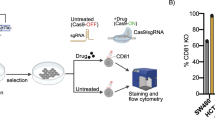
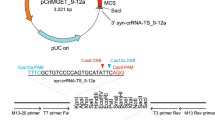
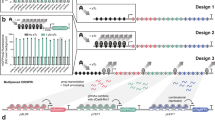
Abstract
CRISPR screens have revolutionized the study of diverse biological processes, particularly in cancer research. Both pooled and arrayed CRISPR screens have facilitated the identification of essential genes for cell survival and proliferation, drivers of drug resistance and synthetic lethal interactions. However, applying loss-of-function CRISPR screening to non-proliferative states remains challenging, largely because of slower editing and the poor sensitivity of identifying guide RNAs that ‘drop out’ in a population of non-dividing cells. Here, we present a detailed protocol to accomplish this, using an inducible Cas9 system that offers precise temporal control over Cas9 expression. This inducible system allows gene editing to occur only after the non-proliferative state is fully established. We describe the complete procedure for generating an inducible Cas9-expressing model and for measuring editing efficiency by using flow cytometry. In addition, we discuss how to optimize key parameters for performing successful CRISPR screens in various non-proliferative states. We describe a detailed workflow for performing a screen in senescent cells to identify senolytic targets. This protocol is accessible to researchers with experience in molecular biology techniques and can be completed in 8–12 weeks, from the generation of an inducible Cas9 cell line clone to the analysis of a CRISPR screen for hit identification. These techniques can be applied by researchers across different fields, including stem cell differentiation, immune cell development, aging and cancer research.
Key points
-
This advanced inducible Cas9 (iCas9) screening platform provides precise temporal control of genome editing, enabling the initiation of editing only after cells have entered a desired non-proliferative state.
-
It addresses key technical considerations to overcome challenges associated with previous methods, including the speed of editing in non-dividing cells, and how to optimize the signal-to-noise ratio for correct hit identification.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
References
Bock, C. et al. High-content CRISPR screening. Nat. Rev. Methods Primers 2, 9 (2022).
Johmura, Y. et al. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science 371, 265–270 (2021).
Colville, A. et al. Death-seq identifies regulators of cell death and senolytic therapies. Cell Metab. 35, 1814–1829.e6 (2023).
Wang, L. et al. cFLIP suppression and DR5 activation sensitize senescent cancer cells to senolysis. Nat. Cancer 3, 1284–1299 (2022).
He, S. & Sharpless, N. E. Senescence in health and disease. Cell 169, 1000–1011 (2017).
He, J. et al. Drug tolerant persister cell plasticity in cancer: a revolutionary strategy for more effective anticancer therapies. Signal Transduct. Target. Ther. 9, 209 (2024).
Lindell, E., Zhong, L. & Zhang, X. Quiescent cancer cells—a potential therapeutic target to overcome tumor resistance and relapse. Int. J. Mol. Sci. 24, 3762 (2023).
Chen, M. et al. Targeting of vulnerabilities of drug-tolerant persisters identified through functional genetics delays tumor relapse. Cell Rep. Med. 5, 101471 (2024).
Zhang, J., Chen, L., Zhang, J. & Wang, Y. Drug inducible CRISPR/Cas systems. Comput. Struct. Biotechnol. J. 17, 1171–1177 (2019).
Childs, B. G., Baker, D. J., Kirkland, J. L., Campisi, J. & van Deursen, J. M. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 15, 1139–1153 (2014).
Davies, D. M., van den Handel, K., Bharadwaj, S. & Lengefeld, J. Cellular enlargement—a new hallmark of aging? Front. Cell Dev. Biol. 10, 1036602 (2022).
Casagrande Raffi, G. et al. An antibiotic that mediates immune destruction of senescent cancer cells. Proc. Natl Acad. Sci. USA 121, e2417724121 (2024).
Reimann, M., Lee, S. & Schmitt, C. A. Cellular senescence: neither irreversible nor reversible. J. Exp. Med. 221, e20232136 (2024).
Park, F., Ohashi, K., Chiu, W., Naldini, L. & Kay, M. A. Efficient lentiviral transduction of liver requires cell cycling in vivo. Nat. Genet. 24, 49–52 (2000).
Miller, D. G., Adam, M. A. & Miller, A. D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol. Cell. Biol. 10, 4239–4242 (1990).
Laustsen, A. & Bak, R. O. Electroporation-based CRISPR/Cas9 gene editing using Cas9 protein and chemically modified sgRNAs. Methods Mol. Biol. 1961, 127–134 (2019).
Xu, C. L., Ruan, M. Z. C., Mahajan, V. B. & Tsang, S. H. Viral delivery systems for CRISPR. Viruses 11, 28 (2019).
