Abstract
Responding to threats in the real world demands a sophisticated orchestration of freeze and flight behaviors dynamically modulated by the neural activity. While the medial prefrontal cortex-basolateral amygdala (mPFC-BLA) network is known to play a pivotal role in coordinating these responses, the mechanisms underlying its population dynamics remain vague. As traditional Pavlovian fear conditioning models fall short in encapsulating the breadth of natural escape behaviors, we introduce a novel dataset to bridge this gap, capturing the defensive strategies of mice against a spider robot in a natural-like environment. The adaptive escape behaviors and concurrent mPFC-BLA activity in eight mice were monitored using wireless local field potential (LFP) and video recordings, both individually and in groups. Our data offers a unique avenue to explore the neural dynamics that govern fear- and vigilance-induced threat responses in isolated and social contexts. Supplemented by detailed methodologies and validation, the dataset allows for the analysis of the transient neural oscillatory dynamics, with prospective implications for the fields of neuroscience, robotics, and artificial intelligence.
Similar content being viewed by others
Background & Summary
Effective responses to threats are imperative for the survival of biological entities, necessitating their ability to dynamically switch between behaviors, such as freezing and fleeing, based on the varying levels of perceived danger and potential outcomes1,2. Existing literature indicates that this behavioral flexibility is facilitated by the precise coordination of multiple brain functions at a fine temporal scale. Specifically, the medial prefrontal cortex (mPFC) and basolateral amygdala (BLA), together constituting the “freeze-or-flight network”, are known to integrate threat-related information and fear memories to guide appropriate defensive actions3,4,5,6,7. However, investigations of these behaviors have primarily relied on conventional Pavlovian fear conditioning paradigms8, which afford limited insights into the full spectrum of natural defensive behaviors. Therefore, a detailed examination of the specific neuronal population dynamics within the mPFC-BLA circuitry is necessary to understand adaptive defensive reactions in natural settings.
To overcome this limitation, we introduce a novel experimental framework that simulates a natural threat using a spider robot. The neural activity of freely behaving mice was measured with the Collective Brain Research platform Aided by Illuminating Neural activity (CBRAIN)9—a wireless local field potential (LFP) measurement apparatus that utilizes a mobile edge computing system for real-time data acquisition. In this experimental setup, we observed the flexible escape behaviors of eight mice when they were confronted with a robotic predator. Our dataset involves the spatial positioning data collected from both the mice and robot, alongside concurrent neural recordings from the mPFC and BLA. Each mouse participated in sixteen experimental sessions, initially in isolation and then in a group setting. This dataset aims to offer insights into the natural, instinctive behaviors of mice under threat and the associated neural dynamics within brain regions responsible for modulating such behaviors. The utility of this dataset has been partially demonstrated in prior research10, wherein we discerned how theta (3–14 Hz) and high beta (22–34 Hz) oscillations within the mPFC-BLA network are modulated through the principles of frequency-division multiplexing. Furthermore, the interregional directionality of LFPs connecting these two brain regions was found to be correlated with the escape strategies in mice.
During the surgical and data collection steps, we adopted several key measures to ensure the quality and reliability of the dataset. Firstly, in addition to post-mortem histological analyses, we verified the precise position of electrodes within the deep BLA during surgeries. This verification was accomplished through intra-operative stimulation tests, which assessed the responsiveness of genetically modified BLA neurons to a series of pulse stimulations before the electrode implantation. Secondly, LFPs and video recordings were initially synchronized based on the time logged in CBRAIN and video data files using the Java-based method (System.currentTimeMillies()). These synchronizations were then fine-tuned by post-hoc correction using the headstage LED blink signals, which were separately identified in two recording modalities (see Methods for detail). Finally, experimental parameters, such as experiment duration and movement speed of a robot, were carefully set to induce BLA gamma responses in all mice without them accommodating to the experiment protocol, as evidenced by the absence of a reduction in responses across multiple trials9.
The dataset we have compiled, as exemplified in its previous applications above, holds considerable promise for elucidating the neural basis of natural defensive behaviors in mice. In particular, the main advantages of this dataset are twofold. First, we captured neural activity in mice within environments that closely mimic their natural habitat, devoid of specific task assignments. Next, the data encompasses observations of mice both in isolation and in social contexts, enabling an analysis of how neural dynamics are influenced by the presence of conspecifics. Given the scarcity of publicly available datasets that offer either of these perspectives, our dataset contributes a distinctive value to the scientific community. Future works addressing the neural mechanisms underlying freeze-or-flight decisions, collision avoidance strategies inspired by natural behaviors, or the development of machine learning (ML) algorithms that exhibit adaptive and flexible responses to perceived threats may all benefit from this resource.
Methods
Data acquisition and processing
The schematic representation of the data recording process and the experimental configuration is depicted in Fig. 1. For a more comprehensive overview of the experimental paradigm and the data acquisition methodologies employed, readers are encouraged to consult the supplementary materials provided in Kim et al.9 and Han et al.10.
