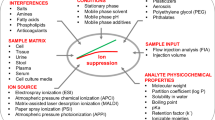
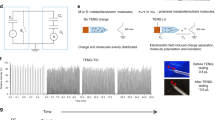
Abstract
The direct coupling of ion-exchange chromatography with mass spectrometry using electrochemical ion suppression creates a hyphenated technique with selectivity and specificity for the analysis of highly polar and ionic compounds. The technique has enabled new applications in environmental chemistry, food chemistry, forensics, cell biology and, more recently, metabolomics. Robust, reproducible and quantitative methods for the analysis of highly polar and ionic metabolites help meet a longstanding analytical need in metabolomics. Here, we provide step-by-step instructions for both untargeted and semi-targeted metabolite analysis from cell, tissue or biofluid samples by using anion-exchange chromatography–high-resolution tandem mass spectrometry (AEC-MS/MS). The method requires minimal sample preparation and is robust, sensitive and selective. It provides comprehensive coverage of hundreds of metabolites found in primary and secondary metabolic pathways, including glycolysis, the pentose phosphate pathway, the tricarboxylic acid cycle, purine and pyrimidine metabolism, amino acid degradation and redox metabolism. An inline electrolytic ion suppressor is used to quantitatively neutralize OH− ions in the eluent stream, after chromatographic separation, enabling AEC to be directly coupled with MS. Counter ions are also removed during this process, creating a neutral pH, aqueous eluent with a simplified matrix optimal for negative ion MS analysis. Sample preparation through to data analysis and interpretation is described in the protocol, including a guide to which metabolites and metabolic pathways are suitable for analysis by using AEC-MS/MS.
Key points
-
This protocol uses an integrated ion-chromatography-mass spectrometry system that incorporates in-line eluent generation and electrochemical ion suppression for the analysis of metabolites in cells, tissues and biofluids.
-
Highly sensitive, selective, reproducible and robust, this protocol provides an alternative to methods such as HILIC-MS and ion-pairing-MS, which can be more limited by analytical conditions and provide different metabolite coverage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The main data discussed in this protocol are available in the supporting primary research papers (https://doi.org/10.1038/s42003-020-0957-6 and https://doi.org/10.1038/s41467-022-34095-x). The raw datasets have also been deposited in publicly available repositories for research purposes, and any further data are available from the corresponding author upon reasonable request. Source data for Fig. 1 is available in the Oxford University Research Archive with the identifiers https://doi.org/10.5287/bodleian:2abVOAvrg and https://doi.org/10.5287/bodleian:eyq4Qj8AR (ref. 19). Source data for Fig. 3 are available in Supplementary Data 6.
References
Bennett, B. D. et al. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat. Chem. Biol. 5, 593–599 (2009).
Hu, T. et al. Metabolic regulation of the immune system in health and diseases: mechanisms and interventions. Signal Transduct. Target. Ther. 9, 268 (2024).
Ayari, A. et al. Influenza infection rewires energy metabolism and induces browning features in adipose cells and tissues. Commun. Biol. 3, 237 (2020).
Haythorne, E. et al. Altered glycolysis triggers impaired mitochondrial metabolism and mTORC1 activation in diabetic β-cells. Nat. Commun. 13, 6754 (2022).
Li, Q. et al. Energy metabolism: a critical target of cardiovascular injury. Biomed. Pharmacother. 165, 115271 (2023).
De Vos, R. C. et al. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2, 778–791 (2007).
Want, E. J. et al. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 8, 17–32 (2013).
Want, E. J. et al. Global metabolic profiling procedures for urine using UPLC–MS. Nat. Protoc. 5, 1005–1018 (2010).
Dunn, W. B. et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 6, 1060–1083 (2011).
Smart, K. F., Aggio, R. B., Van Houtte, J. R. & Villas-Bôas, S. G. Analytical platform for metabolome analysis of microbial cells using methyl chloroformate derivatization followed by gas chromatography–mass spectrometry. Nat. Protoc. 5, 1709–1729 (2010).
Sellick, C. A., Hansen, R., Stephens, G. M., Goodacre, R. & Dickson, A. J. Metabolite extraction from suspension-cultured mammalian cells for global metabolite profiling. Nat. Protoc. 6, 1241–1249 (2011).
Martano, G. et al. Fast sampling method for mammalian cell metabolic analyses using liquid chromatography–mass spectrometry. Nat. Protoc. 10, 1–11 (2015).
Fujii, T. et al. Direct metabolomics for plant cells by live single-cell mass spectrometry. Nat. Protoc. 10, 1445–1456 (2015).
