Abstract
Pituitary neuroendocrine tumors remain one of the most common intracranial tumors. While radiomic research related to pituitary tumors is progressing, public data sets for external validation remain scarce. We introduce an open dataset comprising high-resolution T1 contrast-enhanced MR scans of 136 patients with pituitary tumors, annotated for tumor segmentation and accompanied by clinical, radiological and pathological metadata. This diverse dataset captures variations in tumor size, location, and pathological activity, essential for understanding this complex condition. Expert annotations of both the tumor and adjacent carotid arteries ensure precise delineation, facilitating the development of automated segmentation algorithms. Our initiative addresses the need for standardized data in pituitary oncology, fosters collaboration and innovation, and enables the development and benchmarking of workflows that utilize pituitary radiomics for treatment planning and outcome prediction.
Similar content being viewed by others
Background & Summary
Pituitary neuroendocrine tumors are common lesions found in the sella and parasellar region, comprising about 15% of all intracranial tumors1. Pituitary tumors are categorized based on size, with macroadenomas exceeding 10 mm in diameter and microadenomas being smaller. Additionally, they are classified as functioning (FPAs) if they exhibit significant hormone activity, and as non-functioning (NFPAs) if they do not. Despite their classification as benign neoplasms, they can exhibit aggressive local growth and can result in systemic morbidity2. The challenges of diagnosing and accurately localizing these tumors, especially microadenomas, critically impact both the surgical approach and the prognosis3.
The management of patients with pituitary adenomas relies on a thorough assessment of their clinical and endocrine features. In recent years, tumor radiology has gained importance in guiding therapeutic decisions. Magnetic resonance imaging (MRI) remains the primary imaging modality for assessing pituitary gland abnormalities. It allows for the evaluation of a tumor’s volume and its spatial relationship with critical anatomical structures including the optic chiasm, cavernous sinus, and carotid arteries—key considerations for surgical planning. Moreover, radiomic profiling assists in predicting essential tumor properties such as consistency, invasiveness, hormonal activity, histopathological features, and likelihood of recurrence post-surgery4.
Underpinning radiomic analysis is the need for large-scale high-quality neuroimaging, with accurate lesion segmentation. However, few studies adopt the standards recommended for radiomic research5 and fewer still have pituitary data repositories available for public access. Indeed, large-scale data-sharing is necessary for the validation and full potential that radiomics represents. Additionally, significant variability across different scanner types, imaging sequences, and pre-processing methods underscores the importance of utilizing external, multi-vendor datasets to ensure robustness and generalizability of radiomic analyses6.
Pituitary MR imaging presents distinct challenges stemming from variations in scanning protocols across centers. Pituitary scans are often non-isotropic, limited to coronal and/or sagittal planes with a limited field of view and may lack T2-weighted signal information. This hampers inter-subject registration and limits the acquisition of certain radiomic features. Historical approaches to pituitary tumor annotation have demonstrated considerable variability, likely exacerbated by difficulties in distinguishing the gland from the tumor7. Previous radiomic analyses have also neglected proximity to critical neurovascular structures such as the carotid arteries, which significantly influences surgical outcomes.
To address these challenges, we present an isotropic, sub-millimeter voxel-sampled MR dataset comprising 136 patients. This comprehensive dataset includes whole-brain T1-weighted contrast-enhanced (T1w-CE) images, annotated to mark pituitary tumors and bilateral carotid arteries. Additionally, co-registered axial T2-weighted (T2w) images with matching voxel sizes are provided for the majority of cases.
Methods
Ethics and guidelines
This use of data from this cohort including anonymous data sharing was approved by the South-West regional Frenchay Research Ethics Committee (IRAS 271696). This repository follows, where relevant, the FAIR guidelines for data sharing8 and the radiomic quality score with respect to the source imaging data5,7,8.
Patients
A review of our institutional database (a large tertiary neurosciences center with a high volume of pituitary referrals) was conducted to identify a series of all adult patients between July 2021 and December 2023. Patients meeting the following eligibility criteria were selected: (1) diagnosis of pituitary adenoma (2) high spatial resolution T1w-CE brain MRI performed prior to surgery. Patients who were having an operation for tumor recurrence or any pathology other than adenoma were excluded. All patients had been screened in the pituitary MDT with a consensus agreement for surgery made by attending neurosurgeons, endocrinologists and radiologists based on clinical and imaging information. Patients contemporaneously provide their consent for use of their data.
Clinical and pathological information
Basic demographics (age, sex), clinical phenotype (non-functioning, Cushing’s disease, acromegaly, prolactinoma) were collected from a prospectively maintained surgical database. Histopathological data was obtained via pathology reports detailing cell lineage, cell type and Ki67 proliferative index according to WHO 2023 CNS tumor classifications.
The demographics of the cohort have been summarized in Table 1.
