Abstract
Cardiac organs-on-a-chip (OoCs) or microphysiological systems have the potential to predict cardiac effects of new drug candidates, including unanticipated cardiac outcomes, which are among the main causes for drug attrition. This protocol describes how to prepare and use a cardiac OoC containing cardiomyocytes differentiated from human induced pluripotent stem cells (hiPS cells). The use of cells derived from hiPS cells as reliable sources of human cells from diverse genetic backgrounds also holds great potential, especially when cultured in OoCs that are physiologically relevant culture platforms. To promote the broad adoption of hiPS cell-derived cardiac OoCs in the drug development field, there is a need to first ensure reproducibility in their preparation and use. This protocol aims to provide key information on how to reduce sources of variability during hiPS cell maintenance, differentiation, loading and maturation in OoCs. Variability in these procedures can lead to inconsistent purity after differentiation and variable function between batches of microtissues formed in OoCs. This protocol also focuses on describing the handling and functional assessment of cardiac microtissues using live-cell microscopy approaches to quantify parameters of cellular electrophysiology, calcium transients and contractility. The protocol consists of five stages: (1) thaw and maintain hiPS cells, (2) differentiate hiPS cell cardiomyocytes, (3) load differentiated cells into OoCs, (4) maintain and characterize loaded cells, and (5) evaluate and utilize cardiac OoCs. Execution of the entire protocol takes ~40 days. The required skills to carry out the protocol are experience with sterile techniques, mammalian cell culture and maintaining hiPS cells in a pluripotent state.
Key points
-
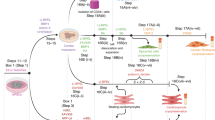
Preparation and use of a cardiac organ-on-a-chip is described in five stages: thawing of human induced pluripotent stem cells, differentiation into cardiomyocytes, loading the microfluidic chip with cardiomyocytes, maturation of microtissues and functional characterization with image-based assays.
-
This protocol reproduces the physiology of the myocardium more robustly than traditional 2D cultures, ensuring reproducibility and reducing variability in results that can limit the use of organs-on-a-chip in drug development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Data supporting the findings of this study are shared in the paper. Source data accompany this paper. Source data are provided with this paper.
Code availability
Codes used for analyzing videos of fluorescent labels in cells are publicly available at Zenodo https://doi.org/10.5281/zenodo.12563741. The graphical user interface for characterizing movement of cells with digital image correlation using brightfield microscopy videos of beating cells has been published and is publicly available18.
References
Tsao, C. W. et al. Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circulation 147, e93–e621 (2023).
Gwathmey, J. K., Tsaioun, K. & Hajjar, R. J. Cardionomics: a new integrative approach for screening cardiotoxicity of drug candidates. Expert Opin. Drug Metab. Toxicol. 5, 647–660 (2009).
Weaver, R. J. & Valentin, J. P. Today’s challenges to de-risk and predict drug safety in human ‘Mind-the-Gap’. Toxicol. Sci. 167, 307–321 (2019).
Vunjak-Novakovic, G., Ronaldson-Bouchard, K. & Radisic, M. Organs-on-a-chip models for biological research. Cell 184, 4597–4611 (2021).
Baran, S. W. et al. Perspectives on the evaluation and adoption of complex in vitro models in drug development: workshop with the FDA and the pharmaceutical industry (IQ MPS Affiliate). ALTEX https://doi.org/10.14573/altex.2112203 (2022).
Huebsch, N. et al. Metabolically driven maturation of human-induced-pluripotent-stem-cell-derived cardiac microtissues on microfluidic chips. Nat. Biomed. Eng. 6, 372–388 (2022).
Charrez, B. et al. Heart muscle microphysiological system for cardiac liability prediction of repurposed COVID-19 therapeutics. Front. Pharmacol. 12, 684252 (2021).
Mathur, A. et al. Human iPSC-based cardiac microphysiological system for drug screening applications. Sci. Rep. 5, 8883 (2015).
Miller, J. M. et al. Heart slice culture system reliably demonstrates clinical drug-related cardiotoxicity. Toxicol. Appl. Pharmacol. 406, 115213 (2020).