Paglia, G. & Astarita, G. Metabolomics and lipidomics using traveling-wave ion mobility mass spectrometry. Nat. Protoc. 12, 797–813 (2017).
Yuan, H. et al. Metabolomics analysis reveals global acetoin stress response of Bacillus licheniformis. Metabolomics 15, 1–12 (2019).
Zhang, J. et al. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat. Protoc. 7, 1068–1085 (2012).
Beckmann, M., Parker, D., Enot, D. P., Duval, E. & Draper, J. High-throughput, nontargeted metabolite fingerprinting using nominal mass flow injection electrospray mass spectrometry. Nat. Protoc. 3, 486–504 (2008).
Dunn, W. B. et al. Mass appeal: metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics 9, 44–66 (2013).
Walsby-Tickle, J. et al. Anion-exchange chromatography mass spectrometry provides extensive coverage of primary metabolic pathways revealing altered metabolism in IDH1 mutant cells. Commun. Biol. 3, 247 (2020).
Walsby-Tickle, J. Investigating Isocitrate Dehydrogenase Mutations in Cancer Using New Metabolomics Methods. University of Oxford thesis (2020).
Kadir, A. A. et al. On the interdependence of ketone body oxidation, glycogen content, glycolysis and energy metabolism in the heart. J. Physiol. 601, 1207–1224 (2023).
Saraç, H. et al. Systematic characterization of chromatin modifying enzymes identifies KDM3B as a critical regulator in castration resistant prostate cancer. Oncogene 39, 2187–2201 (2020).
Winter, H. et al. Identification of circulating genomic and metabolic biomarkers in intrahepatic cholangiocarcinoma. Cancers (Basel) 11, 1895 (2019).
Schulthess, J. et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 50, 432–445.e7 (2019).
Riffelmacher, T. et al. Autophagy-dependent generation of free fatty acids is critical for normal neutrophil differentiation. Immunity 47, 466–480.e5 (2017).
Vaughan-Jackson, A. et al. Density dependent regulation of inflammatory responses in macrophages. Front. Immunol. 13, 895488 (2022).
Yalaz, C. et al. Cone photoreceptor phosphodiesterase PDE6H inhibition regulates cancer cell growth and metabolism, replicating the dark retina response. Cancer Metab. 12, 5 (2024).
Bardella, C. et al. Expression of Idh1R132H in the murine subventricular zone stem cell niche recapitulates features of early gliomagenesis. Cancer Cell 30, 578–594 (2016).
Finelli, M. J. et al. Oxidation resistance 1 modulates glycolytic pathways in the cerebellum via an interaction with glucose-6-phosphate isomerase. Mol. Neurobiol. 56, 1558–1577 (2019).
Dearlove, D. J. et al. The effects of endogenously- and exogenously-induced hyperketonemia on exercise performance and adaptation. Physiol. Rep. 10, e15309 (2022).
Park, K. C. et al. Disrupted propionate metabolism evokes transcriptional changes in the heart by increasing histone acetylation and propionylation. Nat. Cardiovasc. Res. 2, 1221–1245 (2023).
San Millan, A. et al. Integrative analysis of fitness and metabolic effects of plasmids in Pseudomonas aeruginosa PAO1. ISME J. 12, 3014–3024 (2018).
Then, C. K. et al. Dietary fibre supplementation enhances radiotherapy tumour control and alleviates intestinal radiation toxicity. Microbiome 12, 89 (2024).
Sanchez, M. I. G., McCullagh, J., Guy, R. H. & Compton, R. G. Reverse iontophoretic extraction of metabolites from living plants and their identification by ion-chromatography coupled to high resolution mass spectrometry. Phytochem. Anal. 28, 195–201 (2017).
Smith, E. N., McCullagh, J. S. O., Ratcliffe, R. G. & Kruger, N. J. Limitations of deuterium-labelled substrates for quantifying NADPH metabolism in heterotrophic Arabidopsis cell cultures. Metabolites 9, 205 (2019).
Ngere, J. B. et al. Ion-exchange chromatography coupled to mass spectrometry in life science, environmental, and medical research. Anal. Chem. 95, 152–166 (2023).
Xu, Y.-F., Lu, W. & Rabinowitz, J. D. Avoiding misannotation of in-source fragmentation products as cellular metabolites in liquid chromatography–mass spectrometry-based metabolomics. Anal. Chem. 87, 2273–2281 (2015).
