Abstract
The Wadden Sea is the world’s largest intertidal area and a UNESCO World Heritage Site. Macrozoobenthic invertebrates perform key ecological functions within intertidal areas by regulating nutrient cycles, decomposing organic matter, and providing food for fish, birds and humans. To understand ecological processes and human impacts on biodiversity, the Synoptic Intertidal BEnthic Survey (SIBES) has sampled intertidal macrozoobenthos since 2008. On average 4,109 stations across 1,200 km² of Dutch Wadden Sea mudflats are sampled from June to October to quantify the benthic invertebrate community and sediment composition, including species abundance and biomass, and grain size and mud content. The dataset published now contains 51,851 sampled stations with 3,034,760 individuals of 177 species. This paper details data collection, validation and processing methods. SIBES is ongoing and data will be updated yearly. In sharing these data, we hope to enhance collaborations and understanding of the impact of various pressures on macrozoobenthic invertebrates, sediment composition, food webs, the ecosystem, and biodiversity in the Wadden Sea and other intertidal habitats.
Similar content being viewed by others
Background & Summary
Intertidal mudflat ecosystems provide essential foraging, reproduction, and nursery habitat for a variety of organisms including marine mammals, birds, fish, and invertebrates1,2. Throughout history, these areas have also provided essential resources for humans3,4. Due to the growth of human population size and anthropogenic impact during the last century4, many intertidal ecosystems have been lost or are now severely degraded5. Intertidal mudflat ecosystems face a multitude of threats, including sea-level rise6,7, reduced sediment supply and coastal squeeze8,9, warming sea water temperature10, wind farm and coastal development11,12, aquaculture and fishing practices13,14, land subsidence due to salt- and gas mining15, compaction of coastal sediments16, and eutrophication17,18,19. Many of these threats have an impact on biodiversity and the dynamics of food web and ecosystem processes. Because of the natural value of intertidal ecosystems and their importance to humans, understanding the effects of pressures on these habitats is essential for the effective conservation and sustainable use of these areas.
Central to intertidal mudflat ecosystems are macrozoobenthic invertebrates, i.e. invertebrates larger than 1 mm that live in or near the surface of mudflats. These invertebrates regulate nutrient cycles20,21,22, decompose organic matter23, bioturbate24, engineer the ecosystem25,26,27, and serve as an important food source for many animal species including fish, birds and humans28,29,30,31,32. Additionally, macrozoobenthic invertebrates can be useful indicators of environmental health and human impact. They are relatively sessile and mostly short-lived ( <6 years in most cases)33, which limits their ability to escape disturbances and adverse conditions34. Due to their sessile nature, the presence of respective species is often correlated with a specific suite of local environmental conditions35,36,37,38,39, such as inundation time and sediment composition of the mudflat35. If this suite of conditions changes due to human perturbations, it may lead to a shift or disappearance of suitable habitat characteristics, resulting in changes to the macrozoobenthic community. Abundance, species richness, and shifts in community composition of benthic invertebrates and their associated sediments are thus frequently used to assess human disturbances15,40,41,42,43,44,45. Additionally, because benthic invertebrates are important prey for e.g. shorebirds, the implications of human disturbances on their predators can be assessed as well46,47,48.
The Wadden Sea, covering the coastal zones of the Netherlands, Germany and Denmark, is the world’s largest intertidal mudflat ecosystem49. The area is recognized as a UNESCO World Heritage Site because of its exceptional natural capital of global importance, including marine mammals, fish and shorebirds30,49,50,51,52. Despite legal protection and management measures being in place, many economic activities occur in the Dutch Wadden Sea31,53,54 that can have detrimental effects on macrozoobenthic invertebrates and their habitat52. These activities include commercial fishing for worms55, shellfish42,46,56,57, and shrimp58, as well as mining15 that causes (deep) land subsidence59. In combination with large-scale phenomena, such as sea level rise60,61 and global warming10,62, anthropogenic activities can cause habitat alterations and destruction, thus contributing to declines in the abundance of macrozoobenthic invertebrates with effects on the food web and biodiversity40,52,57,63. The Wadden Sea has a rich history of research on macrozoobenthic invertebrates, and their habitat use, role in the food web, and the impact of anthropogenic activities17,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81. Sometimes this research is impressively long-term82, but it is often focused on a particular area. For a comprehensive understanding, it is also important to study macrozoobenthic invertebrates across a large scale with varying habitats and environmental gradients. Such data are, however, currently lacking.
The Royal Netherlands Institute for Sea Research (NIOZ) has sampled macrozoobenthic invertebrate distributions and sediment composition throughout the Dutch Wadden Sea since 2008. As part of SIBES (Synoptic Intertidal BEnthic Survey), more than four thousand stations distributed across the entire Dutch Wadden Sea have been sampled annually. SIBES is the largest intertidal sampling campaign in the world83, and its methodology is now used worldwide, for instance in Portugal, Germany, Australia, Guinea-Bissau, Mauritania, Oman, and China14,18,84,85. The spatial sampling performed by SIBES is useful for representative monitoring and for impact assessment studies. The spatial design avoids the problem of selecting control sites for impact assessment, as the entire sampled area can act as a control86. With this approach and utilizing the large spatial resolution, it was for example shown that sediment composition and the macrozoobenthic community differed between areas with and without land subsidence15. In addition, long-term and large-scale monitoring provides insurance against potentially unmonitored impacts, by providing baseline data for post-hoc comparisons and if unplanned accidents occur87.
To advance fundamental knowledge, buttress collaboration, and assess impacts of human disturbance on intertidal areas, this paper presents SIBES and its continuously growing data set. We describe how the samples are taken in the field and processed in the laboratory, and the procedures for quality assessment and control (Fig. 1). We also describe how the data can be accessed, and how they can be processed within R88.
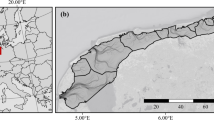
SIBES design with sampling stations and workflow of data collection and processing. The map of the sampling stations shows all stations that have been sampled at least once, with grid (in blue) and random sampling stations (in yellow). The inset shows a map of the detailed design. Tidal basins are separated with black lines. The workflow shows collecting samples in the field, processing them in the laboratory, and curating the data afterwards. Photos from left to right: sampling sediment cores from the boat, sampling by foot, sieved sample, jars with collected samples, and processed samples in crucibles ready for biomass and shell weight measurements in the laboratory. Photos taken by Fred Wiering (left photo) and Kees van de Veen.