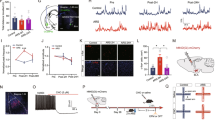
Overview of experimental setup and data acquisition process. (A) The experimental protocol comprises four task stages, each lasting 60 seconds. The illustrations provide a visual representation of the mouse’s behavior within the arena, which is segmented into the threat and safe zones. (B) The panel depicts the recording sites targeted for electrode implantation (left) and the diagram of our measurement device (right). The brain regions highlighted in blue mark the electrode tip locations, along with their anterior-posterior (AP) coordinates relative to the bregma. The electrodes, fixated in place with dental cement, were connected to the CBRAIN headstage device via an electrode-CBRAIN connector. Data transmission was achieved wirelessly through Bluetooth-enabled mobile-edge computing. The mouse brain atlas is taken from Paxinos and Watson’s book27. The image of a telemetry receiver dongle is provided by the courtesy of Nordic Semiconductor (https://www.nordicsemi.com). (C) The experimental conditions are outlined, differentiating between the solitary and group settings. In the solitary condition, a mouse interacts with a robot predator individually, whereas, in the group condition, a cohort of eight mice engage with the robot collectively.
Animals and surgery
A total of eight Thy1-ChR2-YFP B6 mice (B6.Cg-Tg(Thy1-COP4/EYFP)18Gfng/J, stock number 007612, Jackson Laboratory) underwent surgeries for chronic electrode implantation in the mPFC and BLA. All mice were healthy, male, and 8-week-old, weighing more than 25 g at the time of surgery. To confirm the electrode location in the deep BLA during surgeries, we conducted the intraoperative stimulation test (see Fig. S3 in Kim et al.9). After surgery, the mice were kept individually at the Korea Institute of Science and Technology (KIST) Animal Facility on a 12-h light/dark cycle with free access to food and water. The experimental procedures were performed 1–8 weeks after the surgery. All experiments were approved by the KIST Animal Care and Use Committee in advance (permit number: 2019-095) and followed the National Institutes of Health Guidelines to minimize the pain of animals.
Experimental paradigm
The 60 cm × 60 cm arena, divided diagonally into the safe and threat zones by a single gated door (see Fig. 3 in Kim et al.9), was used for the experiment. The experimental procedure consisted of four distinct stages, each with a duration of 60 s. Initially, the mice were placed within the threat zone, where they were allowed to explore the area without any immediate dangers (Stage 1). Next, they encountered a manually controlled spider robot (Model 18143 A, Academy Plastic Model Co. Ltd., South Korea), designed to mimic a predatory threat (Stage 2). Following this, the gate to the safe zone was opened, and the animals were allowed to escape from the robot (Stage 3). In the final stage, the robot was removed from the arena, enabling the mice to freely navigate both the safe and threat zones (Stage 4). This sequence was repeated eight times (4 trials per day over 2 consecutive days) for each mouse, first in the solitary condition and then in the group condition (see Fig. S5 in Kim et al.9). During the experiments, all mice were implanted with LFP electrodes with CBRAIN headstages connected to record the signals. Due to the synchronization issue, the total number of recording sessions was 59 trials for the solitary condition and 48 trials for the group condition (see Synchronization of video and neural data).
A spider robot was selected after testing several robot models, as it elicited consistent escape responses in mice, even after repeated exposures (see Figs. S6-S7 in Kim et al.9). Before commencing the experiments, the mice were acclimated to the CBRAIN device through recordings in an exploratory environment within an unoccupied arena and habituated with group housing. Exemplary videos of these experiments are presented in our previous publications (see Movie S3 in Kim et al.9; Movie S1 in Han et al.10).
LFP and video recording
LFPs were recorded using CBRAIN headstages9, embedded with a 16-channel differential amplifier (RHD2216, 16-bit, INTAN Technologies LLC, Los Angeles, CA, USA). The signals were then transmitted to a Bluetooth System on Chip (SoC) unit (nRF52832, Nordic Semiconductor) via a serial peripheral interface at 8 Mbit/s after a band-pass filtering was applied (with cutoff frequencies of 1 Hz and 4 kHz).
The videos were recorded using a high-speed complementary metal-oxide semiconductor (CMOS) camera (Lt225, Teledyne Lumenera, Ottawa, ON, Canada), installed 1.6 m above the arena and aligned perpendicular to capture a top view. The entire arena was documented using StreamPix 7 software (Norpix Inc., Montreal, Quebec, Canada) at a frame rate of 30 frames per second with a resolution of 1000 × 1000 pixels throughout the experiments. The camera settings were adjusted to reduce color saturation and prevent afterimages. Given the nocturnal tendencies of mice, ambient lighting was kept dim, and the room temperature was maintained at 22–24 °C to provide a comfortable environment for the subjects.
