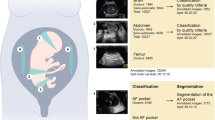
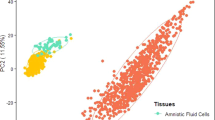
Abstract
Human primary fetal stem cell-derived organoids are used to model developing tissues in vitro. However, ethical and legislative constraints restrict fresh fetal tissue collection in several countries. Amniotic fluid (AF) is easily accessible with minimal ethical and regulatory constraints for collection. Our team recently showed that tissue-specific stem/progenitor cells can be isolated from fetal fluids collected during pregnancy through clinically indicated minimally invasive procedures conducted during the second and third trimesters. These samples consistently generate fetal lung, kidney tubule and gastrointestinal epithelial organoids autologous to the developing fetus. AF-derived organoids (AFOs) allow the investigation of fetal epithelia at developmentally relevant stages. Moreover, AFOs allow research to be conducted on late gestational stages, hardly accessible with other methods. Here, we provide a detailed protocol to establish, characterize and cryopreserve AFOs from viable AF cells. This includes the processing of patient-derived AF samples, viable cell sorting, seeding, establishment of clonal AFO lines, tissue phenotyping, expansion and cryopreservation. Additionally, we describe a straightforward immunofluorescence-based approach to pinpoint the tissue identity of the AFOs in a quick and cost-effective manner. In our hands, the protocol enabled the generation of primary fetal AFOs from 85.71% of samples (62.5% ascribed to the fetal lung, 59.4% to the kidney tubule and 6.2% to the small intestine). It takes 4–6 weeks to implement, requiring only standard equipment and expertise commonly available in cell biology laboratories.
Key points
-
This Protocol describes the derivation, expansion and cryopreservation of primary organoids from second and third trimester human amniotic fluid cells. These samples generate lung, kidney tubule and gastrointestinal epithelial organoids autologous to the developing fetus.
-
The generation of organoids from fetal tissue is often challenging owing to ethical and legal constraints. Generating fetal epithelial organoids from amniotic fluid cells enables overcoming some of these limitations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and the supporting primary research paper9. Source data are provided with this paper.
References
Zhao, O. Z. et al. Organoids. Nat. Rev. Methods Primers 2, 1–21 (2022).
Calà, G., Sina, B., De Coppi, P., Giobbe, G. G. & Gerli, M. F. M. Primary human organoids models: current progress and key milestones. Front. Bioeng. Biotechnol. 11, 320 (2023).
Jensen, K. B. & Little, M. H. Organoids are not organs: sources of variation and misinformation in organoid biology. Stem Cell Rep. 18, 1255–1270 (2023).
Gerrelli, D., Lisgo, S., Copp, A. J. & Lindsay, S. Enabling research with human embryonic and fetal tissue resources. Development 142, 3073–3076 (2015).
Abortion care. RCOG https://www.rcog.org.uk/for-the-public/browse-our-patient-information/abortion-care/ (2012).
Fetal tissue research: a weapon and a casualty in the war against abortion. Guttmacher Institute https://www.guttmacher.org/gpr/2016/fetal-tissue-research-weapon-and-casualty-war-against-abortion (2016).
McCune, J. M. & Weissman, I. L. The ban on US government funding research using human fetal tissues: how does this fit with the nih mission to advance medical science for the benefit of the citizenry? Stem Cell Rep. 13, 777–786 (2019).
Brumbaugh, J., Aguado, B. A., Lysaght, T. & Goldstein, L. S. B. Human fetal tissue is critical for biomedical research. Stem Cell Rep. 18, 2300–2312 (2023).
Gerli, M. F. M. et al. Single-cell guided prenatal derivation of primary fetal epithelial organoids from human amniotic and tracheal fluids. Nat. Med. 30, 875–887 (2024).
Underwood, M. A., Gilbert, W. M. & Sherman, M. P. Amniotic fluid: not just fetal urine anymore. J. Perinat. 25, 341–348 (2005).