Methods
Area description
The Wadden Sea is separated from the North Sea by a series of barrier islands, by which sediment sharing inlet systems connect to back-barrier tidal basins. The area stretches from Den Helder in the Netherlands to Esbjerg in Denmark and spans nearly 500 km linearly following the coast. The total area of the Wadden Sea is about 8000 km2 with approximately 50% of this area consisting of intertidal mudflats89. These mudflats emerge twice per lunar day around low tide. The Dutch part of the Wadden Sea consists of 10 tidal basins which cover a total area about 2500 km2, of which about 50% is intertidal flats38,52. This includes the tidal basin Ems-Dollard, the large estuary that borders the German Wadden Sea in the east (Fig. 1).
Synoptic Intertidal Benthic Survey - SIBES
SIBES is an ongoing ecological monitoring program aimed at monitoring macrozoobenthos and their habitat, i.e. the sediment composition of mudflats (Figs. 2 and 3). Since 2008, every year numerical and biomass density for macrozoobenthic organisms (taxa) are sampled across the entire intertidal Dutch Wadden Sea at a high spatial resolution. These systematic monitoring data have proven fundamental for understanding, for instance, biodiversity76,90, distributions and habitat use69,70,91, species interactions23,77,78,92, the introduction of new species93, sediment composition of mudflats94,95,96,97, and the role of macrozoobenthos in the food web, e.g., as prey to shorebirds98,99,100. In combination with other ecological time-series, for instance on seasonal changes in primary producers101 or the distribution of secondary consumers such as shorebirds102, these long-term data are invaluable for understanding intertidal ecosystems, and the Wadden Sea in particular. SIBES is also essential for management and evidence-based conservation of the Wadden Sea, such as, in relation to studying the effects of gas extraction15,103,104, assessing the European habitat directive105, monitoring the quality status of the Wadden Sea106, and environmental impact assessments107.
Mean biomass and abundance of benthic invertebrates in the Dutch Wadden Sea. (A) Mean annual biomass (g AFDM m−2) calculated across 14 years for the 15 most abundant species and all other species summed, ranked from most abundant to least abundant. (B) Mean annual numerical abundance (# m−2) calculated across 14 years for the 15 most abundant species and all other species summed. (C) Total biomass (g AFDM m−2) per year for the four most abundant species. (D) Total abundance (# m−2) per year for the four most abundant species. Error bars in (A) and (B) denote standard errors of the mean among years. For all species, the colors match between panels.
Sampling design
The spatial sampling design of SIBES encompasses the entire intertidal Dutch Wadden Sea (Fig. 1) with a combination of a grid sampling (systematic sampling) at an inter-sampling distance of 500 m, and ‘random sampling’ with additional stations placed at random distances from grid sampling stations (hereafter referred to as ‘random stations’)108. For logistic convenience, these additional sampling stations were placed on gridlines. To maximize the statistical power to detect changes in abundance of macrozoobenthos, both the grid and random sampling stations were placed at random in the first year and revisited in proceeding years109. That is, with a randomly selected starting position, a full 500-m grid was overlaid onto the Wadden Sea. Stations were then removed if they were not located on an intertidal mudflat. The random stations were placed last. The first years of SIBES had approximately 10% random sampling stations, which was increased to almost 20% from 2014 onwards (Table 1) to increase accuracy of estimating spatial autocorrelation parameters108. Estimating spatial autocorrelation allows for mapping the distribution of benthic invertebrates71,98 (Fig. 4). The Wadden Sea is a dynamic intertidal area in which gullies shift, and mudflats appear and disappear through erosion and sedimentation110. Consequently, sampling stations on intertidal mudflats can become too deep to sample. Yearly, sampling stations are removed that are no longer on mudflats and sampling stations are added on the grid if new mudflats arise, while roughly maintaining the percentage of random stations per tidal basin.
Example of how spatial autocorrelation estimates can be used to interpolate biomass between sampling stations. The spatial sampling design of SIBES with grid and random stations combined allows for accurately estimating autocorrelation. (A) The correlogram showing the spatial autocorrelation for Mudshrimp (Corophiidae) at the Balgzand area in 2011. Correlograms can be used to interpolate the abundance of a species between sampling stations as shown in (B) where Mudshrimp densities (AFDM m−2) were interpolated (mapped) at a 25 × 25 m grid. Actual sampling stations are shown in solid black circles.
Overall, between 2,399 and 4,471 stations have been sampled each year (Table 1). In 2008, the Ems-Dollard estuary (the most eastern tidal basin in Fig. 1) was not sampled. In 2020, due to COVID-19 restrictions that limited the crew on board of the research vessel, the grid was sampled at 1000 meters (twice the normal distance), but all random stations were still visited. Excluding 2020, an average of 4,109 stations have been sampled yearly. Between 2017-2018, due to a lack of funding, stations were sampled but not processed in the laboratory. Recent funding has allowed processing these samples that will be finished in 2025. As these data become available, they will be added to the database.
Field sampling
The NIOZ research vessels RV Navicula (2008–2023) and RV Wim Wolff (from 2024 onwards) were used as a platform to access the sampling areas across the Dutch Wadden Sea. Sampling locations were accessed by rubber dinghies during high-tide or by foot during low-tide (Fig. 1). Because of time constraints and the distances between sampling stations, most samples were taken by boat (Table 1). Sampling stations close to shore were often accessed by foot, as these relatively elevated sites could not be easily reached by boat. Sampling occurred in teams of at least two people and sampling locations were found with a handheld GPS (WGS84 as map datum).
In most years, the sampling was conducted for six to eight weeks between June and September (Fig. 5). To prevent an interaction between sampling location and seasonal change (growth of macrozoobenthos), the aim is to sample each area during the same period each year. In 2008, due to logistic constraints, sediment samples were collected on a 1000-m grid instead of at all stations on the 500-m grid.
At each sampling station, a sample was taken by boat or by foot (‘platform’ in Table 2). First, a sediment sample was taken. This was done with a small jar (Polypropylene container) with a diameter of 33 mm to a depth of 4 cm38,97. Next, a macrozoobenthic sample was taken. At each site sampled by boat, two cores were taken with a combined area of 0.0173 m2 to a depth of ~25 cm. At each site sampled by foot, a single core of 0.0177 m2 was taken to a depth of ~25 cm. Both these methods yield similar results (Kraan et al., 2007). Details on the sampling cores can be found in the appendix (Supplementary Information S1). In the field, the sample cores were sieved on a 1-mm round mesh (Supplementary Information S1). After sieving, large bivalves (shell length approximately >1 cm) that were easily visible were separated and collected in plastic bags to be frozen at −20 °C for later analysis in the laboratory. The remaining macrozoobenthic species were collected in plastic jars (Fig. 1) and preserved using a 4% formaldehyde solution. To preserve shells and identify live material more easily in the laboratory, all formaldehyde solutions were respectively borax-buffered and stained using rose Bengal dye (C.A.S. no. 632-68-8).