Mouse and robot position tracking
The tracking of mouse body positions in a solitary conditioned experiment was conducted using a convolutional neural network (CNN) based on the U-Net architecture11, designed to estimate the center of mass of the mouse body area. The video frames were first extracted and transformed into low-resolution grayscale images (488 × 488 pixels). From these, 500 frames were randomly subsampled to create a training dataset, for which corresponding binary masks were manually crafted. We implemented the U-Net model using Keras 2.2.4 within a Python 3 environment. This network processed an input image matrix of 488 × 488 pixels to generate a probability map of the same dimension as an output. Subsequently, this map was binarized using a threshold value (i.e., probability of 0.4). The position of a mouse was determined as the center of mass within the binarized map. Extracting the time-varying positions of a spider robot followed an identical pipeline but with a reduced training set comprising 100 frames. Details on the specific hyperparameters of the CNN and its design can be found in Han et al.10 For the tracking of mouse positions in the group condition, an OpenCV-based customized Python script was used to track the location of a headstage mounted on each mouse based on its blue LED light (see Fig. S8 in Kim et al.9).
Synchronization of video and neural data
The neural and video data were digitally archived using separate processes (MATLAB custom software and StreamPix7 software, respectively), which led to mismatches in the start times of the recordings across the two data modalities. To address this problem, we implemented a synchronization procedure, aligning the timestamps of neural and video recordings based on the time information of red LED blinking. This red LED, captured by both the neural and video data, was preset to blink when power within the 24–56 Hz range in the mouse BLA increased. At each frame, the blinks were detected by counting red pixels within a square area centered around the mouse position (80² pixels for solitary sessions, 50² pixels for group sessions). The LED time traces extracted from the video data were upsampled to 1024 Hz to match the sampling rate of the LFP data. By computing the cross-correlation of video- and LFP-driven LED time traces and finding the maximum value of cross-correlation functions, the time points of neural data were aligned with the video data. This alignment resulted in time corrections of 0.619 ± 0.969 s (Pearson r = 0.676 ± 0.091) for solitary sessions and 1.520 ± 0.172 s (Pearson r = 0.720 ± 0.115) for group sessions (MATLAB source code for this process is uploaded in subfunc/estimate_timelag.m). Consequently, the temporal discrepancy of neural data was minimized to less than one frame.
Due to inadequate sampling rates in certain neural recordings, specific trial sessions revealed suboptimal LFP recording quality characterized by low correlation values (Pearson’s r < 0.5) and had to be excluded from the dataset. In particular, five single-session neural recordings (Mouse 2 Session 5, Mouse 5 Session 6, Mouse 7 Session 2, Mouse 8 Session 4, Mouse 8 Session 8; Pearson’s r = 0.246 ± 0.114) and 16 group-session neural recordings (Sessions 13 and 16 for all mice; Pearson’s r = 0.134 ± 0.080) were removed. Although we excluded these neural data to prevent any potential challenges that may affect their direct comparison to video data, they are still available upon request. Note that the video recordings from these sessions remain valid and were not subjected to exclusion. These would be useful for analyzing behavioral dynamics and, to a limited extent, for studying neural dynamics through the analysis of red LED blinking.
LFP & behavioral data analysis
Our previous studies9,10,12 delineate a range of analytical methods that has been applied to the dataset presented in this paper. In the following section, we focus on describing the specific methodologies employed in the Technical Validation section.
Neural oscillatory burst detection
To identify transient neural oscillatory bursts in the beta (20–30 Hz) and high gamma (60–92 Hz) frequency bands, we employed a bandpass filtering-based burst detection technique as described in Cho and Choi12. This method was applied to raw LFP signals from the mPFC and BLA at the individual trial level. The signals were filtered using a Hamming-windowed finite impulse response (FIR) filter from EEGLAB (version 2023.0), an open-source software package available at its website (https://sccn.ucsd.edu/eeglab/index.php).
Burst occurrence rates were computed following Kim et al.9, by smoothing a binary vector indicating burst events using a simple moving average with a 1-s time step and a 10-s moving window. The window size was truncated at the endpoints when there were not enough points to fill in the window. These rates were calculated for each mouse, session, and day on an individual trial basis and subsequently averaged across trials to yield trial-averaged rates. Additionally, they were segmented into 1-min bins, each corresponding to an experiment stage, and averaged over a stage duration to derive trial-wise stage-averaged burst occurrence rates under both the solitary and group conditions.
Statistical testing
The changes in burst rates across different stages of an experiment (i.e., upon the introduction of a threat) were statistically tested for the solitary condition (n = 59 trials) using the Friedman test, after checking the basic assumptions of normality and equal variance for every stage-averaged burst rate at each frequency band and stage. The normality was tested by the Kolmogorov-Smirnov test, and the equal variance was tested by Levene’s test. Not all distributions were normal, and the stage-averaged beta and high gamma burst rates did not have equal variances across stages. To account for the multiple comparisons problem, we conducted a post hoc Nemenyi’s test with a Tukey-Kramer critical value to affirm statistical differences in burst rates between two different stages.