Gil, A. et al. Stability of energy metabolites—an often overlooked issue in metabolomics studies: a review. Electrophoresis 36, 2156–2169 (2015).
Schwaiger, M. et al. Anion-exchange chromatography coupled to high-resolution mass spectrometry: a powerful tool for merging targeted and non-targeted metabolomics. Anal. Chem. 89, 7667–7674 (2017).
Nanadikar, M. S. et al. IDH3γ functions as a redox switch regulating mitochondrial energy metabolism and contractility in the heart. Nat. Commun. 14, 2123 (2023).
Grotehans, N. et al. Ribonucleotide synthesis by NME6 fuels mitochondrial gene expression. EMBO J. 42, e113256 (2023).
Floros, D. J., Xu, K., Berthiller, F. & Schwartz-Zimmermann, H. Comparison of chromatographic conditions for the targeted tandem mass spectrometric determination of 354 mammalian metabolites. J. Chromatogr. A 1697, 463985 (2023).
Baghdadi, M. et al. Reduced insulin signaling in neurons induces sex-specific health benefits. Sci. Adv. 9, eade8137 (2023).
Ahola, S. et al. OMA1-mediated integrated stress response protects against ferroptosis in mitochondrial cardiomyopathy. Cell Metab. 34, 1875–1891.e7 (2022).
Liu, X. & Xu, G. Recent advances in using mass spectrometry for mitochondrial metabolomics and lipidomics—a review. Anal. Chim. Acta 1037, 3–12 (2018).
Burgess, K., Creek, D., Dewsbury, P., Cook, K. & Barrett, M. P. Semi-targeted analysis of metabolites using capillary-flow ion chromatography coupled to high-resolution mass spectrometry. Rapid Commun. Mass Spectrom. 25, 3447–3452 (2011).
Corry, T. A., Jackson, B. A. & Ray, A. D. Impurity analysis of 2-butynoic acid by ion chromatography–mass spectrometry. J. Chromatogr. A 1604, 460470 (2019).
Li, X. et al. Mechanistic insights into metabolic function of dynamin-related protein 1. J. Lipid Res. 65, 100633 (2024).
Tan, L., Martinez, S. A., Lorenzi, P. L. & Karlstaedt, A. Quantitative analysis of acetyl-CoA, malonyl-CoA, and succinyl-CoA in myocytes. J. Am. Soc. Mass Spectrom. 34, 2567–2574 (2023).
Chen, Y. et al. Development of a rational strategy for integration of lactate dehydrogenase A suppression into therapeutic algorithms for head and neck cancer. Br. J. Cancer 124, 1670–1679 (2021).
Masschelin, P. M. et al. Vitamin B2 enables regulation of fasting glucose availability. eLife 12, e84077 (2023).
Schwartz-Zimmermann, H. E. et al. Comparison of LC-MS-based methods for the determination of carboxylic acids in animal matrices. Anal. Bioanal. Chem. 416, 1199–1215 (2024).
Carter, B. Z. et al. Targeting MCL-1 dysregulates cell metabolism and leukemia-stroma interactions and resensitizes acute myeloid leukemia to BCL-2 inhibition. Haematologica 107, 58–76 (2022).
Wei, D. et al. Targeting glutamine metabolism with a novel Na+/K+-ATPase inhibitor RX108 in hepatocellular carcinoma. Mol. Cancer Ther. 22, 693–705 (2023).
Wang, J. et al. Metabolomic profiling of anionic metabolites in head and neck cancer cells by capillary ion chromatography with Orbitrap mass spectrometry. Anal. Chem. 86, 5116–5124 (2014).
Sun, Y., Saito, K., Iiji, R. & Saito, Y. Application of ion chromatography coupled with mass spectrometry for human serum and urine metabolomics. SLAS Discov. 24, 778–786 (2019).
Hopsort, G., Latapie, L., Groenen Serrano, K., Loubière, K. & Tzedakis, T. Deciphering the human urine matrix: a new approach to simultaneously quantify the main ions and organic compounds by ion chromatography/mass spectrometry (IC-MS). Anal. Bioanal. Chem. 415, 5337–5352 (2023).
Liu, S. et al. Roles of metal ions in the selective inhibition of oncogenic variants of isocitrate dehydrogenase 1. Commun. Biol. 4, 1243 (2021).
Wishart, D. S. et al. HMDB 5.0: the Human Metabolome Database for 2022. Nucleic Acids Res. 50, D622–D631 (2022).