Laboratory analysis – macrozoobenthic species
Because of the high number of samples processed each year, a trade-off was made between the level of taxonomic detail and the time taken per sample. In practice, this means that all mollusks and larger crustaceans and polychaetes were identified to the species level (Table 3). All other organisms were identified to the finest taxonomical level given in Supplementary Table S1. Identification of the macrozoobenthos species started by using more general keys (e.g. class or order) such as those for Polychaeta by Hartmann-Schröder et al.111, Amphipoda by Lincoln112 and Mollusks by de Bruyne, R. & de Boer, T.W.113, and was completed by using recent family or genus keys. The nomenclature was verified in the World Register of Marine Species. Because of time constraints, sponges, hydroids, sea anemones, barnacles, bryozoans, and sea squirts were not analyzed.
After identification, all individuals were counted (Table 4). Polychaetes were often found fragmented and counting them is not straightforward. Between 2008 and into 2011, the number of polychaetes was derived by counting the loose heads and tails. From 2011 onwards, polychaete species were counted by summing the number of heads. For Lanice conchilega, only whole individuals were counted.
For the species and samples that contained many individuals, a subsample was selected and analyzed. The method of subsampling changed in 2014. For the samples collected up to 2013, a subsample was made by dividing the organisms over a sorting tray and a subsample of at least 30 individuals was taken randomly and the subsample percentage noted down. For the samples collected in 2014 and onwards, a subsample was taken by dividing the organisms equally over the middle 4 bands of the sorting tray. A subsample of around 30 individuals was taken. A dice was thrown to decide where in the tray the subsample was taken. Subsamples were taken for Peringia ulvae, small bivalves, Crustaceans and small worms (including Oligochaeta) which can all occur at high densities.
The body size of species was measured according to the methods in Supplementary Table S1. All crustaceans were identified, but because of time constraints lengths were only taken for shrimps and crabs. All individuals were measured to the nearest mm using a vernier or a digital caliper. For Macoma balthica and Cerastoderma edule shells larger than 8 mm, the width and thickness were also measured, where possible. Occasionally, length could not be measured, for instance, because a shell was broken. In those cases and when possible, length was estimated. The bivalve Ensis leei would often break and the length could not be measured. In such cases the length (mm) was estimated with the width (mm) of the top or bottom following: length = 6.5 × widthtop or 6.6 × widthbottom.
The biomass of individuals was measured as Ash Free Dry Mass (AFDM) (Table 4), that was determined by first drying the sample for 2 to 3 days at 60 °C in a ventilated stove, then measuring dry mass. Following this, the sample was incinerated for 5 hours at 560 °C and then the mass was measured again to calculate AFDM. Measurements of mass were done by hand until July 2011. From July 2011 onwards, most measurements of mass were made by the WULC automatic weighing machine. Only very large crucibles were still weighed by hand. Shell and meat were separated before AFDM was measured, but only for individuals above a minimum size (see Supplementary Table S1 for the thresholds). Bivalves below the minimum size threshold were incinerated together. For worms, biomass was determined for all species in 2008 and 2009. To allow processing many samples, from 2010 onwards, biomass was not measured for the small worms Pygospio elegans, Spio martinensis, Cirratulidae (Aphelochaeta sp. and Tharyx sp.), and Oligochaeta.
Laboratory analysis – sediment samples
The sediment samples were first freeze-dried for up to 96 h, then homogenized with a mortar and pestle. The mass of homogenized samples was measured, and the sample was mixed with degassed water, purified by means of reverse osmosis, and placed into 13-ml polypropylene auto-sampler tubes. Samples were then shaken vigorously with a vortex mixer for 30 s prior to grain sizes being measured using a particle size analyzer that uses laser diffraction and polarization intensity differential scattering technology (Coulter LS 13 320, optical module ‘grey’). Grain sizes were measured from 0.04 to 2000 μm in 126 size classes. From these distributions, median grain size and mud content (volume percentage of particles <63 μm) were extracted (Table 2). Organic matter and calcium carbonate were not removed by treating sediment samples with hydrogen chloride and hydrogen peroxide38.
Data validation and processing
After processing samples in the laboratory, data records were checked for quality through, e.g., outlier analyses. To identify erroneous biomass measurements, for example, biomass was analyzed against length to ensure they followed plausible and understandable patterns of variability (Fig. 6). Extreme abundances, biomass and length values were double checked for mistakes and corrected or deleted if the mistake was unclear. For animals without recorded lengths, boxplots were created to identify outliers, which were then reviewed for potential data entry errors and corrected if necessary. The lengths of bivalves were checked against maximal length values reported in de Bruyne & de Boer (2008)113.
Example of a species-specific allometric relationship used for predicting an individual’s Ash Free Dry Mass (AFDM, g) from (estimated) length (mm). The solid green line is the fitted LOESS-model that is used for predicting AFDM from length. The LOESS curve was fitted using the ‘loess’ function in R88, with a span of 0.6. The model’s residual standard error was 0.15. Open circles are individual measurements (n = 58,989) and grey solid dots are outliers (n = 560; corresponding to 0.9% of the data points) that fall outside of twice the inter quartile range.
In some cases, AFDM measurements could not be made, for instance because the shell was broken, or values were deleted after they were identified as outliers. If a length measure was available, AFDM was predicted from allometric relationships (Fig. 6). Because allometric relationships can be non-linear, they were modelled using LOESS71. Because these biomass-length relationships can differ between years and areas, we used a hierarchical approach for each species or species group (Supplementary Table S1). That is, we first filtered the data for that species or group per season, then per year, then per region, and then per tidal basin. After each step, the number of records with biomass and length measures was determined. The LOESS-model was then fitted at the smallest scale that included at least 75 individual records of biomass and length measured. For species and species groups <11 mm, 45 individual records were sufficient. When no length value was available to predict AFDM for a species or species group, the average biomass was used following the same hierarchical approach to get the most fine-grained input possible, but with a minimum of 30 individual records.
All total abundance (#) and biomass (g AFDM) measurements were standardized per unit area (m−2) by dividing them by the sampled surface area. In the case of subsampling (see section ‘Laboratory analysis – macrozoobenthic species’), abundances were corrected by dividing the densities with the fraction of the subsample. After this standardization, individual measurements were grouped per species or species group and summed per sample (‘sample_id’, Table 2). The underlying individual measurement data as well as raw sediment distribution data are available, but because they are sensitive for erroneous calculations, they are not shared here but available upon request.
