets for approved drugs: how many targets and how many drugs? Mol. Pharmacol. 93, 251–258 (2018).
Zhang, B., Vogelzang, A. & Fagarasan, S. Secreted immune metabolites that mediate immune cell communication and function. Trends Immunol. 43, 990–1005 (2022).
Rashid, M., Singla, D., Sharma, A., Kumar, M. & Raghava, G. P. S. Hmrbase: a database of hormones and their receptors. BMC Genomics 10, 307 (2009).
Pándy-Szekeres, G. et al. GPCRdb in 2023: state-specific structure models using AlphaFold2 and new ligand resources. Nucleic Acids Res. 51, D395–D402 (2023).
Hastings, J. et al. ChEBI in 2016: improved services and an expanding collection of metabolites. Nucleic Acids Res. 44, D1214–D1219 (2016).
Bansal, P. et al. Rhea, the reaction knowledgebase in 2022. Nucleic Acids Res. 50, D693–D700 (2022).
Shilts, J. et al. A physical wiring diagram for the human immune system. Nature 608, 397–404 (2022).
Harding, S. D. et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2022: curating pharmacology for COVID-19, malaria and antibacterials. Nucleic Acids Res. 50, D1282–D1294 (2022).
Breuer, K. et al. InnateDB: systems biology of innate immunity and beyond–recent updates and continuing curation. Nucleic Acids Res. 41, D1228–D1233 (2013).
Orchard, S. et al. The MIntAct project—IntAct as a common curation platform for 11 molecular interaction databases. Nucleic Acids Res. 42, D358–D363 (2014).
UniProt Consortium. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 49, D480–D489 (2021).
Elmentaite, R. et al. Cells of the human intestinal tract mapped across space and time. Nature 597, 250–255 (2021).
Park, J.-E. et al. A cell atlas of human thymic development defines T cell repertoire formation. Science 367, eaay3224 (2020).
Suo, C. et al. Mapping the developing human immune system across organs. Science 376, eabo0510 (2022).
Hie, B., Cho, H., DeMeo, B., Bryson, B. & Berger, B. Geometric sketching compactly summarizes the single-cell transcriptomic landscape. Cell Syst. 8, 483–493.e7 (2019).
Yayon, N. et al. A spatial human thymus cell atlas mapped to a continuous tissue axis. Nature 635, 708–718 (2024).
Nguyen, H. C. T., Baik, B., Yoon, S., Park, T. & Nam, D. Benchmarking integration of single-cell differential expression. Nat. Commun. 14, 1–16 (2023).
Van den Berge, K. et al. Trajectory-based differential expression analysis for single-cell sequencing data. Nat. Commun. 11, 1–13 (2020).
Velten, B. & Stegle, O. Principles and challenges of modeling temporal and spatial omics data. Nat. Methods 20, 1462–1474 (2023).
Kleshchevnikov, V. et al. Cell2location maps fine-grained cell types in spatial transcriptomics. Nat. Biotechnol. 40, 661–671 (2022).
Andersson, A. et al. Single-cell and spatial transcriptomics enables probabilistic inference of cell type topography. Commun. Biol. 3, 565 (2020).
Li, H. et al. A comprehensive benchmarking with practical guidelines for cellular deconvolution of spatial transcriptomics. Nat. Commun. 14, 1548 (2023).
Geevarghese, A. & Herman, I. M. Pericyte-endothelial crosstalk: implications and opportunities for advanced cellular therapies. Transl. Res. 163, 296–306 (2014).
Kats, I., Vento-Tormo, R. & Stegle, O. SpatialDE2: Fast and localized variance component analysis of spatial transcriptomics. Preprint at bioRxiv https://doi.org/10.1101/2021.10.27.466045 (2021).
Dries, R. et al. Giotto: a toolbox for integrative analysis and visualization of spatial expression data. Genome Biol. 22, 78 (2021).
Sun, S., Zhu, J. & Zhou, X. Statistical analysis of spatial expression patterns for spatially resolved transcriptomic studies. Nat. Methods 17, 193–200 (2020).
Birk, S. et al. Preprint at bioRxiv https://doi.org/10.1101/2024.02.21.581428 (2024).
Haviv, D. et al. The covariance environment defines cellular niches for spatial inference. Nat. Biotechnol. 43, 269–280 (2025).
