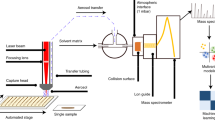
Abstract
The development and analysis of engineered enzymes is greatly assisted by the use of high-throughput screening to quickly determine the efficacy of biotransformations under various conditions. Ambient ionization, particularly desorption electrospray ionization (DESI), coupled to high-resolution mass spectrometry has the advantages of minimal requirements for sample preparation before analysis, which renders it suitable for high-throughput screening, in which the accurate mass and potentially the tandem mass spectrometry (MS) fingerprint for any given product can be used for identification. We present a protocol that permits the application of this method in routine biotechnology and chemical biology laboratories that are using engineered enzymes (such as imine reductases and carboxylic acid reductases, mentioned herein) to produce target compounds from substrates (quinoline moieties and phenyl(piperazinyl) moieties, respectively). Through the use of DESI’s MS imaging capabilities, reaction monitoring can be easily visualized via imaging of selected substrate or product ions in a convenient, user-friendly workflow. We describe here how DESI-MS can be used to directly analyze the activity of biotransformations from crude cell lysate, which we term ‘DiBT-MS’. The DiBT-MS method presented here is 10–1,000 times as fast as liquid chromatography-MS, with the full procedure for 96 samples taking ~2 h and consuming far less solvent and sample. Also demonstrated in this protocol is the impact of solvent spray composition on ionization efficiency of the target analyte, the benefits of a nylon membrane slide and the reusability of sample slides in multiple experiments.
Key points
-
Engineering enzymes to perform specific chemical transformations is still an iterative process that involves expressing mutant proteins and screening them for reactivity under different conditions. The screening process can be time consuming if both the protein and the products need to be enriched before analysis.
-
With desorption electrospray ionization (DESI) mass spectrometry, it is possible to analyze the reaction mixture directly via the accurate mass of the products without extensive sample preparation or work-up.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Raw data files pertaining to Figs. 2, 5, 6 and 7 and the Excel file used to calculate reaction rates from DiBT data are available to download from Figshare https://doi.org/10.6084/m9.figshare.27931572.v1.
References
Takáts, Z., Wiseman, J. M., Gologan, B. & Cooks, R. G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 306, 471–473 (2004).
Hale, O. J. & Cooper, H. J. Native mass spectrometry imaging of proteins and protein complexes by Nano-DESI. Anal. Chem. 93, 4619–4627 (2021).
Ferguson, C. N., Benchaar, S. A., Miao, Z., Loo, J. A. & Chen, H. Direct ionization of large proteins and protein complexes by desorption electrospray ionization-mass spectrometry. Anal. Chem. 83, 6468–6473 (2011).
Morato, N. M., Holden, D. T. & Cooks, R. G. High-throughput label-free enzymatic assays using desorption electrospray-ionization mass spectrometry. Angew. Chem. Int. Ed. Engl. 59, 20459–20464 (2020).
Morato, N. M., Le, M. T., Holden, D. T. & Graham Cooks, R. Automated high-throughput system combining small-scale synthesis with bioassays and reaction screening. SLAS Technol. 26, 555–571 (2021).
Eberlin, L. S. DESI-MS imaging of lipids and metabolites from biological samples. In Mass Spectrometry in Metabolomics: Methods and Protocols (ed. Raftery, D.) 299–311 (Springer New York, New York, New York, USA, 2014).
Loren, B. P. et al. High throughput experimentation using DESI-MS to guide continuous-flow synthesis. Sci. Rep. 9, 14745 (2019).
Logsdon, D. L. et al. High-throughput screening of reductive amination reactions using desorption electrospray ionization mass spectrometry. Org. Process Res. Dev. 24, 1647–1657 (2020).
Fedick, P. W. et al. Screening of the Suzuki cross-coupling reaction using desorption electrospray ionization in high-throughput and in Leidenfrost droplet experiments. J. Am. Soc. Mass Spectrom. 30, 2144–2151 (2019).
Venter, A., Sojka, P. E. & Cooks, R. G. Droplet dynamics and ionization mechanisms in desorption electrospray ionization mass spectrometry. Anal. Chem. 78, 8549–8555 (2006).
Wei, Z. et al. High-throughput bioassays using “Dip-and-Go” multiplexed electrospray mass spectrometry. Angew. Chem. Int. Ed. Engl. 58, 17594–17598 (2019).
Girod, M., Moyano, E., Campbell, D. I. & Cooks, R. G. Accelerated bimolecular reactions in microdroplets studied by desorption electrospray ionization mass spectrometry. Chem. Sci. 2, 501–510 (2011).
Kuo, T.-H., Dutkiewicz, E. P., Pei, J. & Hsu, C.-C. Ambient ionization mass spectrometry today and tomorrow: embracing challenges and opportunities. Anal. Chem. 92, 2353–2363 (2020).
Sinclair, E. et al. Validating differential volatilome profiles in Parkinson’s disease. ACS Cent. Sci. 7, 300–306 (2021).
