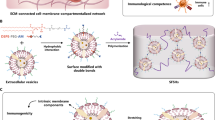
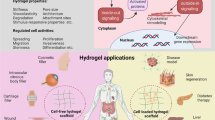
Abstract
Hydrogels, as 3D cross-linked hydrophilic networks that exhibit favorable flexibility, cargo loading and release abilities and structure and function designability, are desirable for diverse biomedical applications. For in vivo implementation, however, hydrogels often suffer from swelling-weakened mechanical strength, uncontrollable cargo release and complex composition, inevitably hindering further translation. Despite different reported synthetic approaches, the development of a facile yet universal method capable of fabricating hydrogels with dynamically adjustable structure and function remains difficult. Recently, inspired by biological tissues, we have developed a versatile biological membrane hybridization strategy to generate structurally and functionally programmable hydrogels. Specifically, biological membranes are used as a cross-linker to form a cross-linked network through a supramolecular-covalent cascade reaction route. This protocol demonstrates the construction of two biological membrane-hybridized hydrogels, including liposome-hybridized muscle-mimicking hydrogels with swelling-strengthening mechanical behavior and extracellular vesicle-hybridized skin-mimicking hydrogels with enhanced mechanical strength, lubricity, antibacterial activity and immunoactivity. We describe the detailed preparation procedures and characterize the structures and functions of the obtained hydrogels. We also expand the applicability of this biological membrane hybridization strategy to further tune the structure and function of the biomimetic hydrogels by incorporating a second network. This protocol provides a robust preparative platform to develop dual structure- and function-tunable hydrogels for different biomedical applications. Excluding the synthesis of reactive group–functionalized biological membranes, the fabrication of muscle-mimicking hydrogels takes ~3 d, while the construction of skin-mimicking hydrogels takes ~1 d. The implementation of the protocol requires expertise in polymer modification, hydrogel preparation, nanoscale vesicles, surface functionalization and cell culture.
Key points
-
This protocol describes a versatile biological membrane hybridization strategy that uses biological membranes as cross-linkers to generate structurally and functionally programmable hydrogels through a supramolecular-covalent cascade reaction procedure.
-
Compared to conventional cross-linking strategies, this protocol shows flexibility and versatility to generate biomimetic hydrogels, such as muscle-mimicking hydrogels with swelling-strengthening mechanical behavior and skin-mimicking hydrogels with enhanced mechanical strength, lubricity, antibacterial activity and immunoactivity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The main data discussed in this protocol are available in the supporting primary research papers (refs. 38,39). Source data are provided with this paper.
References
Daly, A. C., Riley, L., Segura, T. & Burdick, J. A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 5, 20–43 (2020).
Li, J. & Mooney, D. J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 1, 16071 (2016).
Xu, D. et al. High-flexibility, high-toughness double-cross-linked chitin hydrogels by sequential chemical and physical cross-linkings. Adv. Mater. 28, 5844–5849 (2016).
Zang, L. et al. Design and fabrication of an all-solid-state polymer supercapacitor with highly mechanical flexibility based on polypyrrole hydrogel. ACS Appl. Mater. Interfaces 9, 33941–33947 (2017).
Díaz-Marín, C. D. et al. Heat and mass transfer in hygroscopic hydrogels. Int. J. Heat Mass Transf. 195, 123103 (2022).
Liu, R. R. et al. Biomimetic chitin hydrogel via chemical transformation. Nano Res. 17, 771–777 (2024).
Li, X. & Gong, J. P. Design principles for strong and tough hydrogels. Nat. Rev. Mater. 9, 380–398 (2024).
Cao, H., Duan, L., Zhang, Y., Cao, J. & Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 6, 426 (2021).
Choi, C., Yun, E. & Cha, C. Emerging technology of nanofiber-composite hydrogels for biomedical applications. Macromol. Biosci. 23, 2300222 (2023).
Wang, X. Q. et al. Structuring and shaping of mechanically robust and functional hydrogels toward wearable and implantable applications. Adv. Mater. 36, 2309952 (2024).