Beall, M. H., van den Wijngaard, J. P. H. M., van Gemert, M. & Ross, M. G. in Nephrology and Fluid/Electrolyte Physiology 3–18 (Springer, 2019); https://doi.org/10.1016/B978-0-323-53367-6.00001-7.
Deprest, J. A. et al. Randomized trial of fetal surgery for severe left diaphragmatic hernia. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2027030 (2021).
Deprest, J. A. et al. Randomized trial of fetal surgery for moderate left diaphragmatic hernia. New Engl. J. Med. 385, 119–129 (2021).
De Coppi, P. et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 25, 100–106 (2007).
Senat, M.-V. et al. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N. Engl. J. Med. 351, 136–144 (2004).
Adzick, N. S. et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N. Engl. J. Med. 364, 993–1004 (2011).
He, P. et al. A human fetal lung cell atlas uncovers proximal-distal gradients of differentiation and key regulators of epithelial fates. Cell 185, 4841–4860.e25 (2022).
Lim, K. et al. Organoid modeling of human fetal lung alveolar development reveals mechanisms of cell fate patterning and neonatal respiratory disease. Cell Stem Cell 30, 20–37.e9 (2022).
Kraiczy, J. et al. DNA methylation defines regional identity of human intestinal epithelial organoids and undergoes dynamic changes during development. Gut 68, 49–61 (2019).
Elmentaite, R. et al. Single-cell sequencing of developing human gut reveals transcriptional links to childhood Crohn’s disease. Dev. Cell 55, 771–783.e5 (2020).
Wesley, B. T. et al. Single-cell atlas of human liver development reveals pathways directing hepatic cell fates. Nat. Cell Biol. 24, 1487–1498 (2022).
Andersson-Rolf, A. et al. Long-term in vitro expansion of a human fetal pancreas stem cell that generates all three pancreatic cell lineages. Cell https://doi.org/10.1016/j.cell.2024.10.044 (2024).
Miller, A. J. et al. In vitro and in vivo development of the human airway at single-cell resolution. Dev. Cell 53, 117–128.e6 (2020).
Fordham, R. P. et al. Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13, 734–744 (2013).
Sheridan, M. A. et al. Establishment and differentiation of long-term trophoblast organoid cultures from the human placenta. Nat. Protoc. 15, 3441–3463 (2020).
Liang, J. et al. Modeling human thyroid development by fetal tissue-derived organoid culture. Adv. Sci. https://doi.org/10.1002/ADVS.202105568 (2022).
Minimally invasive derivation of primary human epithelial organoids from fetal fluids. Nat. Med. https://doi.org/10.1038/s41591-024-02831-z (2024).
Geurts, M. H. & Clevers, H. CRISPR engineering in organoids for gene repair and disease modelling. Nat. Rev. Bioeng. 1, 32–45 (2023).
Schwank, G. et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13, 653–658 (2013).
Ringel, T. et al. Genome-scale CRISPR screening in human intestinal organoids identifies drivers of TGF-β resistance. Cell Stem Cell 26, 431–440.e8 (2020).
Pagliaro, A., Andreatta, F., Finger, R., Artegiani, B. & Hendriks, D. Generation of human fetal brain organoids and their CRISPR engineering for brain tumor modeling. Nat. Protoc. https://doi.org/10.1038/s41596-024-01107-7 (2025).
Artegiani, B. & Hendriks, D. Organoids from pluripotent stem cells and human tissues: when two cultures meet each other. Dev. Cell 60, 493–511 (2025).
Lim, K. et al. A novel human fetal lung-derived alveolar organoid model reveals mechanisms of surfactant protein C maturation relevant to interstitial lung disease. EMBO J. 44, 639–664 (2025).
Driehuis, E., Kretzschmar, K. & Clevers, H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat. Protoc. 15, 3380–3409 (2020).
Boretto, M. et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 21, 1041–1051 (2019).