Gan, J. et al. Native mass spectrometry of recombinant proteins from crude cell lysates. Anal. Chem. 89, 4398–4404 (2017).
Woolman, M. et al. Rapid determination of the tumour stroma ratio in squamous cell carcinomas with desorption electrospray ionization mass spectrometry (DESI-MS): a proof-of-concept demonstration. Analyst 142, 3250–3260 (2017).
Pirro, V. et al. Intraoperative assessment of tumor margins during glioma resection by desorption electrospray ionization-mass spectrometry. Proc. Natl Acad. Sci. USA 114, 6700–6705 (2017).
Vimer, S., Ben-Nissan, G. & Sharon, M. Direct characterization of overproduced proteins by native mass spectrometry. Nat. Protoc. 15, 236–265 (2020).
Yan, C. et al. Real-time screening of biocatalysts in live bacterial colonies. J. Am. Chem. Soc. 139, 1408–1411 (2017).
Kempa, E. E. et al. Rapid screening of diverse biotransformations for enzyme evolution. JACS Au 1, 508–516 (2021).
Ramsden, J. I. et al. Biocatalytic N-alkylation of amines using either primary alcohols or carboxylic acids via reductive aminase cascades. J. Am. Chem. Soc. 141, 1201–1206 (2019).
Gahloth, D. et al. Structures of carboxylic acid reductase reveal domain dynamics underlying catalysis. Nat. Chem. Biol. 13, 975–981 (2017).
Lubberink, M. et al. Biocatalytic monoacylation of symmetrical diamines and its application to the synthesis of pharmaceutically relevant amides. ACS Catal. 10, 10005–10009 (2020).
Schnepel, C. et al. Thioester-mediated biocatalytic amide bond synthesis with in situ thiol recycling. Nat. Catal. 6, 89–99 (2023).
Alonzi, D. S., Scott, K. A., Dwek, R. A. & Zitzmann, N. Iminosugar antivirals: the therapeutic sweet spot. Biochem. Soc. Trans. 45, 571–582 (2017).
France, S. P. et al. One-pot cascade synthesis of mono- and disubstituted piperidines and pyrrolidines using carboxylic acid reductase (CAR), ω-transaminase (ω-TA), and imine reductase (IRED) biocatalysts. ACS Catal. 6, 3753–3759 (2016).
Morato, N. M. & Cooks, R. G. Inter-platform assessment of performance of high-throughput desorption electrospray ionization mass spectrometry. Talanta Open 4, 100046 (2021).
Steven, R. T. et al. Evaluation of inlet temperature with three sprayer designs for desorption electrospray ionization mass spectrometry tissue analysis. J. Am. Soc. Mass Spectrom. 35, 224–233 (2024).
Leferink, N. G. H. et al. An automated pipeline for the screening of diverse monoterpene synthase libraries. Sci. Rep. 9, 11936 (2019).
Kempa, E. E., Hollywood, K. A., Smith, C. A. & Barran, P. E. High throughput screening of complex biological samples with mass spectrometry—from bulk measurements to single cell analysis. Analyst 144, 872–891 (2019).
Dueñas, M. E. et al. Advances in high‐throughput mass spectrometry in drug discovery. EMBO Mol. Med. 15, e14850 (2023).
Choe, K. & Sweedler, J. V. Workflow for high-throughput screening of enzyme mutant libraries using matrix-assisted laser desorption/ionization mass spectrometry analysis of Escherichia coli colonies. Bio Protoc. 13, e4862 (2023).
Castro, D. C., Xie, Y. R., Rubakhin, S. S., Romanova, E. V. & Sweedler, J. V. Image-guided MALDI mass spectrometry for high-throughput single-organelle characterization. Nat. Methods 18, 1233–1238 (2021).
Wu, X. et al. In vitro ADME profiling using high-throughput rapidfire mass spectrometry: cytochrome P450 inhibition and metabolic stability assays. J. Biomol. Screen. 17, 761–772 (2012).
Pluchinsky, A. J., Wackelin, D. J., Huang, X., Arnold, F. H. & Mrksich, M. High throughput screening with SAMDI mass spectrometry for directed evolution. J. Am. Chem. Soc. 142, 19804–19808 (2020).
Sinclair, I. et al. Acoustic mist ionization platform for direct and contactless ultrahigh-throughput mass spectrometry analysis of liquid samples. Anal. Chem. 91, 3790–3794 (2019).
Myung, S. et al. Coupling desorption electrospray ionization with ion mobility/mass spectrometry for analysis of protein structure: evidence for desorption of folded and denatured states. J. Phys. Chem. B 110, 5045–5051 (2006).
Badu-Tawiah, A., Bland, C., Campbell, D. I. & Cooks, R. G. Non-aqueous spray solvents and solubility effects in desorption electrospray ionization. J. Am. Soc. Mass Spectrom. 21, 572–579 (2010).