Miller, J. M. et al. Biomimetic cardiac tissue culture model (CTCM) to emulate cardiac physiology and pathophysiology ex vivo. Commun. Biol. 5, 934 (2022).
Wu, P. et al. Maturation strategies and limitations of induced pluripotent stem cell-derived cardiomyocytes. Biosci. Rep. https://doi.org/10.1042/BSR20200833 (2021).
Schroer, A., Pardon, G., Castillo, E., Blair, C. & Pruitt, B. Engineering hiPSC cardiomyocyte in vitro model systems for functional and structural assessment. Prog. Biophys. Mol. Biol. 144, 3–15 (2019).
Ronaldson-Bouchard, K. et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556, 239–243 (2018).
Ribeiro, A. J. S. et al. Considerations for an in vitro, cell-based testing platform for detection of drug-induced inotropic effects in early drug development. Part 2: designing and fabricating microsystems for assaying cardiac contractility with physiological relevance using human iPSC-cardiomyocytes. Front. Pharmacol. 10, 934 (2019).
Wang, G. et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat. Med. 20, 616–623 (2014).
Guo, J. et al. Substrate mechanics unveil early structural and functional pathology in iPSC micro-tissue models of hypertrophic cardiomyopathy. iScience 27, 109954 (2024).
Mozneb, M. et al. Multi-lineage heart-chip models drug cardiotoxicity and enhances maturation of human stem cell-derived cardiovascular cells. Lab Chip 24, 869–881 (2024).
Ribeiro, A. J. S. et al. Multi-imaging method to assay the contractile mechanical output of micropatterned human iPSC-derived cardiac myocytes. Circ. Res. 120, 1572–1583 (2017).
Maddah, M. et al. A non-invasive platform for functional characterization of stem-cell-derived cardiomyocytes with applications in cardiotoxicity testing. Stem Cell Rep. 4, 621–631 (2015).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Huang, Y. L., Walker, A. S. & Miller, E. W. A photostable silicon rhodamine platform for optical voltage sensing. J. Am. Chem. Soc. 137, 10767–10776 (2015).
McCain, M. L., Sheehy, S. P., Grosberg, A., Goss, J. A. & Parker, K. K. Recapitulating maladaptive, multiscale remodeling of failing myocardium on a chip. Proc. Natl Acad. Sci. USA 110, 9770–9775 (2013).
Charrez, B. et al. In vitro safety ‘clinical trial’ of the cardiac liability of drug polytherapy. Clin. Transl. Sci. 14, 1155–1165 (2021).
Charwat, V. et al. Validating the arrhythmogenic potential of high-, intermediate-, and low-risk drugs in a human-induced pluripotent stem cell-derived cardiac microphysiological system. ACS Pharmacol. Transl. Sci. 5, 652–667 (2022).
Ferdinandy, P. et al. Definition of hidden drug cardiotoxicity: paradigm change in cardiac safety testing and its clinical implications. Eur. Heart J. 40, 1771–1777 (2019).
Yang, X., Ribeiro, A. J. S., Pang, L. & Strauss, D. G. Use of human iPSC-CMs in nonclinical regulatory studies for cardiac safety assessment. Toxicol. Sci. 190, 117–126 (2022).
Mamoshina, P., Rodriguez, B. & Bueno-Orovio, A. Toward a broader view of mechanisms of drug cardiotoxicity. Cell Rep. Med. 2, 100216 (2021).
Luo, M. & Anderson, M. E. Mechanisms of altered Ca2+ handling in heart failure. Circ. Res. 113, 690–708 (2013).
Leung, C. M. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2, 33 (2022).
Huebsch, N. et al. Automated video-based analysis of contractility and calcium flux in human-induced pluripotent stem cell-derived cardiomyocytes cultured over different spatial scales. Tissue Eng. Part C Methods 21, 467–479 (2015).
Dame, K. & Ribeiro, A. J. S. Microengineered systems with iPSC-derived cardiac and hepatic cells to evaluate drug adverse effects. Exp. Biol. Med. 246, 317–331 (2021).
Arefin, A. et al. Reproducibility of drug-induced effects on the contractility of an engineered heart tissue derived from human pluripotent stem cells. Front. Pharmacol. 14, 1212092 (2023).