Schep, A. N., Wu, B., Buenrostro, J. D. & Greenleaf, W. J. chromVAR: inferring transcription factor-associated accessibility from single-cell epigenomic data. Nat. Methods 14, 975–978 (2017).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Garcia-Alonso, L., Holland, C. H., Ibrahim, M. M., Turei, D. & Saez-Rodriguez, J. Benchmark and integration of resources for the estimation of human transcription factor activities. Genome Res. 29, 1363–1375 (2019).
Müller-Dott, S. et al. Expanding the coverage of regulons from high-confidence prior knowledge for accurate estimation of transcription factor activities. Nucleic Acids Res. 51, 10934–10949 (2023).
Badia-i-Mompel, P. et al. decoupleR: ensemble of computational methods to infer biological activities from omics data. Bioinform. Adv. 2, vbac016 (2022).
Noël, F. et al. Dissection of intercellular communication using the transcriptome-based framework ICELLNET. Nat. Commun. 12, 1089 (2021).
Jin, S. et al. Inference and analysis of cell–cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Armingol, E., Officer, A., Harismendy, O. & Lewis, N. E. Deciphering cell–cell interactions and communication from gene expression. Nat. Rev. Genet. 22, 71–88 (2020).
Tuong, Z. K. & colin-leeyc. Zktuong/ktplots: v1.2.2. Zenodo https://doi.org/10.5281/zenodo.7358897 (2022).
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502 (2015).
Amezquita, R. A. et al. Orchestrating single-cell analysis with Bioconductor. Nat. Methods 17, 137–145 (2019).
Virshup, I., Rybakov, S., Theis, F. J., Angerer, P. & Alexander Wolf, F. anndata: annotated data. Preprint at bioRxiv https://doi.org/10.1101/2021.12.16.473007 (2021).
Guo, C. et al. Single-cell analysis of two severe COVID-19 patients reveals a monocyte-associated and tocilizumab-responding cytokine storm. Nat. Commun. 11, 3924 (2020).
Leader, A. M. et al. Single-cell analysis of human non-small cell lung cancer lesions refines tumor classification and patient stratification. Cancer Cell 39, 1594–1609.e12 (2021).
Tuong, Z. K., colin-leeyc & Feng, T. Zktuong/ktplots: v1.2.3. Zenodo https://doi.org/10.5281/zenodo.7699617 (2023).
Rozenfeld, R. & Devi, L. A. Exploring a role for heteromerization in GPCR signalling specificity. Biochem. J. 433, 11–18 (2011).
Namwanje, M. & Brown, C. W. Activins and inhibins: roles in development, physiology, and disease. Cold Spring Harb. Perspect. Biol. 8, a021881 (2016).
Dimitrov, D. et al. Comparison of methods and resources for cell–cell communication inference from single-cell RNA-seq data. Nat. Commun. 13, 3224 (2022).
Jin, S., Plikus, M. V. & Nie, Q. CellChat for systematic analysis of cell–cell communication from single-cell transcriptomics. Nat. Protocol. 20, 180–219 (2024).
Zhao, W., Johnston, K. G., Ren, H., Xu, X. & Nie, Q. Inferring neuron–neuron communications from single-cell transcriptomics through NeuronChat. Nat. Commun. 14, 1128 (2023).
Jakobsson, J. E. T., Spjuth, O. & Lagerström, M. C. scConnect: a method for exploratory analysis of cell–cell communication based on single-cell RNA-sequencing data. Bioinformatics 37, 3501–3508 (2021).
Zheng, R. et al. MEBOCOST: metabolic cell–cell communication modeling by single cell transcriptome. Preprint at bioRxiv https://doi.org/10.1101/2022.05.30.494067 (2022).
Xing, S., Wallmeroth, N., Berendzen, K. W. & Grefen, C. Techniques for the analysis of protein–protein interactions in vivo. Plant Physiol. 171, 727–758 (2016).
Türei, D., Korcsmáros, T. & Saez-Rodriguez, J. OmniPath: guidelines and gateway for literature-curated signaling pathway resources. Nat. Methods 13, 966–967 (2016).
Armingol, E., Baghdassarian, H. M. & Lewis, N. E. The diversification of methods for studying cell–cell interactions and communication. Nat. Rev. Genet. 25, 381–400 (2024).