Next, we examined the differences in burst rates between the solitary and group conditions at each frequency band and stage, employing the two-tailed Wilcoxon signed-rank test. This choice was made after confirming that the pairwise differences between burst rates across trials have a roughly symmetrical distribution, although they were not without outliers and did not always follow a normal distribution. Only the recording sessions with both the solitary and group data (n = 45 trials) were used for the statistical comparison. To mitigate the errors due to multiple comparisons, we implemented the Bonferroni correction, adjusting for four stages, to validate the statistical differences in burst rates between the experiment conditions at each stage. For all statistical tests, we set the alpha significance level at 0.05 (before corrections) to determine statistical significance.
Data Records
Our dataset is structured according to the Brain Imaging Data Structure (BIDS)13 standard and is hosted on the GIN G-Node repository14. It comprises three distinct types of data for each subject: (i) raw data files of the mPFC and BLA LFPs, recorded at a sampling rate of 1024 Hz and stored in the EEGLAB15 format (i.e., .fdt and .set files), (ii) positional coordinates of both the mice and robotic spider, captured at a sampling rate of 30 Hz, and (iii) video footage of the experimental sessions. Additionally, we provide a montage.csv file, which includes the spatial coordinates of the electrode channels. The structure of our dataset is summarized in Fig. 2, and detailed descriptions of the format and contents of each data file can be found on our G-Node repository14. It is important to note that the dataset uses the term “EEG” specifically to denote LFP data to ensure compatibility with the EEGLAB software and maintain consistency with the BIDS format.
Dataset structure. The LFP and behavioral data are organized according to the EEG-BIDS standard. The LFP signals of each mouse are stored in a directory denoted as ‘sub-**’, which contains 16 session data that span four trials across two days under two distinct task conditions (solitary vs. group). Sessions labeled ‘ses-**’ contains 2-channel LFP recordings (*.set / *.fdt) and a 1-channel LED blinking indicator (preset to blink slightly after detection of power increase in 24–56 Hz from BLA channel; see Kim et al.9 for further details) with each session lasting 4 minutes. The behavioral data, consisting of video recordings and mouse tracking information, are saved in a separate ‘stimuli’ folder. Note that LFP data files are stored within directories named ‘eeg’ to ensure their compatibility with the EEGLAB software and BIDS formatting.
The dataset can be downloaded from the GIN website directly or by using the gin command-line interface. On the website, a step-by-step tutorial is available in the form of a README markdown file and a MATLAB how_to_start.mlx file. This guide provides detailed instructions for users on how to access and handle the raw data files after they are downloaded, covering basic file management procedures, steps for installing the EEGLAB toolbox within the MATLAB environment, and introductory analysis scripts encompassing time-frequency analysis and movement trajectory visualizations to survey the data. Prospective users are strongly encouraged to review this guide online to familiarize themselves with the dataset before initiating their analyses.
Users should be aware that LFP and video data are organized differently. LFP data follows a ses-01 to ses-16 numbering system in separate directories per subject to comply with EEG-BIDS format, while video data uses Day*-Trial*-Mouse* for solitary (single) recordings and Day*-Trial*-Group for group recordings. Consequently, (1) ses-01 to ses-04 correspond to single/day 1/trials 1–4, (2) ses-05 to ses-08 correspond to single/day 2/trials 1–4, (3) ses-09 to ses-12 correspond to group/day 1/trials 1-4, and (4) ses-13 to ses-16 correspond to group/day 2/trials 1-4.
Technical Validation
The presented dataset has been partly validated in our previous works, and all data were manually inspected for corruption and omissions prior to data analysis. Notably, we examined the BLA gamma activity in the solitary and group conditions under threat9 and subsequently investigated the neural dynamics within the mPFC-BLA circuit in response to the freeze-or-flight behaviors10. The analyses of LFP and behavioral data implemented in these studies demonstrate the integrity of our dataset. To provide further transparency and expound on the integrity of measured data, we discuss below the validity of the histological data probed in prior studies.
Additionally, our methodological work12 has applied multiple burst detection algorithms to the dataset. However, this research primarily focused on evaluating the algorithms and did not inspect how neural burst events extracted from our data relate to rodent behaviors. As a further quality control, we conduct the neural burst analysis to elucidate the potential implications of our LFP recordings to the neuroscience community. This analysis aims to affirm the utility of the dataset in the investigation of transient neural oscillations and, more generally, dynamic neural processes underpinning animal behaviors.