Data Records
Here, we present the currently available SIBES data that spans 14 years (2008–2021). SIBES has continued beyond these years and those data will also be made available in the future (see the section “Usage Notes”). With 5,107 unique sampling stations (‘sampling_station_id’ in Table 2), the dataset currently includes 51,851 sampled stations (‘sample_id’ in Table 2) with biomass and abundance of 177 taxa (‘sibes_id’ in Table 3) and 48,186 of sediment composition. The measurements of biomass and abundance presented here were based on 3,034,760 individuals. These data are available in three ‘csv’ files that can be downloaded from the NIOZ Data Archive System DAS114. The files contain (1) metadata of sampling stations and sediment characteristics (‘sample.csv’, Table 2), (2) species found on the mudflats of the Dutch Wadden Sea (‘species.csv’, Table 3), and (3) biomass and abundance per species per sample station (‘biota.csv’, Table 4).
Technical Validation
Before starting SIBES in 2008, a simulation study108 was conducted to estimate the optimal sampling design for different (sometimes conflicting) objectives while maximizing statistical power and efficiency of sampling. Building on existing intertidal benthic monitoring programs in the Dutch Wadden Sea64,83,115,116, three objectives were identified: (1) estimating population sizes, monitoring of population trends, and comparisons of populations/trends between years or areas, (2) mapping species distributions, and (3) accurately estimating spatial autocorrelation parameters, which is required for mapping abundances. Four commonly used sampling designs were compared: simple random, grid, and two types of transects. An additional fifth novel design was also included: grid with random replacements. Sampling designs were analyzed using Monte Carlo simulations at four levels of naturally occurring spatial autocorrelation. To compare sampling designs, the following criteria were used: (1) minimum detectable difference in mean between two time periods or two areas, (2) mean prediction error and (3) estimation bias of autocorrelation parameters. It was shown that grid sampling with a number of random samples was accurate and effective, and the optimal sampling design catering for all three objectives. Therefore, when SIBES started in 2008, the optimal design of combined grid sampling with a percentage of random stations was chosen. In the simulation study, these random samples were replaced to maintain equal samples sizes and a fair comparison between sampling designs. With SIBES, due to logistic efficiency in the field, these were additional stations placed on gridlines (Fig. 1).
To further validate the SIBES sampling scheme, data were compared with traditional transect sampling that occurred in Balgzand, the western most tidal basin of the Wadden Sea64,82. These analyses indicated that standardized (m−2) abundance and biomass estimates were, on average, similar but with two striking differences117: i) SIBES showed larger accuracy with lower variance around the estimates, and ii) the densities of deeper living species, such as Mya arenaria and Arenicola marina, were underestimated with SIBES. A potential cause of the latter difference could be that sampling depth is reduced when a rubber boat is needed to access and sample the station.
To continuously ensure a high and standardized data quality, sampling and processing are conducted with protocols in accordance with NEN-EN-ISO 9001:2015. Since 2010, the SIBES quality management system externally evaluated (Kiwa) each year according to ISO 9001 standards.
Usage Notes
Because SIBES is ongoing, the dataset will be updated, and data will be made available online yearly114. To allow ongoing research to be completed (e.g. PhD-candidates publishing their work), there is a delay of three years after data collection. However, the most recent data are also available upon request. Likewise, the underlying data of individual measurements as well as raw sediment distribution are available upon request. When using these data, people are urged to cite this data paper in any resulting product (e.g. publication or report). These products and collaborations will help us to maintain this valuable monitoring program that requires substantial effort and resources. More information and documentation on SIBES can be found online (www.nioz.nl/sibes).
Code availability
To aid the use and exploration of SIBES data using the software package R88, we have developed an R-library that will continue to be updated. The latest version can be accessed on: https://github.com/allertbijleveld/SIBES. With data included in the R-library, examples are provided for preparing the data, exploring trends, and making maps.
References
Levin, L. A. et al. The function of marine critical transition zones and the importance of sediment biodiversity. Ecosyst. 4, 430–451, https://doi.org/10.1007/s10021-001-0021-4 (2001).
Dissanayake, N. G., Frid, C. L. J., Drylie, T. P. & Caswell, B. A. Ecological functioning of mudflats: global analysis reveals both regional differences and widespread conservation of functioning. Mar. Ecol. Prog. Ser. 604, 1–20, https://doi.org/10.3354/meps12728 (2018).
Lotze, H. K. et al. Human transformations of the Wadden Sea ecosystem through time: a synthesis. Helgol. Mar. Res. 59, 84–95, https://doi.org/10.1007/s10152-004-0209-z (2005).
Lotze, H. K. et al. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312, 1806–1809, https://doi.org/10.1126/science.1128035 (2006).
Murray, N. J. et al. The global distribution and trajectory of tidal flats. Nature 565, 222–225, https://doi.org/10.1038/s41586-018-0805-8 (2019).
Vermeersen, B. L. et al. Sea-level change in the Dutch Wadden Sea. Neth. J. Geosci. 97, 79–127, https://doi.org/10.1017/njg.2018.7 (2018).
van der Wegen, M., Jaffe, B., Foxgrover, A. & Roelvink, D. Mudflat morphodynamics and the impact of sea level rise in south San Francisco bay. Estuar. Coast. 40, 37–49, https://doi.org/10.1007/s12237-016-0129-6 (2017).
Delafontaine, M. T., Flemming, B. W. & Mai, S. in Proceedings in Marine Science Vol. 2 (eds B. W. Flemming, M. T. Delafontaine, & G. Liebezeit) 273-286 (Elsevier, 2000).
Wang, H., Ge, Z., Yuan, L. & Zhang, L. Evaluation of the combined threat from sea-level rise and sedimentation reduction to the coastal wetlands in the Yangtze Estuary, China. Ecol. Eng. 71, 346–354, https://doi.org/10.1016/j.ecoleng.2014.07.058 (2014).
Chust, G. et al. Cross-basin and cross-taxa patterns of marine community tropicalization and deborealization in warming European seas. Nat. Commun. 15, 2126, https://doi.org/10.1038/s41467-024-46526-y (2024).
Seto, K. C., Fragkias, M., Güneralp, B. & Reilly, M. K. A meta-analysis of global urban land expansion. PLoS One 6, e23777, https://doi.org/10.1371/journal.pone.0023777 (2011).
Stewart, G. B., Pullin, A. S. & Coles, C. F. Poor evidence-base for assessment of windfarm impacts on birds. Environ. Conserv. J. 34, 1–11, https://doi.org/10.1017/S0376892907003554 (2007).
Beninger, P. G. in Mudflat Ecology (ed Peter G. Beninger) 339-363 (Springer International Publishing, 2018).
Peng, H. B. et al. Mollusc aquaculture homogenizes intertidal soft‐sediment communities along the 18,400 km long coastline of China. Divers. Distrib. 27, 1553–1567, https://doi.org/10.1111/ddi.13302 (2021).
de la Barra, P., Aarts, G. & Bijleveld, A. I. The effects of gas extraction under intertidal mudflats on sediment and macrozoobenthic communities. J. Appl. Ecol. 61, 390–405, https://doi.org/10.1111/1365-2664.14530 (2024).