Liu, Z., Sun, D. & Wang, C. Evaluation of cell–cell interaction methods by integrating single-cell RNA sequencing data with spatial information. Genome Biol. 23, 218 (2022).
Wang, Y. et al. iTALK: an R package to characterize and illustrate intercellular communication. Preprint at bioRxiv https://doi.org/10.1101/507871 (2019).
Cabello-Aguilar, S. et al. SingleCellSignalR: inference of intercellular networks from single-cell transcriptomics. Nucleic Acids Res. 48, e55 (2020).
Hou, R., Denisenko, E., Ong, H. T., Ramilowski, J. A. & Forrest, A. R. R. Predicting cell-to-cell communication networks using NATMI. Nat. Commun. 11, 1–11 (2020).
Raredon, M. S. B. et al. Computation and visualization of cell–cell signaling topologies in single-cell systems data using Connectome. Sci. Rep. 12, 4187 (2022).
Armingol, E. et al. Context-aware deconvolution of cell–cell communication with Tensor-cell2cell. Nat. Commun. 13, 1–15 (2022).
Jerby-Arnon, L. & Regev, A. DIALOGUE maps multicellular programs in tissue from single-cell or spatial transcriptomics data. Nat. Biotechnol. 40, 1467–1477 (2022).
Flores, R. O. R., Lanzer, J. D., Dimitrov, D., Velten, B. & Saez-Rodriguez, J. Multicellular factor analysis of single-cell data for a tissue-centric understanding of disease. eLife 12, e93161 (2023).
Browaeys, R., Saelens, W. & Saeys, Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat. Methods 17, 159–162 (2020).
Xin, Y. et al. LRLoop: a method to predict feedback loops in cell–cell communication. Bioinformatics 38, 4117–4126 (2022).
Baruzzo, G., Cesaro, G. & Di Camillo, B. Identify, quantify and characterize cellular communication from single-cell RNA sequencing data with scSeqComm. Bioinformatics 38, 1920–1929 (2022).
Hu, Y., Peng, T., Gao, L. & Tan, K. CytoTalk: de novo construction of signal transduction networks using single-cell transcriptomic data. Sci. Adv. 7, eabf1356 (2021).
Palla, G. et al. Squidpy: a scalable framework for spatial omics analysis. Nat. Methods 19, 171–178 (2022).
Tanevski, J., Flores, R. O. R., Gabor, A., Schapiro, D. & Saez-Rodriguez, J. Explainable multiview framework for dissecting spatial relationships from highly multiplexed data. Genome Biol. 23, 1–31 (2022).
Pham, D. et al. Robust mapping of spatiotemporal trajectories and cell–cell interactions in healthy and diseased tissues. Nat. Commun. 14, 7739 (2023).
Mason, K. et al. Niche-DE: niche-differential gene expression analysis in spatial transcriptomics data identifies context-dependent cell–cell interactions. Genome Biol. 25, 1–33 (2024).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Van de Sande, B. et al. A scalable SCENIC workflow for single-cell gene regulatory network analysis. Nat. Protoc. 15, 2247–2276 (2020).
Bravo, G.-B. C. et al. SCENIC+: single-cell multiomic inference of enhancers and gene regulatory networks. Nat. Methods 20, 1355–1367 (2023).
Troulé, K. et al. CellphoneDB-data. CellPhoneDB database of interactions. GitHub https://github.com/ventolab/CellphoneDB-data.
Troulé, K. et al. NatureProtocols2024 Case Studies. GitHub https://github.com/ventolab/CellphoneDB/tree/master/NatureProtocols2024_case_studies.
Troulé, K. et al. CellPhoneDB python package. GitHub https://github.com/ventolab/CellphoneDB.
Troulé, K. et al. CellPhoneDBViz. GitHub https://github.com/ventolab/CellphoneDBViz.
Troulé, K. et al. ktplots visualisation package. GitHub https://github.com/zktuong/ktplots.