Ebisudani, T. et al. Direct derivation of human alveolospheres for SARS-CoV-2 infection modeling and drug screening. Cell Rep. 35, 109218 (2021).
Kim, J., Koo, B. K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 21, 571–584 (2020).
Hu, C. et al. Organoids and organoids-on-a-chip as the new testing strategies for environmental toxicology—applications & advantages. Environ. Int. 184, 108415 (2024).
Wang, Y., Wang, L., Zhu, Y. & Qin, J. Human brain organoid-on-a-chip to model prenatal nicotine exposure. Lab Chip 18, 851–860 (2018).
Winkler, A. S. et al. Human airway organoids and microplastic fibers: a new exposure model for emerging contaminants. Environ. Int. 163, 107200 (2022).
Verstegen, M. M. A. et al. Clinical applications of human organoids. Nat. Med. 31, 409–421 (2025).
Sachs, N. et al. Long-term expanding human airway organoids for disease modeling. EMBO J. 38, e100300 (2019).
Schutgens, F. et al. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat. Biotechnol. 37, 303–313 (2019).
Roos, F. J. M. et al. Human bile contains cholangiocyte organoid-initiating cells which expand as functional cholangiocytes in non-canonical Wnt stimulating conditions. Front. Cell Dev. Biol. https://doi.org/10.3389/fcell.2020.630492 (2021).
Cindrova-Davies, T. et al. Menstrual flow as a non-invasive source of endometrial organoids. Commun. Biol. 4, 1–8 (2021).
Huang, X.-Z. et al. Single-cell sequencing of ascites fluid illustrates heterogeneity and therapy-induced evolution during gastric cancer peritoneal metastasis. Nat. Commun. 14, 1–22 (2023).
Liang, J., Li, X., Dong, Y. & Zhao, B. Modeling human organ development and diseases with fetal tissue-derived organoids. Cell Transplant. 31, 9636897221124481 (2022).
Haniffa, M. et al. A roadmap for the Human Developmental Cell Atlas. Nature 597, 196–205 (2021).
de Coppi, P. et al. Regenerative medicine: prenatal approaches. Lancet Child Adolesc. Health 6, 643–653 (2022).
Kretzschmar, K. & Clevers, H. Wnt/β-catenin signaling in adult mammalian epithelial stem cells. Dev. Biol. 428, 273–282 (2017).
Clevers, H. Modeling development and disease with organoids. Cell 165, 1586–1597 (2016).
Beumer, J. & Clevers, H. Hallmarks of stemness in mammalian tissues. Cell Stem Cell 31, 7–24 (2024).
Rossi, G., Manfrin, A. & Lutolf, M. P. Progress and potential in organoid research. Nat. Rev. Genet. 19, 671–687 (2018).
Combes, A. N., Zappia, L., Er, P. X., Oshlack, A. & Little, M. H. Single-cell analysis reveals congruence between kidney organoids and human fetal kidney. Genome Med. 11, 1–15 (2019).
Navaratnam, K. & Alfirevic, Z. Amniocentesis and chorionic villus sampling. BJOG 129, e1–e15 (2022).
Cao, J. et al. A human cell atlas of fetal gene expression. Science https://doi.org/10.1126/science.aba772 (2020).
Camp, J. G., Wollny, D. & Treutlein, B. Single-cell genomics to guide human stem cell and tissue engineering. Nat. Methods 15, 661–667 (2018).
Wapner, R. J. et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N. Engl. J. Med. 367, 2175–2184 (2012).
Shear, M. A., Robinson, P. N. & Sparks, T. N. Fetal imaging, phenotyping, and genomic testing in modern prenatal diagnosis. Best. Pract. Res. Clin. Obstet. Gynaecol. 98, 102575 (2025).
Beall, M. H., van den Wijngaard, J. P. H. M., van Gemert, M. J. C. & Ross, M. G. Amniotic fluid water dynamics. Placenta 28, 816–823 (2007).