Harrieder, E.-M., Kretschmer, F., Böcker, S. & Witting, M. Current state-of-the-art of separation methods used in LC-MS based metabolomics and lipidomics. J. Chromatogr. B 1188, 123069 (2022).
Hu, Q., Tang, H. & Wang, Y. Challenges in analysis of hydrophilic metabolites using chromatography coupled with mass spectrometry. J. Anal. Test. 4, 140–162 (2020).
Psychogios, N. et al. The human serum metabolome. PLoS One 6, e16957 (2011).
Contrepois, K., Jiang, L. & Snyder, M. Optimized analytical procedures for the untargeted metabolomic profiling of human urine and plasma by combining hydrophilic interaction (HILIC) and reverse-phase liquid chromatography (RPLC)–mass spectrometry. Mol. Cell. Proteom. 14, 1684–1695 (2015).
Hosseinkhani, F. et al. Systematic evaluation of HILIC stationary phases for global metabolomics of human plasma. Metabolites 12, 165 (2022).
Amer, B., Deshpande, R. R. & Bird, S. S. Simultaneous quantitation and discovery (SQUAD) analysis: combining the best of targeted and untargeted mass spectrometry-based metabolomics. Metabolites 13, 648 (2023).
Hvinden, I. C. Exploring Metabolic Vulnerability and Therapeutic Potential in Cancers with Isocitrate Dehydrogenase Mutations. University of Oxford thesis (2022).
Reinbold, R. et al. Resistance to the isocitrate dehydrogenase 1 mutant inhibitor ivosidenib can be overcome by alternative dimer-interface binding inhibitors. Nat. Commun. 13, 4785 (2022).
Lin, S.-C. & Hardie, D. G. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 27, 299–313 (2018).
McCullagh, J. & Probert, F. New analytical methods focusing on polar metabolite analysis in mass spectrometry and NMR-based metabolomics. Curr. Opin. Chem. Biol. 80, 102466 (2024).
Vuckovic, D. Current trends and challenges in sample preparation for global metabolomics using liquid chromatography–mass spectrometry. Anal. Bioanal. Chem. 403, 1523–1548 (2012).
Barri, T. & Dragsted, L. O. UPLC-ESI-QTOF/MS and multivariate data analysis for blood plasma and serum metabolomics: effect of experimental artefacts and anticoagulant. Anal. Chim. Acta 768, 118–128 (2013).
Bruggink, C. & Jensen, D. Combining ion chromatography with mass spectrometry and inductively coupled plasma-mass spectrometry: annual review 2020. Anal. Sci. Adv. 2, 238–249 (2020).
Thonusin, C. et al. Evaluation of intensity drift correction strategies using MetaboDrift, a normalization tool for multi-batch metabolomics data. J. Chromatogr. A 1523, 265–274 (2017).
Xia, J. & Wishart, D. S. Metabolomic data processing, analysis, and interpretation using MetaboAnalyst. Curr. Protoc. Bioinforma. 34, 14.10.11–14.10.48 (2011).
Pang, Z. et al. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 17, 1735–1761 (2022).
Cooper, B. & Yang, R. An assessment of AcquireX and Compound Discoverer software 3.3 for non-targeted metabolomics. Sci. Rep. 14, 4841 (2024).
Forsberg, E. M. et al. Data processing, multi-omic pathway mapping, and metabolite activity analysis using XCMS Online. Nat. Protoc. 13, 633–651 (2018).
Heuckeroth, S. et al. Reproducible mass spectrometry data processing and compound annotation in MZmine 3. Nat. Protoc. 19, 2597–2641 (2024).
Wu, Y. & Li, L. Sample normalization methods in quantitative metabolomics. J. Chromatogr. A 1430, 80–95 (2016).
Liu, X., Ser, Z., Cluntun, A. A., Mentch, S. J. & Locasale, J. W. A strategy for sensitive, large scale quantitative metabolomics. J. Vis. Exp. 2014, 51358 (2014).
Szymańska, E., Saccenti, E., Smilde, A. K. & Westerhuis, J. A. Double-check: validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 8, 3–16 (2012).
Schrimpe-Rutledge, A. C., Codreanu, S. G., Sherrod, S. D. & McLean, J. A. Untargeted metabolomics strategies—challenges and emerging directions. J. Am. Soc. Mass Spectrom. 27, 1897–1905 (2016).