Acknowledgements
The authors thank J. Shilts for introducing new interactions in the CellphoneDB database, E. Armingol for insightful discussion of the manuscript, A. García from Bio-Graphics for scientific illustrations, A. Maartens for proofreading and R. Vilarrasa for her feedback on the scoring methodology. We are grateful to all the Vento-Tormo lab members for their advice. This project was supported by the Chan Zuckerberg Initiative DAF grant 2022-249429 (S.T., L.G-A. and R.V.-T.), the Wellcome Trust grant 220540/Z/20/A, (R.V.-T.) and the UK Research and Innovation (UKRI) under the UK government’s Horizon Europe funding guarantee EP/Y009924/1 (R.V.T.).
Author information
Authors and Affiliations
Contributions
K.T., L.G.-A., S.T. and R.V.-T. conceived and developed the protocol and wrote the manuscript. M.P. and R.P. implemented and optimized CellPhoneDB v5. R.P. developed CellPhoneDBViz. K.T., L.G.-A., J.C. and S.T. contributed to the manual revision of the CellphoneDB database. A.H. and Z.K.T. developed ktplots and ktplotspy. S.T., L.G.-A. and R.V.-T. cosupervised the work.
Corresponding authors
Ethics declarations
Competing interests
In the past 3 years, S.A.T. has consulted or been a member of scientific advisory boards at Roche, Genentech, Biogen, GlaxoSmithKline, Qiagen and ForeSite Labs, is an equity holder of Transition Bio and is a cofounder of Ensocell Therapeutics.
Peer review
Peer review information
Nature Protocols thanks Lucile Massenet, Vassili Soumelis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Key references
Garcia-Alonso, L. et al. Nat. Genet. 53, 1698–1711 (2021): https://doi.org/10.1038/s41588-021-00972-2
Garcia-Alonso, L. et al. Nature 607, 540–547 (2022): https://doi.org/10.1038/s41586-022-04918-4
Marečková, M. et al. Nat. Genet 56, 1925–1937 (2024): https://doi.org/10.1038/s41588-024-01873-w
This protocol is an update to: Nat. Protoc. 15, 1484–1506 (2020): https://doi.org/10.1038/s41596-020-0292-x
Supplementary information
Supplementary Information
Supplementary Information containing Supplementary Notes, Figs. and Tables. Supplementary Notes. Three case studies using the novel features of CellPhoneDB v5, including method 3 and the three prioritization strategies CellSign, microenvironments and specificity scoring. Supplementary Note 1 describes case example 1 exploring germ-somatic communication during ovarian development, by combining method 3, CellSign and spatial microenvironments. Supplementary Note 2 describes case example 2 inferring cell–cell communication in the spatial niches of the endometrium, by combining method 3 and spatiotemporal microenvironments. Supplementary Note 3 describes case example 3, which aims to identify overexpressed cell–cell interactions between trophoblasts and maternal vessels by combining method 2 and scoring approach. Supplementary Fig. 1. CellPhoneDBViz web overview. Screenshots of CellPhoneDBViz reporting CellPhoneDB results for the case study 1 (Supplementary Note 1). a, Stankey plot and dotplot depicting cell types (second row or y axis) per microenvironment (thirds row or x axis). b, Chord plots showing the total number of interactions identified for each cell-type pair in each microenvironment (same data coils be represented as heat maps, a feature that is customizable in CellPhoneDBViz). c, Mean expression for a subset of selected interactions (y axis) per cell-type pair (x axis). If the interaction involves a receptor which downstream TF is active (CellSign) then an outer green ring is shown. The cell-type pairs are colored according to their microenvironment definition. Supplementary Table. Description of the output files (a) and the meaning of the columns (b).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Troulé, K., Petryszak, R., Cakir, B. et al. CellPhoneDB v5: inferring cell–cell communication from single-cell multiomics data. Nat Protoc 20, 3412–3440 (2025). https://doi.org/10.1038/s41596-024-01137-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-024-01137-1
This article is cited by
-
Single-cell analysis reveals multi-faceted features of B cell development, together with age-associated B cell subpopulations
Communications Biology (2026)
-
Advancing spatial cellular communication inference with ligand diffusion and transport model
Communications Biology (2026)
-
Cell–cell interactions as predictive and prognostic markers for drug responses in cancer
Genome Medicine (2025)
-
Systematic assessment of microenvironment-dependent transcriptional patterns and intercellular communication
Genome Biology (2025)
-
Cross-expression meta-analysis of mouse brain slices reveals coordinated gene expression across spatially adjacent cells
Genome Biology (2025)