Lin, D. et al. Multifunctional hydrogel based on silk fibroin promotes tissue repair and regeneration. Adv. Funct. Mater. 34, 2405255 (2024).
Kharaziha, M., Baidya, A. & Annabi, N. Rational design of immunomodulatory hydrogels for chronic wound healing. Adv. Mater. 33, 2100176 (2021).
Rizzo, F. & Kehr, N. S. Recent advances in injectable hydrogels for controlled and local drug delivery. Adv. Healthc. Mater. 10, e2001341 (2021).
Cully, M. Hydrogel drug delivery for inflammatory bowel disease. Nat. Rev. Drug Discov. 14, 678–679 (2015).
Macdougall, L. J., Perez-Madrigal, M. M., Arno, M. C. & Dove, A. P. Nonswelling thiol–Yne cross-linked hydrogel materials as cytocompatible soft tissue scaffolds. Biomacromolecules 19, 1378–1388 (2018).
Zhang, Y., Li, Y. & Liu, W. Dipole–dipole and H-bonding interactions significantly enhance the multifaceted mechanical properties of thermoresponsive shape memory hydrogels. Adv. Funct. Mater. 25, 471–480 (2015).
Hezaveh, H. & Muhamad, I. Controlled drug release via minimization of burst release in pH-response kappa-carrageenan/polyvinyl alcoholhydrogels. Comput. Chem. Eng. 91, 508–519 (2012).
Manghnani, P. N. et al. From burst to controlled release: using hydrogel crosslinking chemistry to tune release of micro-crystalline active pharmaceutical ingredients. RSC Pharm. 2, 94–101 (2025).
Thoniyot, P., Tan, M. J., Karim, A. A., Young, D. J. & Loh, X. J. Nanoparticle–hydrogel composites: concept, design, and applications of these promising, multi-functional materials. Adv. Sci. (Weinh.) 2, 1400010 (2015).
O’Shea, T. M., Aimetti, A. A., Kim, E., Yesilyurt, V. & Langer, R. Synthesis and characterization of a library of in-situ curing, nonswelling ethoxylated polyol thiol-ene hydrogels for tailorable macromolecule delivery. Adv. Mater. 27, 65–72 (2015).
Jia, F., Kubiak, J. M., Onoda, M., Wang, Y. & Macfarlane, R. J. Design and synthesis of quick setting nonswelling hydrogels via brush polymers. Adv. Sci. (Weinh.) 8, 2100968 (2021).
Zhang, J. et al. Highly stretchable and biocompatible wrinkled nanoclay-composite hydrogel with enhanced sensing capability for precise detection of myocardial infarction. Adv. Mater. 35, 2209497 (2022).
Kaixiang, S. et al. Nanocomposite conductive hydrogels with robust elasticity and multifunctional responsiveness for flexible sensing and wound monitoring. Mater. Horiz. 10, 2096–2108 (2023).
Kamata, H., Akagi, Y., Kayasuga-Kariya, Y., Chung, U.-i. & Sakai, T. ‘Nonswellable’ hydrogel without mechanical hysteresis. Science 343, 873–875 (2014).
Bignotti, F., Baldi, F., Grassi, M., Abrami, M. & Spagnoli, G. Hydrophobically-modified PEG hydrogels with controllable hydrophilic/hydrophobic balance. Polymers (Basel) 13, 1489 (2021).
Hao, Z. et al. Supramolecular peptide nanofiber hydrogels for bone tissue engineering: from multihierarchical fabrications to comprehensive applications. Adv. Sci. (Weinh.) 9, 2103820 (2022).
Alsaid, Y. et al. Tunable sponge-like hierarchically porous hydrogels with simultaneously enhanced diffusivity and mechanical properties. Adv. Mater. 33, e2008235 (2021).
Panja, S., Dietrich, B. & Adams, D. J. Controlling syneresis of hydrogels using organic salts. Angew. Chem. Int. Ed. Engl. 61, e202115021 (2022).