Imaging
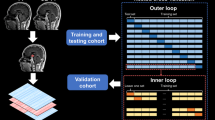
The salient steps involved in acquisition and processing of the pituitary imaging data have been outlined in Fig. 1.
Scanning protocol
All patients had high spatial resolution T1w-CE sequences used for intraoperative neuronavigation compatible with the Medtronic Stealth 8® navigation system. These scans were generally performed internally of which the majority were performed 1-2 days prior to surgery. The original voxel dimensions for the Stealth sequence were 0.488 mm × 0.488 mm and 1.5 mm slice thickness. These scans were obtained on a Siemens Skyra or Prisma scanner at 3 T, with 512 × 512 × 114 matrix dimensions. The majority of patients also had a conventional T2w whole-brain turbo-spin echo (TSE) sequence performed. These were obtained on either a 1.5 or 3 T scanners with a range of matrix dimensions. A minority of patients had T2w imaging in pituitary-specific views. Each patient’s individual scanning information has been outlined in detail in the repository spreadsheet.
Pre-processing
Scans were downloaded from the institution’s PACS system in DICOM (Digital Imaging and Communications in Medicine) format. They were converted to NifTI format using the dcm2niix package9, at which point the imaging header data which includes patient identifiable information were stripped. All scans were reformatted using the fslorient2std function and then bias corrected using FMRIB’s Automated Segmentation Tool (FAST)10, both part of the FMRIB software library (FSL). T2-weighted images were co-registered to its T1W-CE counterpart using a 6 degree of freedom linear transformation applying a tri-linear interpolation and normalized mutual information cost function using FLIRT (FMRIB’s Linear Image Registration Tool). Image segmentation was then performed using these scans. All images were then resampled to 0.5 × 0.5 × 0.5 mm voxel sizes to ensure isotropy. We intentionally avoided cohort signal intensity normalization as (1) this is a step that can easily be performed by the user if they wish, (2) may significantly reduce the variance within the dataset and (3) was unlikely to affect the annotation process.
Post-processing
Following image annotation, images were then face stripped using cropping tools to ensure patient anonymity.
Data Records
Imaging and associated clinical and pathological metadata are available on Figshare11. Pre-processed T1-weighted, contrast-enhanced MR images have been stored in NifTI format, alongside manual/semi-automated segmentations of (1) the pituitary tumor-gland complex and (2) bilateral intracranial carotid arteries. Co-registered T2-weighted imaging, if available, has also been uploaded. Therefore, for each patient, there are 3 or 4 imaging files with the following endings, prefixed by their anonymised identifier: [pid]_T1.nii.gz, [pid]_T2.nii.gz, [pid]_tumour.nii.gz, [pid]_carotids.nii.gz. One supplementary excel file containing: (1) relevant clinical metadata, (2) scanning parameters (3) neuropathological information and (4) basic radiomic data.
Technical Validation
In developing this repository, rigorous measures were implemented to uphold the quality and validity of the dataset. Each MR image was inspected at several time points as part of the processing framework (Fig. 1). The initial inspection occurred at the point of conversion from DICOM to NiFTI. Subsequent evaluations were conducted after image pre-processing and intra-subject registration, followed by a final check after face-stripping. Accordingly, patients were excluded if images were degraded by motion or other scanning artifacts, if registration or pre-processing had failed, or if face-stripping appeared insufficient.
Image segmentation
Image segmentation was performed using ITK-Snap12, a widely used image annotation tool for neurosurgical lesions13 including pituitary tumors14 which has both manual and semi-automated segmentation functions.
To segment tumors, a region of interest on isovolumetric T1 encompassing the tumor was supersampled by a factor of 2 in ITK-snap. Within this region, an intensity window was set to isolate the greatest extent of the tumor soft tissue from the carotids and surrounding CSF. Then a ‘region competitive snake’ segmentation was initialized using manually placed spheres covering the tumor and iteratively reduced to fit the tumor volume (balloon force of 0.1 and curvature force of 0.9).
Carotid segmentations were approached similarly, however using a lower limit intensity threshold, to isolate the typically higher intensity carotids. These were initialized using a string of smaller spheres to cover the desired length of the carotids and iterated over, using the same region’s competitive parameters. For both carotids and tumors, manual adjustments were made using both T1- and T2- weighted images where required. Segmentations were smoothed using a full width at half maximum (FWHM) Gaussian kernel and eroded, followed by a clustering algorithm to remove stray voxels.
Segmentation quality
Annotations were performed by neurosurgical residents and students familiar to the pituitary operative workflow and who were pre-trained on a dataset not used for this repository. Segmentation quality was then assessed by both an attending board-certified neurosurgeon with a subspeciality interest in pituitary and anterior skull base tumors and attending board-certified neuroradiologists with subspeciality interests in neuro-oncology and anterior skull base. Therefore, in total, each patient’s images were reviewed by a neurosurgical resident at least four times and by subject matter experts twice.