Sobreira, T. J. P. et al. High-throughput screening of organic reactions in microdroplets using desorption electrospray ionization mass spectrometry (DESI-MS): hardware and software implementation. Anal. Methods 12, 3654–3669 (2020).
Furey, A., Moriarty, M., Bane, V., Kinsella, B. & Lehane, M. Ion suppression; a critical review on causes, evaluation, prevention and applications. Talanta 115, 104–122 (2013).
Cappiello, A., Famiglini, G., Palma, P. & Trufelli, H. Matrix effects in liquid chromatography-mass spectrometry. J. Liq. Chromatogr. Relat. Technol. 33, 1067–1081 (2010).
Needham, S. R. Microspray and microflow liquid chromatography: the way forward for LC–MS bioanalysis. Bioanalysis 9, 1935–1937 (2017).
Cooks, R. G., Ouyang, Z., Takats, Z. & Wiseman, J. M. Ambient mass spectrometry. Science 311, 1566–1570 (2006).
Acknowledgements
The authors would like to acknowledge Waters Corporation, and in particular E. Jones, for their support of our MS development. We acknowledge the Engineering and Physical Sciences Research Council (EPSRC), the Biotechnology and Biological Sciences Research Council (BBSRC) and AstraZeneca plc for funding under the Prosperity Partnership EP/S005226/1. We acknowledge the support of EPSRC through the strategic equipment award EP/T019328/1 and BBSRC for funding the Centre for Synthetic Biology of Fine and Speciality Chemicals BB/M017702/1. R. Smith acknowledges Bristol Myers Squib and the Department of Chemistry for funding her PhD studentship.
Author information
Authors and Affiliations
Contributions
R.K. and R. Smith developed the sprayer head solvent optimization methods, acquired all spectra and drafted the manuscript. E.E.K. contributed to method development while studying at The University of Manchester and paper drafting while under employment by AstraZeneca plc. C.S. prepared CAR enzymes, and P.E.B., R. Smith, R. Spiess, C.S., S.L.F. and N.J.T. contributed to method development and paper revisions.
Corresponding author
Ethics declarations
Competing interests
E.E.K. is an employee of AstraZeneca and owns or has the option to own stocks in this company.
Peer review
Peer review information
Nature Protocols thanks Manfred Reetz, László Csaba Bencze and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Yan, C. et al. J. Am. Chem. Soc. 139, 1408–1411 (2017): https://doi.org/10.1021/jacs.6b12165
Kempa, E. E. et al. JACS Au 1, 508–516 (2021): https://doi.org/10.1021/jacsau.1c00027
Extended data
Extended Data Fig. 1 Generating slide area and optimizing visualization parameters within HD Imaging.
a, Screenshots of the import and slide selection process (described in protocol Steps 14–17) for HD Imaging. A photo of the nylon slide can be imported to the acquisition computer by pen drive or email. 1. Select ‘DESI-MS’ as the experiment type and choose the slide position on the stage. 2. Align the DESI stage movements with the correct position of the slide. 3. Finalize the slide area and double-check boundaries. b, Screenshots of protocol Steps 18–22. The instrument parameters must be customized by mass range, analyzer mode and type of MS experiment (ion MS versus MS versus tandem MS). The area selected for recording (red rectangle) should include a margin of nylon membrane outside the target area, to ensure complete recording of the sample wells. Finally, the default scanning parameters (and associated time for recording the experiment) are displayed.
Extended Data Fig. 2 HD imaging data analysis window overview for 6,7-dimethoxymethyl-3,4-dihydroisoquinoline.
Top left square: List of imported acquired files (after protocol Steps 33–37). A preview of each file is shown as a small black image, with a species highlighted. The TIC over the complete slide and for the ions of interest is shown in Fig. S2 (Supplementary Data). The current file that is visualized in the rest of the HD Imaging window is highlighted in blue (bottom of this square). Bottom left square: Molecular ions associated with the selected imported data file, categorized by summed ion intensity (high to low), with the top 1,000 ions displayed. The highest ion selected corresponds to the reactant depicted in Fig. 2. Top right rectangle: Image selection tools to normalize visualization across image files. Additional options include blending results for multiple ions in the same image (data set blend) or producing a smoothed pixel image (image smoothing, linear interpolation). Bottom right rectangle: Mass spectra for the acquired file selected in the top right. The selected ion (highlighted in purple) at m/z 206.13 corresponds to the protonated starting material for the IRED biotransformation displayed in Fig. 2 (and Figs. S2 and S3) and is also the most abundant ion recorded. The corresponding selected ion chromatogram is shown in Fig. S4.
Supplementary information
Supplementary Information
Supplementary data in support of Extended Data Fig. 2 and Fig. 5; Supplementary notes on DESI stage control for the different DESI sources; Supplementary Figs. S1–S15
Supplementary Data 1
Supplementary data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Knox, R., Smith, R., Kempa, E.E. et al. Direct analysis of biotransformations with mass spectrometry—DiBT-MS. Nat Protoc (2025). https://doi.org/10.1038/s41596-025-01161-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-025-01161-9