Syvitski, J. P. M. et al. Sinking deltas due to human activities. Nat. Geosci. 2, 681–686, https://doi.org/10.1038/ngeo629 (2009).
Philippart, C. J. et al. Impacts of nutrient reduction on coastal communities. Ecosyst. 10, 96–119, https://doi.org/10.1007/s10021-006-9006-7 (2007).
Singer, A. et al. Long-term response of coastal macrofauna communities to de-eutrophication and sea level rise mediated habitat changes (1980s versus 2018). Front. Mar. Sci. 9, https://doi.org/10.3389/fmars.2022.963325 (2023).
Beukema, J. & Cadée, G. Growth rates of the bivalve Macoma balthica in the Wadden Sea during a period of eutrophication: relationships with concentrations of pelagic diatoms and flagellates. Mar. Ecol. Prog. Ser. 68, 249–256 (1991).
Herman, P., Middelburg, J., van de Koppel, J. & Heip, C. Ecology of estuarine macrobenthos. Adv. Ecol. Res. 29, 195–240 (1999).
Asmus, H. & Asmus, R. The importance of grazing food chain for energy flow and production in three intertidal sand bottom communities of the northern Wadden Sea. Helgolander Meeresun 39, 273–301, https://doi.org/10.1007/BF01992775 (1985).
Verwey, J. On the ecology of distribution of cockle and mussel in the Dutch Waddensea, their role in sedimentation and the source of their food supply. Arch. Neerl. Zool. 10, 171–239 (1954).
Riekenberg, P. M., van der Heide, T., Holthuijsen, S. J., van der Veer, H. W. & van der Meer, M. T. J. Compound-specific stable isotope analysis of amino acid nitrogen reveals detrital support of microphytobenthos in the Dutch Wadden Sea benthic food web. Front Ecol. Evol. 10, https://doi.org/10.3389/fevo.2022.951047 (2022).
Meysman, F. J. R., Middelburg, J. J. & Heip, C. H. R. Bioturbation: a fresh look at Darwin’s last idea. Trends Ecol. Evol. 21, 688–695, https://doi.org/10.1016/j.tree.2006.08.002 (2006).
Bouma, T. J., Olenin, S., Reise, K. & Ysebaert, T. Ecosystem engineering and biodiversity in coastal sediments: posing hypotheses. Helgol. Mar. Res. 63, 95–106, https://doi.org/10.1007/s10152-009-0146-y (2009).
Eriksson, B. K. et al. Facilitation by ecosystem engineers enhances nutrient effects in an intertidal system. Ecosphere 8, e02051, https://doi.org/10.1002/ecs2.2051 (2017).
van der Zee, E. M. et al. How habitat-modifying organisms structure the food web of two coastal ecosystems. Proc. R. Soc. B 283, 20152326, https://doi.org/10.1098/rspb.2015.2326 (2016).
Zwarts, L. & Wanink, J. H. How the food-supply harvestable by waders in the Wadden Sea depends on the variation in energy density, body-weight, biomass, burying depth and behavior of tidal-flat invertebrates. Neth. J. Sea Res. 31, 441–476, https://doi.org/10.1016/0077-7579(93)90059-2 (1993).
Piersma, T. Production by intertidal benthic animals and limits to their predation by shorebirds: a heuristic model. Mar. Ecol. Prog. Ser. 38, 183–196 (1987).
van de Kam, J., Ens, B., Piersma, T. & Zwarts, L. Shorebirds. An illustrated behavioral ecology. (KNNV Publishers, 2004).
Wolff, W. J. The exploitation of living resources in the Dutch Wadden Sea: a historical overview. Helgol. Mar. Res. 59, 31–38, https://doi.org/10.1007/s10152-004-0204-4 (2005).
Poiesz, S. S. H., Witte, J. I. J. & van der Veer, H. W. Only a few key prey species fuel a temperate coastal fish food web. Mar. Ecol. Prog. Ser. 653, 153–166, https://doi.org/10.3354/meps13472 (2020).
Gusmao, J. B. et al. Comparing taxonomic and functional trait diversity in marine macrozoobenthos along sediment texture gradients. Ecol. Indic. 145, 109718, https://doi.org/10.1016/j.ecolind.2022.109718 (2022).
Beauchard, O. et al. A generic approach to develop a trait-based indicator of trawling-induced disturbance. Mar. Ecol. Prog. Ser. 675, 35–52, https://doi.org/10.3354/meps13840 (2021).
Kraan, C., Aarts, G., van der Meer, J. & Piersma, T. The role of environmental variables in structuring landscape-scale species distributions in seafloor habitats. Ecology 91, 1583–1590, https://doi.org/10.1890/09-2040.1 (2010).
Compton, T. J. et al. Repeatable sediment associations of burrowing bivalves across six European tidal flat systems. Mar. Ecol. Prog. Ser. 382, 87–98, https://doi.org/10.3354/meps07964 (2009).
Ysebaert, T. & Herman, P. M. J. Spatial and temporal variation in benthic macrofauna and relationships with environmental variables in an estuarine, intertidal soft-sediment environment. Mar. Ecol. Prog. Ser. 244, 105–124, https://doi.org/10.3354/meps244105 (2002).
Compton, T. J. et al. Distinctly variable mudscapes: distribution gradients of intertidal macrofauna across the Dutch Wadden Sea. J. Sea Res. 82, 103–116, https://doi.org/10.1016/j.seares.2013.02.002 (2013).
Thrush, S. F. et al. Habitat change in estuaries: predicting broad-scale responses of intertidal macrofauna to sediment mud content. Mar. Ecol. Prog. Ser. 263, 101–112, https://doi.org/10.3354/meps263101 (2003).
Beukema, J. J. Expected changes in the benthic fauna of Wadden Sea tidal flats as a result of sea-level rise or bottom subsidence. J. Sea Res. 47, 25–39, https://doi.org/10.1016/S1385-1101(01)00095-8 (2002).
van der Veer, H. W., Bergman, M. J. N. & Beukema, J. J. Dredging activities in the Dutch Wadden Sea: effects on macrobenthic infauna. Neth. J. Sea Res. 19, 183–190, https://doi.org/10.1016/0077-7579(85)90022-5 (1985).
Piersma, T. et al. Long-term indirect effects of mechanical cockle-dredging on intertidal bivalve stocks in the Wadden Sea. J. Appl. Ecol. 38, 976–990, https://doi.org/10.1046/j.1365-2664.2001.00652.x (2001).