For tumors and carotid arteries, the quality of annotations was assessed using criteria described in Tables 2, 3 respectively. If any of the checkpoints were not met, the segmentation was edited before re-assessment.
Usage Notes
The provided dataset is valuable for pituitary tumor radiomics, offering opportunities for training and testing algorithms in lesion segmentation and treatment evaluation. Additionally, it serves as a versatile resource for translational research, enabling investigations into pathological correlations, population trends, and hypothesis validation. By ensuring the entirety of the tumor is captured and also its spatial relationship with the internal carotid arteries, this method of segmentation was felt to be most relevant for neuronavigation and surgical application. As scan resolution and image processing improve and the database is updated, the accuracy of the segmentation will iteratively increase facilitating closer approximation to the true tumor ground truth.
Code availability
The repository has been made available in whole, with associated pre-processing scripts without undue reservation on Figshare11.
References
Melmed, S. Pituitary-Tumor Endocrinopathies. N. Engl. J. Med. 382, 937–950 (2020).
Bioletto, F. et al. Markers of Aggressiveness in Pituitary Tumors: Update and Perspectives. J. Clin. Med. 11, 6508 (2022).
Khan, D. Z. et al. Current and Future Advances in Surgical Therapy for Pituitary Adenoma. Endocr. Rev. 44, 947–959 (2023).
Bioletto, F. et al. Radiomic Analysis in Pituitary Tumors: Current Knowledge and Future Perspectives. J. Clin. Med. 13, 336 (2024).
Lambin, P. et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 14, 749–762 (2017).
Bluemke, D. A. et al. Assessing Radiology Research on Artificial Intelligence: A Brief Guide for Authors, Reviewers, and Readers—From the Radiology Editorial Board. Radiology 294, 192515 (2019).
Won, S. Y. et al. Quality reporting of radiomics analysis in pituitary adenomas: promoting clinical translation. Br. J. Radiol. 95, 20220401 (2022).
Wilkinson, M. D. et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 3, 160018 (2016).
Li, X., Morgan, P. S., Ashburner, J., Smith, J. & Rorden, C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J. Neurosci. Methods 264, 47–56 (2016).
Zhang, Y., Brady, M. & Smith, S. Segmentation of Brain MR Images Through a Hidden Markov Random Field Model and the Expectation-Maximization Algorithm. IEEE Trans. Méd. Imaging 20, 45 (2001).
Pandit, A. S. et al. Mapping Pituitary Neuroendocrine Tumors: An Annotated MRI Dataset Profiling Tumor and Carotid Characteristics. figshare. Online resource. https://doi.org/10.6084/m9.figshare.27894084 (2024).
Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 31, 1116–1128 (2006).
Street, J. S., Pandit, A. S. & Toma, A. K. Predicting vasospasm risk using first presentation aneurysmal subarachnoid hemorrhage volume: A semi-automated CT image segmentation analysis using ITK-SNAP. PLOS ONE 18, e0286485 (2023).
Lindberg, K. et al. Three-Dimensional Volumetric Segmentation of Pituitary Tumors: Assessment of Inter-rater Agreement and Comparison with Conventional Geometric Equations. J. Neurol. Surg. Part B: Skull Base 79, 475–481 (2018).
Knosp, E., Steiner, E., Kitz, K. & Matula, C. Pituitary Adenomas with Invasion of the Cavernous Sinus Space: A Magnetic Resonance Imaging Classification Compared with Surgical Findings. Neurosurgery 33, 610–618 (1993).
Author information
Authors and Affiliations
Contributions
A.S.P., D.Z.K., P.N. and H.J.M. designed and conceived the manuscript and repository. A.S.P., A.K., D.Z.K., G.R. and M.A.K. performed data extraction. A.S.P. performed image pre- and post-processing. A.S.P., A.K. and N.Y. performed annotation. I.D., H.H. and H.J.M. performed quality assessment. A.S.P. composed the first draft of the manuscript. Z.J., A.B., N.L.D., S.E.B., P.N. and H.J.M. reviewed the manuscript and confirmed the data. P.N. and H.J.M. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pandit, A.S., Keenlyside, A., Khan, D.Z. et al. Mapping Pituitary Neuroendocrine Tumors: An Annotated MRI Dataset Profiling Tumor and Carotid Characteristics. Sci Data 12, 80 (2025). https://doi.org/10.1038/s41597-024-04218-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41597-024-04218-8
This article is cited by
-
Development and validation of a machine learning model based on interpretable clinical characteristics for preoperative prediction of Ki-67 expression in pituitary adenomas
Neurological Sciences (2026)
-
MRI annotation using an inversion-based preprocessing for CT model adaptation
European Radiology Experimental (2025)