Dobreva, M. P., Pereira, P. N. G., Deprest, J. & Zwijsen, A. On the origin of amniotic stem cells: of mice and men. Int. J. Dev. Biol. 54, 761–777 (2010).
Gosden, C. M. Amniotic fluid cell types and culture. Br. Med. Bull. 39, 348–354 (1983).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Pleguezuelos-Manzano, C. et al. Establishment and culture of human intestinal organoids derived from adult stem cells. Curr. Protoc. Immunol. 130, e106 (2020).
Blokzijl, F. et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature 538, 260–264 (2016).
Roerink, S. F. et al. Intra-tumour diversification in colorectal cancer at the single-cell level. Nature 556, 457–462 (2018).
Meran, L., Tullie, L., Eaton, S., De Coppi, P. & Li, V. S. W. Bioengineering human intestinal mucosal grafts using patient-derived organoids, fibroblasts and scaffolds. Nat. Protoc. https://doi.org/10.1038/s41596-022-00751-1 (2022).
Gao, N., White, P. & Kaestner, K. H. Establishment of intestinal identity and epithelial–mesenchymal signaling by Cdx2. Dev. Cell 16, 588 (2009).
Grainger, S., Savory, J. G. A. & Lohnes, D. Cdx2 regulates patterning of the intestinal epithelium. Dev. Biol. 339, 155–165 (2010).
Kumar, N. et al. The lineage-specific transcription factor CDX2 navigates dynamic chromatin to control distinct stages of intestine development. Development 146, dev172189 (2019).
Bouchard, M., Souabni, A., Mandler, M., Neubüser, A. & Busslinger, M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 16, 2958–2970 (2002).
Narlis, M., Grote, D., Gaitan, Y., Boualia, S. K. & Bouchard, M. Pax2 and Pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J. Am. Soc. Nephrol. 18, 1121–1129 (2007).
Lazzaro, D., Price, M., De Felice, M. & Di Lauro, R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development 113, 1093–1104 (1991).
Minoo, P. et al. TTF-1 regulates lung epithelial morphogenesis. Dev. Biol. 172, 694–698 (1995).
Hawkins, F. et al. Prospective isolation of NKX2-1–expressing human lung progenitors derived from pluripotent stem cells. J. Clin. Invest. 127, 2277–2294 (2017).
Dekkers, J. F. et al. Long-term culture, genetic manipulation and xenotransplantation of human normal and breast cancer organoids. Nat. Protoc. 16, 1936–1965 (2021).
Han, H., Zhan, T., Guo, N., Cui, M. & Xu, Y. Cryopreservation of organoids: Strategies, innovation, and future prospects. Biotechnol. J. 19, e2300543 (2024).
Xue, W. et al. Effective cryopreservation of human brain tissue and neural organoids. Cell Rep. Methods 4, 100777 (2024).
Mashouf, P., Tabibzadeh, N., Kuraoka, S., Oishi, H. & Morizane, R. Cryopreservation of human kidney organoids. Cell. Mol. Life Sci. 81, 1–23 (2024).
Dekkers, J. F. et al. High-resolution 3D imaging of fixed and cleared organoids. Nat. Protoc. 14, 1756–1771 (2019).