Lian, X. et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl Acad. Sci. USA 109, E1848–E1857 (2012).
Pointon, A. et al. Cardiovascular microphysiological systems (CVMPS) for safety studies—a pharma perspective. Lab Chip 21, 458–472 (2021).
Liu, S., Fang, C., Zhong, C., Li, J. & Xiao, Q. Recent advances in pluripotent stem cell-derived cardiac organoids and heart-on-chip applications for studying anti-cancer drug-induced cardiotoxicity. Cell Biol. Toxicol. 39, 2527–2549 (2023).
Arslan, U., Orlova, V. V. & Mummery, C. L. Perspectives for future use of cardiac microtissues from human pluripotent stem cells. ACS Biomater. Sci. Eng. 8, 4605–4609 (2022).
Wauchop, M. et al. Maturation of iPSC-derived cardiomyocytes in a heart-on-a-chip device enables modeling of dilated cardiomyopathy caused by R222Q-SCN5A mutation. Biomaterials 301, 122255 (2023).
Mastikhina, O. et al. Human cardiac fibrosis-on-a-chip model recapitulates disease hallmarks and can serve as a platform for drug testing. Biomaterials 233, 119741 (2020).
Mourad, O., Yee, R., Li, M. & Nunes, S. S. Modeling heart diseases on a chip: advantages and future opportunities. Circ. Res. 132, 483–497 (2023).
Ergir, E. et al. Generation and maturation of human iPSC-derived 3D organotypic cardiac microtissues in long-term culture. Sci. Rep. 12, 17409 (2022).
Volmert, B. et al. A patterned human primitive heart organoid model generated by pluripotent stem cell self-organization. Nat. Commun. 14, 8245 (2023).
Varzideh, F. et al. Human cardiomyocytes undergo enhanced maturation in embryonic stem cell-derived organoid transplants. Biomaterials 192, 537–550 (2019).
Lewis-Israeli, Y. R. et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun. 12, 5142 (2021).
Ma, Z. et al. Contractile deficits in engineered cardiac microtissues as a result of MYBPC3 deficiency and mechanical overload. Nat. Biomed. Eng. 2, 955–967 (2018).
Caspi, O. et al. Modeling of arrhythmogenic right ventricular cardiomyopathy with human induced pluripotent stem cells. Circ. Cardiovasc. Genet. 6, 557–568 (2013).
Yazawa, M. et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471, 230–234 (2011).
Huebsch, N. et al. Miniaturized iPS-cell-derived cardiac muscles for physiologically relevant drug response analyses. Sci. Rep. 6, 24726 (2016).
Lee-Montiel, F. T. et al. Integrated isogenic human induced pluripotent stem cell-based liver and heart microphysiological systems predict unsafe drug-drug interaction. Front. Pharmacol. 12, 667010 (2021).
Veldhuizen, J., Cutts, J., Brafman, D. A., Migrino, R. Q. & Nikkhah, M. Engineering anisotropic human stem cell-derived three-dimensional cardiac tissue on-a-chip. Biomaterials 256, 120195 (2020).
Liu, Y. et al. Human heart-on-a-chip microphysiological system comprising endothelial cells, fibroblasts, and iPSC-derived cardiomyocytes. Sci. Rep. 14, 18063 (2024).
Ang, Y. S. et al. Disease model of GATA4 mutation reveals transcription factor cooperativity in human cardiogenesis. Cell 167, 1734–1749 e1722 (2016).
Judge, L. M. et al. A BAG3 chaperone complex maintains cardiomyocyte function during proteotoxic stress. JCI Insight 2, e94623 (2017).
Yang, X., Pabon, L. & Murry, C. E. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Res. 114, 511–523 (2014).
Biendarra-Tiegs, S. M., Secreto, F. J. & Nelson, T. J. Addressing variability and heterogeneity of induced pluripotent stem cell-derived cardiomyocytes. Adv. Exp. Med. Biol. 1212, 1–29 (2020).
Lyra-Leite, D. M. et al. A review of protocols for human iPSC culture, cardiac differentiation, subtype-specification, maturation, and direct reprogramming. STAR Protoc. 3, 101560 (2022).