Vitaliano, J. J., Fromm, S. A., Packer, D. B., Reid, R. N. & Pikanowski, R. A. Recovery of benthic macrofauna from sewage sludge disposal in the New York Bight. Mar. Ecol. Prog. Ser. 342, 27–40, https://doi.org/10.3354/meps342027 (2007).
Bedini, R., Batistini, F., Nannelli, A. & Piazzi, L. Assessment of anthropogenic disturbance on soft-bottom macroinvertebrate assemblages across different spatial scales. J. Mar. Biol. Assoc. U.K. 92, 439–448, https://doi.org/10.1017/S0025315411001184 (2012).
Thrush, S. F., Hewitt, J. E., Cummings, V. J. & Dayton, P. K. The impact of habitat disturbance by scallop dredging on marine benthic communities: what can be predicted from the results of experiments? Mar. Ecol. Prog. Ser. 129, 141–150, https://doi.org/10.3354/meps129141 (1995).
Kraan, C. et al. Landscape-scale experiment demonstrates that Wadden Sea intertidal flats are used to capacity by molluscivore migrant shorebirds. J. Anim. Ecol. 78, 1259–1268, https://doi.org/10.1111/j.1365-2656.2009.01564.x (2009).
Rakhimberdiev, E. et al. Fuelling conditions at staging sites can mitigate Arctic warming effects in a migratory bird. Nat Commun 9, 1–10, https://doi.org/10.1038/s41467-018-06673-5 (2018).
Mathot, K. J., Piersma, T. & Elner, R. W. in Mudflat ecology 309-338 (Springer, 2018).
Reise, K. et al. The Wadden Sea a universally outstanding tidal wetland. Report No. 0946-896X, 7-24 (Common Wadden Sea Secretariat, Wilhelmshaven, Germany, 2010).
van der Veer, H. W. et al. Changes over 50 years in fish fauna of a temperate coastal sea: degradation of trophic structure and nursery function. Estuar. Coast. Shelf. S. 155, 156–166, https://doi.org/10.1016/j.ecss.2014.12.041 (2015).
Aarts, G. et al. Top-down pressure on a coastal ecosystem by harbor seals. Ecosphere 10, e02538, https://doi.org/10.1002/ecs2.2538 (2019).
Wolff, W. J. Causes of extirpations in the Wadden Sea, an estuarine area in the Netherlands. Conserv. Biol. 14, 876–885, https://doi.org/10.1046/j.1523-1739.2000.98203.x (2000).
Daams, M. N. & Sijtsma, F. J. Planting the SEED: towards a Spatial Economic Ecological Database for a shared understanding of the Dutch Wadden area. J. Sea Res. 82, 153–164, https://doi.org/10.1016/j.seares.2012.12.002 (2013).
Kabat, P. et al. The Wadden Sea region: towards a science for sustainable development. Ocean. Coast. Manag. 68, 4–17, https://doi.org/10.1016/j.ocecoaman.2012.05.022 (2012).
Beukema, J. J. Long-term effects of mechanical harvesting of lugworms Arenicola marina on the zoobenthic community of a tidal flat in the Wadden Sea. Neth. J. Sea Res. 33, 219–227, https://doi.org/10.1016/0077-7579(95)90008-X (1995).
Beukema, J. J. & Dekker, R. Annual cockle Cerastoderma edule production in the Wadden Sea usually fails to sustain both wintering birds and a commercial fishery. Mar. Ecol. Prog. Ser. 309, 189–204, https://doi.org/10.3354/meps309189 (2006).
Camphuysen, C. J. et al. Mass mortality of common eiders Somateria mollissima in the Dutch Wadden Sea, winter 1999/2000: starvation in a commercially exploited wetland of international importance. Biol. Conserv. 106, 303–317, https://doi.org/10.1016/S0006-3207(01)00256-7 (2002).
Tulp, I., Glorius, S., Rippen, A., Looije, D. & Craeymeersch, J. Dose-response relationship between shrimp trawl fishery and the macrobenthic fauna community in the coastal zone and Wadden Sea. J. Sea Res. 156, 101829, https://doi.org/10.1016/j.seares.2019.101829 (2020).
Fokker, P. A., Van Leijen, F. J., Orlic, B., Van Der Marel, H. & Hanssen, R. F. Subsidence in the Dutch Wadden Sea. Neth. J. Geosci. 97, 129–181, https://doi.org/10.1017/njg.2018.9 (2018).
Huismans, Y. et al. Development of intertidal flats in the Dutch Wadden Sea in response to a rising sea level: spatial differentiation and sensitivity to the rate of sea level rise. Ocean. Coast. Manag. 216, 105969, https://doi.org/10.1016/j.ocecoaman.2021.105969 (2022).
Wang, Z. B., Elias, E. P., van der Spek, A. J. & Lodder, Q. J. Sediment budget and morphological development of the Dutch Wadden Sea: impact of accelerated sea-level rise and subsidence until 2100. Neth. J. Geosci. 97, 183–214, https://doi.org/10.1017/njg.2018.8 (2018).
Zhou, Z. et al. Temporal dynamics of heatwaves are key drivers of sediment mixing by bioturbators. Limnol. Oceanogr. 68, 1105–1116, https://doi.org/10.1002/lno.12332 (2023).
van der Meer, J., Beukema, J. J. & Dekker, R. Using stochastic population process models to predict the impact of climate change. J. Sea Res. 82, 117–121, https://doi.org/10.1016/j.seares.2012.08.011 (2013).
Beukema, J. J. Seasonal changes in the biomass of the macro-benthos of a tidal flat area in the Dutch Wadden Sea. Neth. J. Sea Res. 8, 94–107, https://doi.org/10.1016/0077-7579(74)90028-3 (1974).
Zwarts, L. Burying depth of the benthic bivalve Scrobicularia plana (da Costa) in relation to siphon-cropping. J. Exp. Mar. Biol. Ecol. 101, 25–39, https://doi.org/10.1016/0022-0981(86)90040-7 (1986).
Zwarts, L. Seasonal-variation in body-weight of the bivalves Macoma balthica, Scrobicularia plana, Mya arenaria and Cerastoderma edule in the Dutch Wadden Sea. Neth. J. Sea Res. 28, 231–245, https://doi.org/10.1016/0077-7579(91)90021-R (1991).
Piersma, T., Verkuil, Y. I. & Tulp, I. Resources for long-distance migration of Knots Calidris canutus islandica and C.c. canutus: how broad is the temporal exploitation window of benthic prey in the western and eastern Wadden Sea. Oikos 71, 393–407, https://doi.org/10.2307/3545827 (1994).
van Gils, J. A. et al. Reversed optimality and predictive ecology: burrowing depth forecasts population change in a bivalve. Biol. Lett. 5, 5–8, https://doi.org/10.1098/rsbl.2008.0452 (2009).