Quality assessment of surgical procedures and data collection
We recorded LFP signals from the mPFC and BLA by implanting electrodes targeting the prelimbic cortex (1.7 ± 0.2 mm anterior, 0.4 ± 0.1 mm lateral, −2.3 ± 0.2 mm ventral relative to the bregma) and basolateral nucleus (−1.8 ± 0.2 mm anterior, 2.8 ± 0.3 mm lateral, −4.5 ± 0.3 mm ventral relative to the bregma). The recording sites of these regions were both situated in the right hemisphere. To ascertain accurate electrode placement within the BLA pre-mortem, an intraoperative stimulation test was performed during electrode implantation surgery (see Fig. S3 in Kim et al.9; Fig. S14 in Han et al.10). After the mice were anesthetized and stabilized in a stereotaxic apparatus, a custom-made optrode—an epoxy-insulated tungsten electrode paired with a fiber optic cannula—was inserted from the top of the BLA, advancing in 4-mm intervals along the dorsoventral axis. Within the depth range of 4.2 to 4.9 mm, where the BLA is typically located, stimulation tests were conducted at 0.1-mm intervals. LFPs were recorded simultaneously at a sampling rate of 30 kHz and analyzed in real-time to detect evoked potentials. The final electrode position was determined at the depth where the largest signal voltage was observed. Following the experiments, the precise locations of the mPFC and BLA electrodes were histologically verified post-mortem, as reported in the supplementary material of Han et al.10 (see Fig. S14), affirming the correct insertion of the electrodes at the targeted locations.
Quality assessment of LFP recordings
To demonstrate whether the threat instigated by a predatory spider robot evokes changes in beta and high gamma burst rates in the mouse mPFC and BLA, we detected burst events from bandpass-filtered LFP signals and quantified their occurrence rates over time (Fig. 3A). In the mPFC, trial-averaged burst occurrence rates indicated an elevation in gamma burst rates with the threat onset, shortly followed by an increase in beta burst rates (Fig. 3B). These increases in burst rates were contingent on specific experimental stages for both frequency bands (Fig. 3C). For the beta band, the burst rates in Stage 2 were significantly higher than those in every other stage (Friedman’s: χ2 = 60.85, df = 3, p = 3.866e-13; Stage 2 vs. Stage 1: p = 1.039e-10, vs. Stage 3: p = 2.519e-5, vs. Stage 4: p = 6.274e-11). Likewise, for the gamma band, the burst rates in Stage 2 significantly surpassed those in other stages (Friedman’s: χ2 = 46.75, df = 3, p = 3.930e-10; Stage 2 vs. Stage 1: p = 7.853e-7, vs. Stage 3: p = 1.289e-9, vs. Stage 4: p = 4.168e-5).
Stage-dependent changes in beta and gamma burst rates in mPFC and BLA. (A) Overview of the burst detection procedure. Left: Anatomical regions of the mPFC and BLA. Middle: Raw LFP time traces for each region, with corresponding beta (20–30 Hz) and gamma (60–92 Hz) bursts marked on the filtered signals. Right: Summary of the burst detection algorithm, adapted from Cho & Choi12. (B) mPFC beta and gamma burst rates, averaged over all trials (n = 59), plotted against task time. The onset of the robot attack is indicated by a vertical dashed line. (C) mPFC beta (left) and gamma (right) burst rates averaged over the duration of each stage (1 min). The bar plots depict the mean burst rates, with their standard errors illustrated in gray error bars. Significant differences between stages are denoted by asterisks. Statistical significance was determined using the Friedman test, followed by the post-hoc Nemenyi’s test accounting for multiple comparisons. (D) The same analysis as in (B) is performed on the BLA data. (E) The same analysis as in (C) is performed on the BLA data. Throughout the plots, orange and green signify the beta and gamma bands, respectively. Significance levels are marked by *** for p < 0.001, ** for p < 0.01, and * for p < 0.05. The mouse head illustration and curved arrows in (A) are credited to Luigi Petrucco (https://scidraw.io/) and SANB (https://flatiocon.com/), respectively.
In the BLA, however, trial-averaged burst occurrence rates demonstrated a noticeable increase only in the beta burst rates under a threat (Fig. 3D). While the beta burst rates in Stage 2 mirrored the significant increase observed in the mPFC (Friedman’s: χ2 = 50.37, df = 3, p = 6.669e-11; Stage 2 vs. Stage 1: p = 5.043e-6, vs. Stage 3: p = 3.220e-4, vs. Stage 4: p = 3.157e-11), gamma burst rates exhibited a significant decrease in Stage 3 compared to other stages (Friedman’s: χ2 = 12.82, df = 3, p = 5.034e-3; Stage 3 vs. Stage 1: p = 0.0415, vs. Stage 2: p = 0.0146, vs. Stage 4: p = 0.0116) (Fig. 3E). Together, these patterns in the mPFC and BLA elucidated a clear stage-dependence of burst rates under the presence of a threat, highlighting changes in neural activities across time and brain regions.
Next, we assessed the quality of the LFP data in relation to the experiment conditions to determine if the neural activity of mice alters across different social environments. Specifically, we investigated whether the rates of beta and gamma bursts within the mPFC and BLA exhibit significant variations when mice are exposed to threats either alone or in a group. Our findings revealed that during Stage 2, beta burst rates in BLA were significantly higher in the solitary condition compared to the group condition (Table 1). In mPFC, significantly higher beta burst rates in the solitary condition were observed during Stage 4. In contrast, no significant difference in the high gamma burst rates was observed in both mPFC and BLA, indicating that the mean gamma burst rates did not differ between the solitary and group conditions across all stages.