Jochems, F. et al. The Cancer SENESCopedia: a delineation of cancer cell senescence. Cell Rep. 36, 109441 (2021).
Wang, L. et al. High-throughput functional genetic and compound screens identify targets for senescence induction in cancer. Cell Rep. 21, 773–783 (2017).
Ahler, E. et al. Doxycycline alters metabolism and proliferation of human cell lines. PLoS ONE 8, e64561 (2013).
Petinari, L., Kohn, L. K., de Carvalho, J. E. & Genari, S. C. Cytotoxicity of tamoxifen in normal and tumoral cell lines and its ability to induce cellular transformation in vitro. Cell Biol. Int. 28, 531–539 (2004).
Brinkman, E. K. & van Steensel, B. Rapid quantitative evaluation of CRISPR genome editing by TIDE and TIDER. Methods Mol. Biol. 1961, 29–44 (2019).
Conant, D. et al. Inference of CRISPR edits from Sanger trace data. CRISPR J. 5, 123–130 (2022).
Joung, J. et al. Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat. Protoc. 12, 828–863 (2017).
Satam, H. et al. Next-generation sequencing technology: current trends and advancements. Biology 12, 997 (2023).
Abid, T. et al. Genome-wide pooled CRISPR screening in neurospheres. Nat. Protoc. 18, 2014–2031 (2023).
Doench, J. G. et al. Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat. Biotechnol. 32, 1262–1267 (2014).
Hanna, R. E. & Doench, J. G. Design and analysis of CRISPR-Cas experiments. Nat. Biotechnol. 38, 813–823 (2020).
Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 15, 554 (2014).
Costa-Silva, J., Domingues, D. & Lopes, F. M. RNA-Seq differential expression analysis: an extended review and a software tool. PLoS ONE 12, e0190152 (2017).
Kuijpers, L., van Veen, E., van der Pol, L. A. & Dekker, N. H. Automated cell counting for Trypan blue-stained cell cultures using machine learning. PLoS ONE 18, e0291625 (2023).
Wang, B., Xu, Y., Wan, A. H., Wan, G. & Wang, Q. P. Integrating genome-wide CRISPR screens and in silico drug profiling for targeted antidote development. Nat. Protoc. 19, 2739–2770 (2024).
Masramon, L. et al. Genetic instability and divergence of clonal populations in colon cancer cells in vitro. J. Cell Sci. 119, 1477–1482 (2006).
Kuiken, H. J. et al. Clonal populations of a human TNBC model display significant functional heterogeneity and divergent growth dynamics in distinct contexts. Oncogene 41, 112–124 (2022).
Dias, M. H. et al. Paradoxical activation of oncogenic signaling as a cancer treatment strategy. Cancer Discov. 14, 1276–1301 (2024).
McDade, J. R., Waxmonsky, N. C., Swanson, L. E. & Fan, M. Practical considerations for using pooled lentiviral CRISPR libraries. Curr. Protoc. Mol. Biol. 115, 31.5.1–31.5.13 (2016).
Pascarella, G., Straniero, L., Frith, M. & Carninci, P. Capture-seq protocol and TE-reX pipeline guidelines for detection of recombination of repeat elements in short- and long-DNA reads libraries. STAR Protoc. 4, 102027 (2023).
Evers, B. et al. CRISPR knockout screening outperforms shRNA and CRISPRi in identifying essential genes. Nat. Biotechnol. 34, 631–633 (2016).
Jastrzebski, K., Evers, B. & Beijersbergen, R. L. Pooled shRNA screening in mammalian cells as a functional genomic discovery platform. Methods Mol. Biol. 1470, 49–73 (2016).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Colic, M. et al. Identifying chemogenetic interactions from CRISPR screens with drugZ. Genome Med. 11, 52 (2019).
Wang, B. et al. Integrative analysis of pooled CRISPR genetic screens using MAGeCKFlute. Nat. Protoc. 14, 756–780 (2019).
von Mering, C. et al. STRING: a database of predicted functional associations between proteins. Nucleic Acids Res. 31, 258–261 (2003).
Szklarczyk, D. et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 51, D638–D646 (2023).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Griss, J. et al. ReactomeGSA—efficient multi-omics comparative pathway analysis. Mol. Cell. Proteomics 19, 2115–2125 (2020).
Jassal, B. et al. The reactome pathway knowledgebase. Nucleic Acids Res. 48, D498–D503 (2020).
Fabregat, A. et al. Reactome graph database: efficient access to complex pathway data. PLoS Comput. Biol. 14, e1005968 (2018).
Otten, A. B. C. & Sun, B. K. Research techniques made simple: CRISPR genetic screens. J. Invest. Dermatol. 140, 723–728.e1 (2020).