Compton, T. et al. Burrowing behavior of a deposit feeding bivalve predicts change in intertidal ecosystem state. Front. Ecol. Evol. 4, https://doi.org/10.3389/fevo (2016).
Compton, T. J. et al. Shifting baselines in the Ems Dollard estuary: a comparison across three decades reveals changing benthic communities. J. Sea Res. 127, 119–132, https://doi.org/10.1016/j.seares.2017.06.014 (2017).
Bijleveld, A. I., Twietmeyer, S., Piechocki, J., van Gils, J. A. & Piersma, T. Natural selection by pulsed predation: survival of the thickest. Ecology 96, 1943–1956, https://doi.org/10.1890/14-1845.1 (2015).
Armonies, W. & Reise, K. Empty habitat in coastal sediments for populations of macrozoobenthos. Helgol. Mar. Res. 56, 279–287, https://doi.org/10.1007/s10152-002-0129-8 (2003).
Ólafsson, E. B., Peterson, C. H. & Ambrose, W. G. Does recruitment limitation structure populations and communities of macroinvertebrates in marine soft sediments: the relative significance of presettlement and postsettlement processes. Oceanogr. Mar. Biol. 32, 65–109 (1994).
Kröncke, I., Dippner, J., Heyen, H. & Zeiss, B. Long-term changes in macrofaunal communities off Norderney (East Frisia, Germany) in relation to climate variability. Mar. Ecol. Prog. Ser. 167, 25–36, https://doi.org/10.3354/meps167025 (1998).
Jensen, K. T. Density-dependent growth in cockles Cerastoderma edule: evidence from interannual comparisons. J. Mar. Biol. Assoc. U.K. 73, 333–342, https://doi.org/10.1017/S0025315400032896 (1993).
Klunder, L. et al. Quantification of marine benthic communities with metabarcoding. Mol. Ecol. Resour. 22, 1043–1054, https://doi.org/10.1111/1755-0998.13536 (2022).
Kwakernaak, C. et al. Ragworms Hediste diversicolor limit eelgrass Zostera marina seedling settlement: Implications for seed-based restoration. J. Exp. Mar. Biol. Ecol. 560, 151853, https://doi.org/10.1016/j.jembe.2022.151853 (2023).
Nauta, J. et al. Mutual facilitation between foundation species Mytilus edulis and Lanice conchilega promotes habitat heterogeneity on tidal flats. Front. Mar. Sci. 11, https://doi.org/10.3389/fmars.2024.1354009 (2024).
van der Meer, J., Beukema, J. J. & Dekker, R. Population dynamics of two marine polychaetes: the relative role of density dependence, predation, and winter conditions. ICES J. Mar. Sci. 57, 1488–1494, https://doi.org/10.1006/jmsc.2000.0912 (2000).
Dekker, R. & Beukema, J. J. Long-term and large-scale variability in productivity of the tellinid bivalve Macoma balthica on Wadden Sea tidal flats. Mar. Ecol. Prog. Ser. 337, 117–134, https://doi.org/10.3354/meps337117 (2007).
Flach, E. C. The influence of the cockle, Cerastoderma edule, on the macrozoobenthic community of tidal flats in the Wadden Sea. Mar. Ecol. 17, 87–98, https://doi.org/10.1111/j.1439-0485.1996.tb00492.x (1996).
Beukema, J. & Dekker, R. Half a century of monitoring macrobenthic animals on tidal flats in the Dutch Wadden Sea. Mar. Ecol. Prog. Ser. 656, 1–18, https://doi.org/10.3354/meps13555 (2020).
Kraan, C., van der Meer, J., Dekinga, A. & Piersma, T. Patchiness of macrobenthic invertebrates in homogenized intertidal habitats: hidden spatial structure at a landscape scale. Mar. Ecol. Prog. Ser. 383, 211–224, https://doi.org/10.3354/meps07994 (2009).
Oudman, T. et al. Resource landscapes explain contrasting patterns of aggregation and site fidelity by red knots at two wintering sites. Mov. Ecol. 6, 24, https://doi.org/10.1186/s40462-018-0142-4 (2018).
Bom, R. A. et al. The intertidal mudflats of Barr Al Hikman, Sultanate of Oman, as feeding, reproduction and nursery grounds for brachyuran crabs. Hydrobiologia 847, 4295–4309, https://doi.org/10.1007/s10750-020-04418-4 (2020).
Underwood, A. The mechanics of spatially replicated sampling programmes to detect environmental impacts in a variable world. Aust. J. Ecol. 18, 99–116, https://doi.org/10.1111/j.1442-9993.1993.tb00437.x (1993).
Wiens, J. A. & Parker, K. R. Analyzing the effects of accidental environmental impacts: approaches and assumptions. Ecol. Appl. 5, 1069–1083, https://doi.org/10.2307/2269355 (1995).
R Core Team. R: a language and environment for statistical computing. (2020).
De Jonge, V. N., Essink, K. & Boddeke, R. The Dutch Wadden Sea: a changed ecosystem. Hydrobiologia 265, 45–71, https://doi.org/10.1007/BF00007262 (1993).
Christianen, M. J. A. et al. Biodiversity and food web indicators of community recovery in intertidal shellfish reefs. Biol. Conserv. 213, 317–324, https://doi.org/10.1016/j.biocon.2016.09.028 (2017).
Bijleveld, A. I. et al. Presence-absence of marine macrozoobenthos does not generally predict abundance and biomass. Sci. Rep. 8, 3039, https://doi.org/10.1038/s41598-018-21285-1 (2018).
Christianen, M. J. et al. Benthic primary producers are key to sustain the Wadden Sea food web: stable carbon isotope analysis at landscape scale. Ecology 98, 1498–1512, https://doi.org/10.1002/ecy.1837 (2017).
Klunder, L. et al. Distribution of the dwarf surf clam Mulinia lateralis (Say, 1822) in the Wadden Sea after first introduction. BioInvasions Record 8, https://doi.org/10.3391/bir.2019.8.4.10 (2019).
Colina Alonso, A., van Maren, D. S., Elias, E. P. L., Holthuijsen, S. J. & Wang, Z. B. The contribution of sand and mud to infilling of tidal basins in response to a closure dam. Mar. Geol. 439, 106544, https://doi.org/10.1016/j.margeo.2021.106544 (2021).
Colina Alonso, A. et al. The existence and origin of multiple equilibria in sand-mud sediment beds. Geophys. Res. Lett. 49, e2022GL101141, https://doi.org/10.1029/2022GL101141 (2022).