In summary, exposure to a predatory threat seemed to induce an increase in the beta burst rates within the mouse mPFC and BLA when the mice were isolated. These beta burst rates in the mPFC and BLA exhibited significant differences between experiment conditions; however, no condition-dependent changes in high-gamma burst rates were observed for both brain regions.
These analyses underscore our dataset as a novel avenue for investigating transient neural bursts as units that encode information from the external environment and are associated with diverse behaviors. The burgeoning theoretical and empirical evidence supports our claim. Previous studies16,17 have established that external stimuli can be encoded through distinct properties of burst events such as their rates and amplitudes, and theoretical models18,19,20 have been proposed to elucidate the role of transient oscillations across multiple frequency bands in mediating information routing. Furthermore, correlations between burst events and behaviors were observed in tasks involving perception, motion, and cognition across multiple species17,21,22.
On a last note, our previous study9 revealed that the frequency range encompassing the high beta and low gamma (i.e., 24 – 56 Hz) bursts in mice are associated with fear responses and are influenced by their social context (i.e., solitary or in groups). However, this observation did not differentiate between high beta and low gamma frequencies, and it was also limited to analysis in the BLA. Here, we broaden our validation to include both the mPFC and BLA and examine multiple frequency bands. Our dataset thus provides an opportunity to explore the hypothesis that low and high gamma bands are associated with distinct behavioral mechanisms, namely fear and vigilance, respectively. Previous research9,23,24 has hinted at the role of transient oscillatory activities in modulating behaviors related to fear and vigilance. These studies suggest that in scenarios involving a spider robot threat, the primary emotional response in mice is fear. This observation is evidenced by the weaker statistical significance associated with BLA gamma burst rates, which are often linked to vigilance, under the threat-induced condition (Fig. 3E). Furthermore, significant changes in BLA high gamma burst rates were observed during Stage 3, a phase in which mice had the option to escape from a robot in the arena, potentially dampening their vigilance further as they move away from the source of a threat.
Therefore, the burst detection analysis here highlights the potential of this dataset for studying the transient neural dynamics of mice in natural-like environments, where putative fear and vigilance-based emotional behaviors are observed and vary according to social contexts. This approach promises to facilitate a more nuanced understanding of fear and vigilance from a transient neural recording perspective, especially within naturalistic settings and at systematic levels. While our earlier work has shed light on neural correlates of fear and fear-related behaviors, future research could further investigate their relationship to vigilance. Moreover, questions about how these findings align with theoretical models, as well as how information is encoded and transferred within and between the mPFC and BLA, remain open. Additionally, with experimental paradigms designed to record cell spiking data or focus on vigilance-related tasks, our dataset promises to be an invaluable complementary resource.
Demonstration: behavioral state-dependent changes in theta peak frequency
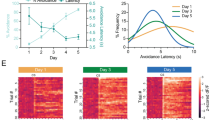
In one of our previous publications, we discovered that the theta frequency of the mPFC-BLA circuit varies depending on the type of defensive behavior (prominent 5 Hz low theta during freezing, prominent 10 Hz high theta during flight) within the solitary condition. To determine whether this previous finding (as shown in Fig. 4e in Han et al.10) can be replicated in the group condition, we aimed to demonstrate it by examining changes in the LFP amplitude spectrum as a function of locomotion speed (i.e., a key variable distinguishing between freeze/flight behavior).
To investigate the relationship between locomotion speed and neural activity in the mPFC-BLA circuit, we divided the mouse’s speed into 10 bins and analyzed the LFP amplitude spectrum for each bin. This approach allowed us to assess neural activity changes across different behavioral states (Fig. 4). Trial-by-trial analysis on the speed-LFP relationship showed that the theta peak frequency shifted from about 5 Hz (low theta) in the slowest bin (green) to around 11 Hz (high theta) in the fastest bin (red) during a solitary recording session (Fig. 4C). A similar pattern appeared in a group recording session for the same mouse, with the theta peak shifting from low theta to high theta (Fig. 4D). We quantified this shift by computing the theta amplitude ratio (8–12 Hz amplitude divided by 3–8 Hz amplitude), which was lower in the slow bin (resembling freezing) and higher in the fast bin (resembling flight) (Fig. 4E). This pattern was consistent in both solitary (Spearman’s ρ = 0.8876, p = 5.8416e-26) and group (Spearman’s ρ = 0.8876, p = 5.4833e-28) sessions. Grand-averaging across all eight mice confirmed a positive correlation between speed and theta amplitude ratio (Fig. 4F).