Acknowledgements
We thank all those who have been involved and contributed to establishing IC-MS in the McCullagh Lab, in particular, J. Harvey, E. Smith, A. Nazeer and J. Gannon. We are grateful to all the staff at the Mass Spectrometry Research Facility, Department of Chemistry, for their support. We also thank E. Haythorne (University of Edinburgh) and F. Ashcroft (University of Oxford) for their collaboration exploring pancreatic islet beta-cell function using AEC-MS/MS. J.S.O.M. acknowledges funding from the University of Oxford John Fell Fund (JFF142/116) to develop a metabolites standards database; a Wellcome Trust Seed Award in Science (204483/Z/16/Z), which helped establish the method; and BBSRC funding (BB/R013829/1), which provided equipment that supported this project. J.S.O.M. and R.W. acknowledge a Thermo Fisher Scientific Industry Collaboration, which provided funding and equipment that have supported recent parts of this work. I.C.H. thanks the Anne Grete Eidsvig and Kjell Inge Røkke Foundation for Education for an Aker Scholarship, which funded her D.Phil. at Oxford, where this protocol was developed. T.K. acknowledges an EPSRC Doctoral Training Partnership (EP/W524311/1) and Numares AG (Am Biopark 9, 93053 Regensburg-Graß, Germany) for funding her D. Phil. We thank T. Christison, W. C. Man, N. Rumachik, B. Amer, R. R. Deshpande, V. Jespers and S. S. Bird at Thermo Fisher Scientific for their continued help and support. Special thanks to A. Huhmer, who helped get the ball rolling!
Author information
Authors and Affiliations
Contributions
J.S.O.M. and J.W.-T. conceived and developed the original method. R.W. provided major updates. R.W., J.W.-T., I.C.H., J.B.N., T.K., J.S. and J.S.O.M. performed laboratory experiments. R.W., J.W.-T., I.C.H., M.M., K.V.L., E.P., T.K., D.H. and J.S. developed methods and tested workflows. J.W.-T., I.C.H., I.L., D.H., J.S., T.K., R.W., T.C.-H. and J.S.O.M. worked on the data-analysis pipeline. J.S.O.M. supervised the project and co-wrote the manuscript with R.W. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.S.O.M. has a research collaboration with Thermo Fisher Scientific, and R.W.’s D.Phil. is supported by funding from Thermo Fisher Scientific. J.B.N. contributed to this research while a postdoc in the McCullagh group. She currently works for Thermo Fisher Scientific.
Peer review
Peer review information
Nature Protocols thanks Gary Williamson, Heidi Schwartz-Zimmermann and Markus Aigensberger for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Walsby-Tickle, J. et al. Commun. Biol. 3, 247 (2020): https://doi.org/10.1038/s42003-020-0957-6
Haythorne, E. et al. Nat. Commun. 13, 6754 (2022): https://doi.org/10.1038/s41467-022-34095-x
Schulthess, J. et al. Immunity 50, 432–445.e7 (2019): https://doi.org/10.1016/j.immuni.2018.12.018
Then, C. et al. Microbiome 12, 89 (2024): https://doi.org/10.1186/s40168-024-01804-1
Ngere, J. et al. Anal. Chem. 95, 152–166 (2023): https://doi.org/10.1021/acs.analchem.2c04298
Supplementary information
Supplementary Information
Supplementary Figures 1 and 2 and Supplementary Method 1
Supplementary Table 1
Validation metrics for a selection of metabolite standards analyzed on the AEC-MS/MS method including sensitivity, linearity and RT stability. Supplementary Table 2 Selection of metrics to assess system suitability for citrate, lactose, fructose-1,6-bisphosphate and 2-hydroxyglutarate from analysis of our metabolite standard mixes. Supplementary Data 1 Chemical compositions of our metabolite standard mixes. Supplementary Data 2 Additional autosampler parameters for the AEC-MS/MS method Supplementary Data 3 AEC-MS/MS parameters for an ICS-6000 coupled to an Exploris 240; AEC-MS/MS RT database for metabolite identification. Supplementary Data 4 Example output from Progenesis QI after metabolite identification Supplementary Data 5 Example of the modified Progenesis QI output for use with Statistical Analysis within MetaboAnalyst. Supplementary Data 6 Example of the modified Progenesis QI output for use with Functional Analysis within MetaboAnalyst
Supplementary Method 2
Workflow template for use with AEC-MS/MS data in Compound Discoverer 3.3
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Williams, R., Walsby-Tickle, J., Hvinden, I.C. et al. Metabolomics using anion-exchange chromatography mass spectrometry for the analysis of cells, tissues and biofluids. Nat Protoc (2025). https://doi.org/10.1038/s41596-025-01222-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-025-01222-z