Park, B. S., Jeon, H., Chi, S. G. & Kim, T. Efficient prioritization of CRISPR screen hits by accounting for targeting efficiency of guide RNA. BMC Biol. 21, 45 (2023).
Munoz, D. M. et al. CRISPR screens provide a comprehensive assessment of cancer vulnerabilities but generate false-positive hits for highly amplified genomic regions. Cancer Discov. 6, 900–913 (2016).
Behan, F. M. et al. Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens. Nature 568, 511–516 (2019).
Chan, E. M. et al. WRN helicase is a synthetic lethal target in microsatellite unstable cancers. Nature 568, 551–556 (2019).
Gilad, Y. et al. A genome-scale CRISPR Cas9 dropout screen identifies synthetically lethal targets in SRC-3 inhibited cancer cells. Commun. Biol. 4, 399 (2021).
Prahallad, A. et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 483, 100–103 (2012).
Jansen, R. A. et al. Small-molecule inhibition of MAP2K4 is synergistic with RAS inhibitors in KRAS-mutant cancers. Proc. Natl Acad. Sci. USA 121, e2319492121 (2024).
Wang, L., Lankhorst, L. & Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 22, 340–355 (2022).
Wiecek, A. J. et al. Genomic hallmarks and therapeutic implications of G0 cell cycle arrest in cancer. Genome Biol. 24, 128 (2023).
Liu, K. I. et al. A chemical-inducible CRISPR-Cas9 system for rapid control of genome editing. Nat. Chem. Biol. 12, 980–987 (2016).
Chylinski, K. et al. CRISPR-Switch regulates sgRNA activity by Cre recombination for sequential editing of two loci. Nat. Commun. 10, 5454 (2019).
Sun, N. et al. Development of drug-inducible CRISPR-Cas9 systems for large-scale functional screening. BMC Genomics 20, 225 (2019).
Senturk, S. et al. Rapid and tunable method to temporally control gene editing based on conditional Cas9 stabilization. Nat. Commun. 8, 14370 (2017).
Acknowledgements
We thank members of the Wang, Bernards and Beijersbergen laboratories for helpful discussion and insightful feedback. This work was supported by the 2024ZD0525004 from the National Health Commission of the PRC-Noncommunicable Chronic Diseases-National Science and Technology Major Project (to L.W.); 2024YFA0918403 from the National Key Research and Development Program of China (to L.W.); W2432050, W2421020 and 82372695 from the National Natural Science Foundation of China (to L.W.); 2024A04J6484 from the Guangdong Basic and Applied Basic Research Foundation (to L.W.); YTP-SYSUCC-0055 from the Young Talents Program of Sun Yat-sen University Cancer Center (to L.W.); the European Research Council as ERC-787925 (to R.B.); 19-051-ASP from the Mark Foundation (to R.B.); ASP-II grant-Bernards 2023 (to R.B.); KWF-12539 from the Dutch Cancer Society (to R.B.); LSH-TKI-LSHM20083 from Health Holland (to R.B.); and KWF lnfrastructure lnitiatives ScreeninC 12539 (to R.L.B.).
Author information
Authors and Affiliations
Contributions
G.C.R., R.L.B., R.B., H.J.K., C.L. and L.W. contributed to the conceptualization of the manuscript. All authors contributed to the evaluation, execution, writing and revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
R.B. and L.W. are shareholders of Oncosence. The other authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Roland Rad and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Wang, L. et al. Nat. Cancer 3, 1284–1299 (2022): https://doi.org/10.1038/s43018-022-00462-2
Casagrande Raffi, G. et al. Proc. Natl Acad. Sci. USA 121, e2417724121 (2024): https://doi.org/10.1073/pnas.2417724121
Chen, M. et al. Cell Rep. Med. 5, 101471 (2024): https://doi.org/10.1016/j.xcrm.2024.101471
Source data
Source Data Fig. 4
Images of SA β-Gal quantification (Fig. 4a, available in cited literature), unprocessed western blots (Fig. 4b), values of curve growth for iCas9 clones (Fig. 4c), unprocessed images and gating strategy for flow cytometry images (Fig. 4d), data for screen analysis (Fig. 4e,f, available as source data in cited literature), values of gene hits for senolytic screen (Fig. 4g) and senescent versus parental arm hit ranking (Fig. 4h)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Casagrande Raffi, G., Kuiken, H.J., Lieftink, C. et al. Inducible CRISPR–Cas9 screening platform to interrogate non-proliferative cellular states. Nat Protoc (2025). https://doi.org/10.1038/s41596-025-01251-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41596-025-01251-8
This article is cited by
-
Investigating non-proliferative cell states with inducible CRISPR screens
Nature Protocols (2025)