Tagle, D. A. The NIH microphysiological systems program: developing in vitro tools for safety and efficacy in drug development. Curr. Opin. Pharmacol. 48, 146–154 (2019).
Tomlinson, L. et al. Considerations from an international regulatory and pharmaceutical industry (IQ MPS Affiliate) workshop on the standardization of complex in vitro models in drug development. Adv. Biol. https://doi.org/10.1002/adbi.202300131 (2023).
Kuo, H. H. et al. Negligible-cost and weekend-free chemically defined human iPSC culture. Stem Cell Rep. 14, 256–270 (2020).
Ohnuki, M., Takahashi, K. & Yamanaka, S. Generation and characterization of human induced pluripotent stem cells. Curr. Protoc. Stem Cell Biol. https://doi.org/10.1002/9780470151808.sc04a02s9 (2009).
Pantazis, C. B. et al. A reference human induced pluripotent stem cell line for large-scale collaborative studies. Cell Stem Cell 29, 1685–1702 e1622 (2022).
Viana, M. P. et al. Integrated intracellular organization and its variations in human iPS cells. Nature 613, 345–354 (2023).
Rivera, T., Zhao, Y., Ni, Y. & Wang, J. Human-induced pluripotent stem cell culture methods under cGMP conditions. Curr. Protoc. Stem Cell Biol. 54, e117 (2020).
Richards, C., Sarkar, S., Kandell, J., Snyder, R. & Lakshmipathy, U. Assessing the suitability of cell counting methods during different stages of a cell processing workflow using an ISO 20391-2 guided study design and analysis. Front. Bioeng. Biotechnol. 11, 1223227 (2023).
Warnecke, N. et al. Generation of bi-allelic MYBPC3 truncating mutant and isogenic control from an iPSC line of a patient with hypertrophic cardiomyopathy. Stem Cell Res. 55, 102489 (2021).
Marty, I. & Faure, J. Excitation-contraction coupling alterations in myopathies. J. Neuromuscul. Dis. 3, 443–453 (2016).
Butler, L. et al. Enhanced characterization of contractility in cardiomyocytes during early drug safety assessment. Toxicol. Sci. 145, 396––406 (2015).
Chung, J. H., Biesiadecki, B. J., Ziolo, M. T., Davis, J. P. & Janssen, P. M. Myofilament calcium sensitivity: role in regulation of in vivo cardiac contraction and relaxation. Front. Physiol. 7, 562 (2016).
Tandon, N. et al. Electrical stimulation systems for cardiac tissue engineering. Nat. Protoc. 4, 155–173 (2009).
Drubin, D. G. & Hyman, A. A. Stem cells: the new ‘model organism’. Mol. Biol. Cell 28, 1409–1411 (2017).
Fonoudi, H., Lyra-Leite, D. M., Javed, H. A. & Burridge, P. W. Generating a cost-effective, weekend-free chemically defined human induced pluripotent stem cell (hiPSC) culture medium. Curr. Protoc. Stem Cell Biol. 53, e110 (2020).
Wakabayashi, S. et al. Overexpression of Na+/H+ exchanger 1 specifically induces cell death in human iPS cells via sustained activation of the Rho kinase ROCK. J. Biol. Chem. 294, 19577–19588 (2019).
Anderson, P. A., Malouf, N. N., Oakeley, A. E., Pagani, E. D. & Allen, P. D. Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ. Res. 69, 1226–1233 (1991).
Garcia, M. I., Chen, J. J. & Boehning, D. Genetically encoded calcium indicators for studying long-term calcium dynamics during apoptosis. Cell Calcium 61, 44–49 (2017).
Garcia, M. I. & Boehning, D. Cardiac inositol 1,4,5-trisphosphate receptors. Biochim. Biophys. Acta Mol. Cell Res. 1864, 907–914 (2017).
Marks, A. R. Cardiac intracellular calcium release channels: role in heart failure. Circ. Res. 87, 8–11 (2000).
Marks, A. R. Calcium and the heart: a question of life and death. J. Clin. Invest. 111, 597–600 (2003).
He, J. Q., Ma, Y., Lee, Y., Thomson, J. A. & Kamp, T. J. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ. Res. 93, 32–39 (2003).
Endoh, M. Force-frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance. Eur. J. Pharmacol. 500, 73–86 (2004).