Li, C. et al. A covalent organic framework/graphene dual-region hydrogel for enhanced solar-driven water generation. J. Am. Chem. Soc. 144, 3083–3090 (2022).
Yi, Y. et al. Hydrogels totally from inorganic nanosheets and water with mechanical robustness, self-healing, controlled lubrication and anti-corrosion. Nano Res. 16, 1533–1544 (2022).
Zhao, P. et al. Versatile hydrogel dressing with skin adaptiveness and mild photothermal antibacterial activity for methicillin-resistant Staphylococcus aureus-infected dynamic wound healing. Adv. Sci. (Weinh.) 10, e2206585 (2023).
He, M., Wang, Q., Zhao, W. & Zhao, C. A substrate-independent ultrathin hydrogel film as an antifouling and antibacterial layer for a microfiltration membrane anchored via a layer-by-layer thiol-ene click reaction. J. Mater. Chem. B 6, 3904–3913 (2018).
Burdick, J. A. & Murphy, W. L. Moving from static to dynamic complexity in hydrogel design. Nat. Commun. 3, 1269 (2012).
van Oosten, A. S. G. et al. Emergence of tissue-like mechanics from fibrous networks confined by close-packed cells. Nature 573, 96–101 (2019).
Jatav, V., Singh, H. & Singh, S. Recent trends on hydrogel in human body. Int. J. Res. Pharm. Biomed. Sci. 2, 442–447 (2011).
Gouaux, E. & MacKinnon, R. Principles of selective ion transport in channels and pumps. Science 310, 1461–1465 (2005).
Chernomordik, L. V. & Kozlov, M. M. Mechanics of membrane fusion. Nat. Struct. Mol. Biol. 15, 675–683 (2008).
Wu, F., Pang, Y. & Liu, J. Swelling-strengthening hydrogels by embedding with deformable nanobarriers. Nat. Commun. 11, 4502 (2020).
Wu, F. et al. Generating dual structurally and functionally skin-mimicking hydrogels by crosslinking cell-membrane compartments. Nat. Commun. 15, 802 (2024).
Xiao, L. et al. Hyaluronic acid-based hydrogels containing covalently integrated drug depots: implication for controlling inflammation in mechanically stressed tissues. Biomacromolecules 14, 3808–3819 (2013).
Xiao, L., Zhu, J., Londono, J. D., Pochan, D. J. & Jia, X. Mechano-responsive hydrogels crosslinked by block copolymer micelles. Soft Matter 8, 10233–10237 (2012).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22425505 to J.L., 22375127 to Y.P., 22475131 to F.W. and 22305152 to H.C.), the Shanghai Rising-Star Program (23QA1408600 to F.W.) and the Innovative Research Team of High-Level Local Universities in Shanghai (SHSMU-ZDCX20210700 to Y.P.).
Author information
Authors and Affiliations
Contributions
Y.P., J.L. and F.W. conceived and managed the manuscript preparation. H.C. helped with the analysis of the results. Y.P., J.L. and F.W. revised the manuscript with input from all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.L. and F.W. have applied for a patent (202311812159.9) from the State Intellectual Property Office (China). The authors declare that they have no competing interests.
Peer review
Peer review information
Nature Protocols thanks Junjie Deng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Wu, F. et al. Nat. Commun. 11, 4502 (2020): https://doi.org/10.1038/s41467-020-18308-9
Wu, F. et al. Nat. Commun. 15, 802 (2024): https://doi.org/10.1038/s41467-024-45006-7
Supplementary information
Source data
Source Data Figs. 3–8
Subsheet: Fig. 3a–g. Subsheet: Fig. 4a–j. Subsheet: Fig. 5a–e. Subsheet: Fig. 6a–i. Subsheet: Fig. 7a–n. Subsheet: Fig. 8a–l
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, F., Chen, H., Liu, J. et al. Generating structurally and functionally programmable hydrogels by biological membrane hybridization. Nat Protoc (2025). https://doi.org/10.1038/s41596-025-01247-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41596-025-01247-4