Acknowledgements
The work in M.F.M.G.’s laboratory is supported by an Academy of Medical Science Springboard Award (grant no. SBF009\1011). M.F.M.G. held a H2020 Marie Skłodowska-Curie Fellowship (grant no. 843265, AmnioticID) and received support from UCL Therapeutic Acceleration Support (TAS Call 10), the NIHR Great Ormond Street Hospital Biomedical Research Centre (NIHR GOSH BRC) and the Rosetrees Trust. G.D. is supported by an EMBO Scientific Exchange Grant (grant no. 11127). K.Y.S. is supported by a Kidney Research UK PhD Scholarship (grant no. Paed_ST_005_220221129). C.C. and G.D.’s work on this project was supported by the Italian Ministry of Health through the ‘cinque per mille’ research fund. A.L.D. is supported by the NIHR UCLH Biomedical Research Centre. A.F.P. and A.M. are supported by the European Research Council (ERC) under the European Union’s Horizon Europe research and innovation program (grant no. 101126209-3D.FETOPRINT). G.G.G. is supported by the UCL Therapeutic Acceleration Support (TAS) LifeArc Fund Rare Diseases Call (TRO award no.: 184646) and the NIHR GOSH BRC. P.D.C. is supported by the CDH-UK (grant no. 16ICH03) and the BREATH Consortium (grant no. 552269), by the National Institute for Health and Care Research (grant no. NIHR-RP-2014-04-046), H2020 (grant no. 668294, INTENS), OAK Foundation (grant no. W1095/OCAY-14-191), GOSH-CC (grant no. V5201) and NIHR GOSH BRC. Research at Great Ormond Street Hospital NHS Foundation Trust and UCL Great Ormond Street Institute of Child Health is made possible by the NIHR GOSH BRC. We are grateful to V. Karaluka, to A. Eddaoudi and the GOSICH Flow Cytometry facility, to the GOSICH Imaging facility and to UCL Genomics for the support provided. We are grateful to the patients and clinical teams who helped with collecting the AF samples: the UCL Hospital (UCLH) Fetal Medicine Unit (FMU) midwife and clinical team; the biobank staff at Ospedale Pediatrico Bambino Gesù, in particular, T. Franchin, G. Di Giovamberardino and V. Marcellini; and the King’s College Hospital staff, in particular, K. Nicolaides and the Fetal Medicine Foundation.
Author information
Authors and Affiliations
Contributions
G.C., G.G.G., M.F.M.G. and P.D.C. conceived and developed the Protocol. G.C. performed the experiments and analyzed the data with the help of G.D. and K.Y.S. G.J.Z., G.M.C. and A.M. helped with the quantifications and supported the experimental work. G.C. and M.F.M.G. wrote the manuscript with the support of A.F.P., G.G.G. and P.D.C. A.L.D., C.C., I.F., R.B., P.S., M.P. and P.D.C. helped with fluid collection and data interpretation. M.F.M.G. and P.D.C. supervised the study and secured the necessary funding.
Corresponding authors
Ethics declarations
Competing interests
M.F.M.G., G.G.G., P.D.C. and G.C. are inventors of the patent ‘Derivation of Primary Organoids from the Fetal Fluids’ filed by UCL on 18 April 2023, with application number GB2305703.7.
Peer review
Peer review information
Nature Protocols thanks Yuanyuan Zhang and Bing Zhao for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key reference
Gerli, M. F. M. et al. Nat. Med. 30, 875–887 (2024): https://doi.org/10.1038/s41591-024-02807-z
Extended data
Extended Data Fig. 1 Antibody panel validation.
a, Immunofluorescence images of a PAX8 positive KAFO displaying dim signal for NKX2-1. b, Immunofluorescence images of CDX2 staining on SiAFO, LAFO and KAFO. c, Immunofluorescence images of CDX2 staining on control fetal tissue-derived small intestinal (FSiO), lung (FLO) and kidney (FKO) organoids. d, Immunofluorescence images of NKX2-1/PAX8 co-staining on FLO, FKO, FSiO and SiAFO. e, Example of non-specific extracellular/cytoplasmic NKX2-1 or PAX8 signal in SiAFO (SiAFO_2, SiAFO_3). All scale bars, 50 µm.
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Source data
Source Data Fig. 2,3,6
Data analysis and quantifications.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Calà, G., D’Ariano, G., Sun, K.Y. et al. Derivation, expansion and cryopreservation of primary fetal organoids from second and third trimester human amniotic fluid cells. Nat Protoc (2025). https://doi.org/10.1038/s41596-025-01227-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41596-025-01227-8