Acknowledgements
The authors thank J. Florian from the Division of Applied Regulatory Science at the US Food and Drug Administration (FDA) for his valuable suggestions during the writing of the manuscript; E. Miller (Berkeley College of Chemistry) for supplying the voltage sensing dye BeRST; J. Berger from the FDA Library for her editorial support, and Z. Ma for his valuable assistance during the manuscript preparation.
Author information
Authors and Affiliations
Contributions
M.I.G., A.J.S.R., K.D., V.C., R.Y. and B.A.S. developed and optimized the protocol. M.I.G. and A.J.S.R. wrote the manuscript. M.I.G. performed experiments. V.C., B.A.S. and K.E.H. provided microfluidic chambers and protocols for handling and use. H.F. and S.T.W. contributed to the development of software used for this protocol. All authors contributed to the manuscript and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
Work for the manuscript was completed by A.J.S.R., K.D., and R.Y. while employees of the FDA. A.J.S.R is currently employed by Hovione PharmaScience Ltd. K.D. is currently employed by United Therapeutics Corporation. R.Y. is currently part of the Department of Biomedical Engineering and Mechanics at Virginia Polytechnic Institute and State University. K.E.H., V.C., S.T.W., H.F. and B.A.S. have either a financial or equity relationship with Organos Inc. and both they and the company may benefit from commercialization of the results of this research. All other authors declare that they have no competing financial interests and that the article reflects the views of the authors and should not be construed to represent the FDA’s views or policies.
Peer review
Peer review information
Nature Protocols thanks Brian Timko, Hidetoshi Masumoto, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Mathur, A. et al. Sci. Rep. 5, 8883 (2015): https://doi.org/10.1038/srep08883
Charrez, B. et al. Front. Pharmacol. 12, 684252 (2021): https://doi.org/10.3389/fphar.2021.684252
Huebsch, N. et al. Nat. Biomed. Eng. 6, 372–388 (2022): https://doi.org/10.1038/s41551-022-00884-4
Arefin, A. et al. Front. Pharmacol. 14, 1212092 (2023): https://doi.org/10.3389/fphar.2023.1212092
Supplementary information
Supplementary Information
Section 1. Supplementary Figs. 1–4, Section 2. Supplementary Tables 1 and 2, Section 3. Supplementary Methods, Section 4. Supplementary Software. Section 5. Supplementary References.
Supplementary Video 1
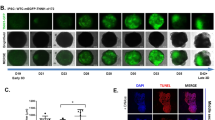
Brightfield video of differentiated hiPS cell-cardiomyocytes. Cardiomyocyte contractions were first observed by light microscopy at day 7 of differentiation and were easily observed by naked eye without microscopic magnification from day 8 onward. Sheet-like appearance is expected to start spontaneously contract as a single sheet starting day 7 of differentiation.
Supplementary Video 2
Video of differentiated hiPS cell-cardiomyocytes expressing GCaMP6f fluorescent protein. For cells expressing GCaMP6f, changes in fluorescent intensity are typically apparent. Using eGFP channel, intracellular calcium typically seems to flow evenly within the beating sheet of cells. Fluorescent variations usually start being observed on day 7 of differentiation.
Supplementary Video 3
Video example of over excited tissue before and after 20 min rest. Representative videos showing over excited tissue after handling.
Supplementary Video 4
Video example of over excited tissue before and after 20 min rest. Representative video shows how tissue goes back to baseline spontaneous contraction after 20 min rest on the microscope.
Source data
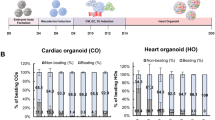
Source Data Fig. 6
Source data for contraction, intracellular calcium, action potential and raw data for calcium trace.
Source Data Fig. 7
Source data for contraction, intracellular calcium, action potential and raw data for contraction displacement trace.
Rights and permissions
About this article
Cite this article
Garcia, M.I., Dame, K., Charwat, V. et al. Human induced pluripotent stem cell-derived cardiomyocytes and their use in a cardiac organ-on-a-chip to assay electrophysiology, calcium and contractility. Nat Protoc (2025). https://doi.org/10.1038/s41596-025-01166-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-025-01166-4