Entrainment of low/high theta rhythm according to different locomotive states. (A) Example frame of a group session recording and the position trace of 8 mice and a robot. Colored squares indicate past positions (from −1 s to 0 s). Positions further in the past are represented in dim colors, while recent positions are depicted vividly. Note that even in the same threat situation, there were mice actively fleeing (e.g., M3) while others remained motionless in corners (e.g., M8). (B) Example of LFP traces in the time domain (left) and frequency domain (right) recorded from the mPFC. Note that, within the recent 1-second relative to the frame, high theta (~10 Hz) activity is prominent in M3 (flight-like locomotion), whereas low theta (~5 Hz) activity is prominent in M8 (freezing-like immobility). (C) Example LFP amplitude spectra as a function of mouse movement speed (Greenish color: slow movement; Reddish color: fast movement) from a solitary recording. (D) Similar to (C), but in a group recording. (E) Theta ratio (i.e., amplitude of 8–12 Hz divided by 3–8 Hz amplitude) derived from (C) and (D). (F) Grand-averaged theta ratio derived from the entire dataset. Error bars indicate ± 1 the standard deviation of the mean.
This demonstration successfully replicates the previous finding from solitary recordings in the new data obtained from group recordings. The MATLAB code used for this data analysis is provided in the how_to_start.mlx file, which will facilitate efforts to analyze behavior and neural dynamics together using this dataset.
Usage Notes
In this paper, we present simultaneous recordings of mouse behaviors and LFP neural activity collected during a robot-based escape experiment, accompanied by the software tools used for technical validation. As detailed above, exhaustive descriptions of the experimental protocols and more advanced analytical methodologies are documented in our earlier publications. In this section, we showcase select examples of these methodologies and explore their potential applications.
Experiment replication
Although our dataset contains a total of 128 recording sessions, the size of the subject pool (n = 8) may appear small for some prospective users. This constraint arose from the intricate nature of conducting experiments within a social framework, which demanded considerable time investment for animal acclimatization and control over the habituation of mouse groups. If there is an interest in replicating or expanding upon this experiment using the same setups (e.g., to assemble a larger cohort of mice), an additional version of the protocol can be obtained further through the Bio-protocol system, as delineated in our publication9, on top of this paper and its related works.
Advanced behavioral analysis techniques
Beyond using stage distinctions and positional data, more sophisticated behavioral analyses can be conducted by categorizing behavior states. Han et al.10 highlights several methodologies, including the calculation of bout durations and movement speed to differentiate between freeze and flight behaviors, as well as the clustering of flight trajectories using a variational autoencoder. There is also a wide array of ML-based tools for behavior labeling and pose estimation, such as DeepLabCut25, which capitalizes on deep residual networks, and B-SOID26, which employs the unsupervised learning algorithm based on t-distributed stochastic neighbor embedding (t-SNE). By correlating a broad spectrum of behaviors with neural activity, it would be possible to construct a richer picture connecting neural dynamics with the emotional responses and natural behaviors of mice.
Acknowledged limitations
The behavioral data presented here is subject to several limitations. Firstly, the methodology employed for tracking mouse positions varies between the solitary and group settings (see Methods). This discrepancy has the potential to introduce algorithm-based variance that has not been accounted for in previous analyses. Secondly, the neural data we provide consists solely of field potentials, thereby describing neural activity at the population level, in contrast to the cell-specific spike data. Lastly, it should be noted that the duration of the experiment sessions spans just over 4 minutes. Hence, it is not guaranteed that our dataset captures changes in neural activity or behaviors resulting from the long-term learning and adaptation of the mice.
Code availability
The MATLAB scripts used for technical validation and data visualizations are accessible under the MIT license through the GitHub repository (https://github.com/jeelabKIST/Cho2024_MouseEscapeData). Comprehensive instructions for the installation and execution of these scripts are provided within the same repository.
References
Cannon, W. B. Bodily changes in pain, hunger, fear and rage: An account of recent researches into the function of emotional excitement. (D Appleton & Company, 1915).
Darwin, C. The expression of the emotions in man and animals. (John Murray, 1872).
Bukalo, O. et al. Prefrontal inputs to the amygdala instruct fear extinction memory formation. Sci Adv 1, https://doi.org/10.1126/sciadv.1500251 (2015).
Courtin, J. et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 505, 92–96, https://doi.org/10.1038/nature12755 (2014).
Mobbs, D. et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science 317, 1079–1083, https://doi.org/10.1126/science.1144298 (2007).
Rozeske, R. R. et al. Prefrontal-Periaqueductal Gray-Projecting Neurons Mediate Context Fear Discrimination. Neuron 97, 898–910 e896, https://doi.org/10.1016/j.neuron.2017.12.044 (2018).
Tovote, P. et al. Midbrain circuits for defensive behaviour. Nature 534, 206–212, https://doi.org/10.1038/nature17996 (2016).
Sun, Y., Gooch, H. & Sah, P. Fear conditioning and the basolateral amygdala. F1000Res 9, https://doi.org/10.12688/f1000research.21201.1 (2020).
Kim, J. et al. A bird’s-eye view of brain activity in socially interacting mice through mobile edge computing (MEC). Sci Adv 6, https://doi.org/10.1126/sciadv.abb9841 (2020).
Han, H. B., Shin, H. S., Jeong, Y., Kim, J. & Choi, J. H. Dynamic switching of neural oscillations in the prefrontal-amygdala circuit for naturalistic freeze-or-flight. Proc Natl Acad Sci USA 120, e2308762120, https://doi.org/10.1073/pnas.2308762120 (2023).
Ronneberger, O., Fischer, P. & Brox, T. 234–241 (Springer International Publishing).
Cho, S. & Choi, J. H. A guide towards optimal detection of transient oscillatory bursts with unknown parameters. J Neural Eng 20, https://doi.org/10.1088/1741-2552/acdffd (2023).
Pernet, C. R. et al. EEG-BIDS, an extension to the brain imaging data structure for electroencephalography. Sci Data 6, 103, https://doi.org/10.1038/s41597-019-0104-8 (2019).
Han, H.-B., Cho, S., Jung, D. & Choi, J. H. Dataset of mouse escape responses and mPFC-BLA LFP recordings. G-Node https://doi.org/10.12751/g-node.2q1eh7 (2024).
Delorme, A. & Makeig, S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134, 9–21, https://doi.org/10.1016/j.jneumeth.2003.10.009 (2004).
Lundqvist, M., Herman, P., Warden, M. R., Brincat, S. L. & Miller, E. K. Gamma and beta bursts during working memory readout suggest roles in its volitional control. Nat Commun 9, 394, https://doi.org/10.1038/s41467-017-02791-8 (2018).
Lundqvist, M. et al. Gamma and Beta Bursts Underlie Working Memory. Neuron 90, 152–164, https://doi.org/10.1016/j.neuron.2016.02.028 (2016).
Bastos, A. M., Lundqvist, M., Waite, A. S., Kopell, N. & Miller, E. K. Layer and rhythm specificity for predictive routing. Proc Natl Acad Sci USA 117, 31459–31469, https://doi.org/10.1073/pnas.2014868117 (2020).
Nandi, B., Swiatek, P., Kocsis, B. & Ding, M. Inferring the direction of rhythmic neural transmission via inter-regional phase-amplitude coupling (ir-PAC). Sci Rep 9, 6933, https://doi.org/10.1038/s41598-019-43272-w (2019).
Palmigiano, A., Geisel, T., Wolf, F. & Battaglia, D. Flexible information routing by transient synchrony. Nat Neurosci 20, 1014–1022, https://doi.org/10.1038/nn.4569 (2017).
Shin, H., Law, R., Tsutsui, S., Moore, C. I. & Jones, S. R. The rate of transient beta frequency events predicts behavior across tasks and species. Elife 6, https://doi.org/10.7554/eLife.29086 (2017).
Tinkhauser, G. et al. Beta burst dynamics in Parkinson’s disease OFF and ON dopaminergic medication. Brain 140, 2968–2981, https://doi.org/10.1093/brain/awx252 (2017).
Amir, A., Headley, D. B., Lee, S. C., Haufler, D. & Pare, D. Vigilance-Associated Gamma Oscillations Coordinate the Ensemble Activity of Basolateral Amygdala Neurons. Neuron 97, 656–669 e657, https://doi.org/10.1016/j.neuron.2017.12.035 (2018).
Stujenske, J. M., Likhtik, E., Topiwala, M. A. & Gordon, J. A. Fear and safety engage competing patterns of theta-gamma coupling in the basolateral amygdala. Neuron 83, 919–933, https://doi.org/10.1016/j.neuron.2014.07.026 (2014).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci 21, 1281–1289, https://doi.org/10.1038/s41593-018-0209-y (2018).
Hsu, A. I. & Yttri, E. A. B-SOiD, an open-source unsupervised algorithm for identification and fast prediction of behaviors. Nat Commun 12, 5188, https://doi.org/10.1038/s41467-021-25420-x (2021).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates. (Elsevier Science, 2013).
Acknowledgements
This research was supported by the National Research Foundation of Korea Grant (NRF-2022R1A2C3003901, 2022R1A6A3A01085957), the KIST Intramural Grant (2E32211), and the ETRI Non-CMOS Neuromorphic Device Basic Technology Grant (21YB3210).
Author information
Authors and Affiliations
Contributions
J.S.K. and J.H.C. designed the behavior paradigm. J.S.K. collected the dataset. H.B.H. synchronized and standardized the raw data into a dataset. S.C. and H.B.H. analyzed the dataset. S.C., D.J. and H.B.H. validated and uploaded the dataset. S.C., H.B.H. and J.H.C. wrote the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cho, S., Han, HB., Jung, D. et al. Mouse Escape Behaviors and mPFC-BLA Activity Dataset: Understanding Flexible Defensive Strategies Under Threat. Sci Data 11, 861 (2024). https://doi.org/10.1038/s41597-024-03688-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41597-024-03688-0