Colina Alonso, A. et al. A mud budget of the Wadden Sea and its implications for sediment management. Commun. Earth Environ. 5, 153, https://doi.org/10.1038/s43247-024-01315-9 (2024).
Folmer, E. O. et al. Space–time analyses of sediment composition reveals synchronized dynamics at all intertidal flats in the Dutch Wadden Sea. Estuar. Coast. Shelf. S. 285, 108308, https://doi.org/10.1016/j.ecss.2023.108308 (2023).
Bijleveld, A. I. et al. Understanding spatial distributions: negative density-dependence in prey causes predators to trade-off prey quantity with quality. Proc. R. Soc. B 283, 20151557, https://doi.org/10.1098/rspb.2015.1557 (2016).
Bakker, W. et al. Connecting foraging and roosting areas reveals how food stocks explain shorebird numbers. Estuar. Coast. Shelf. S. 259, 107458, https://doi.org/10.1016/j.ecss.2021.107458 (2021).
Penning, E., Verkuil, Y. I., Klunder, L. & Reneerkens, J. Sanderlings feed on a diverse spectrum of prey worldwide but primarily rely on brown shrimp in the wadden sea. Ardea 110, 187–199, https://doi.org/10.5253/arde.2022.a11 (2022).
Jacobs, P., Kromkamp, J. C., van Leeuwen, S. M. & Philippart, C. J. M. Planktonic primary production in the western Dutch Wadden Sea. Mar. Ecol. Prog. Ser. 639, 53–71, https://doi.org/10.1016/0077-7579(88)90051-8 (2020).
Bijleveld, A. I. et al. WATLAS: high-throughput and real-time tracking of many small birds in the Dutch Wadden Sea. Anim. Biotelemetry 10, 36, https://doi.org/10.1186/s40317-022-00307-w (2022).
Duijns, S. et al. Monitoring van het voor vogels oogstbare voedselaanbod in de kombergingen van het Pinkegat en Zoutkamperlaag Rapportage t/m monitoringjaar 2023. (Sovon report 2024/16, Sovon Vogelonderzoek Nederland, Nijmegen, 2024).
van de Pol, M. et al. Gaswinning onder Waddenzee heeft ecologische impact. De Levende Natuur (2025).
Baptist, M. J., Van Der Wal, J. T., Folmer, E. O., Gräwe, U. & Elschot, K. An ecotope map of the trilateral Wadden Sea. J. Sea Res. 152, 101761, https://doi.org/10.1016/j.seares.2019.05.003 (2019).
Drent, J. et al. Macrozoobenthos. (Common Wadden Sea Secretariat, Wilhelmshaven, Germany, 2017).
Herman, P. M. J. et al. Statistical analysis of effects of MSC Zoe incident on populations of protected species in Wadden Sea and North Sea. 165 (Deltares, 2021).
Bijleveld, A. I. et al. Designing a benthic monitoring programme with multiple conflicting objectives. Methods Ecol. Evol. 3, 526–536, https://doi.org/10.1111/j.2041-210X.2012.00192.x (2012).
van der Meer, J. Sampling design of monitoring programmes for marine benthos: a comparison between the use of fixed versus randomly selected stations. J. Sea Res. 37, 167–179, https://doi.org/10.1016/S1385-1101(97)00007-5 (1997).
Elias, E., Van der Spek, A., Wang, Z. B. & De Ronde, J. Morphodynamic development and sediment budget of the Dutch Wadden Sea over the last century. Neth. J. Geosci. 91, 293–310, https://doi.org/10.1017/S0016774600000457 (2012).
Hartmann-Schröder, G., Dahl, F. & Schumann, H. Annelida, Borstenwürmer, Polychaeta. (Fischer, 1996).
Lincoln, R. J. British marine Amphipoda: Gammaridea. (British Museum (Natural History), 1979).
de Bruyne, R. & de Boer, T. W. Schelpen van de Waddeneilanden: Overzicht van de mariene autochtone weekdieren (Mollusca) en aangespoelde schelpen van de Nederlandse Waddeneilanden Texel, Vlieland, Terschelling, Ameland en Schiermonnikoog (plus incidentele vondsten elders uit het Nederlandse waddengebied). (‘s-Graveland Fontaine Uitgevers, 2008).
Bijleveld, A. I., Tacoma, M. & Koolhaas, A. SIBES dataset https://doi.org/10.25850/nioz/7b.b.ug (2024).
Piersma, T. et al. Scale and intensity of intertidal habitat use by knots Calidris canutus in the Western Wadden Sea in relation to food, friends and foes. Neth. J. Sea Res. 31, 331–357, https://doi.org/10.1016/0077-7579(93)90052-T (1993).
Zwarts, L., Blomert, A.-M. & Wanink, J. H. Annual and seasonal variation in the food supply harvestable by knot Calidris canutus staging in the Wadden Sea in late summer. Mar. Ecol. Prog. Ser., 129-139 (1992).
van der Meer, J. A comparison between the SIBES and the Beukema/Dekker benthos sampling program at Balgzand. (NIOZ Royal Netherlands Institute for Sea Research, Report Version 20150924, 2015).
Acknowledgements
Special thanks to Jaap van der Meer and Henk van der Veer who have been instrumental for SIBES in the past. We are also grateful to all current and former employees and the many volunteers and students who have ensured that the SIBES program has continued. The RV Navicula was, and the RV Wim Wolff will be, essential for collecting the samples, and particularly we thank the crew Wim Jan Boon, Klaas Jan Daalder, Bram Fey, Hendrik Jan Lokhorst, Eduard Porringa and Hein de Vries. Funding from the Dutch Research Council (NWO), in combination with funding from NAM (the Dutch petroleum company) as well as NIOZ, enabled SIBES to begin as the first-ever, spatially extensive monitoring program of the Dutch Wadden Sea. Funding from the Dutch Research council lasted until 2015. From 2019 until present, Rijkswaterstaat (the Dutch water board) also funds SIBES. Currently, the funding is guaranteed until 2025 and is shared between NAM, Rijkswaterstaat, and Royal NIOZ.
Author information
Authors and Affiliations
Contributions
The author contributions are provided following CRediT (Contributor Roles Taxonomy). Allert I. Bijleveld: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Paula de la Barra: Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. Hailley Danielson-Owczynsky: Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. Livia Brunner: Investigation, Writing – review & editing. Anne Dekinga: Investigation, Methodology, Project administration, Supervision, Writing – review & editing. Sander Holthuijsen: Data curation, Methodology, Investigation, Project administration, Supervision, Writing – review & editing. Job ten Horn: Data curation, Investigation, Writing – review & editing. Anne de Jong: Investigation, Writing – review & editing. Loran Kleine Schaars: Data curation, Investigation, Project administration, Supervision, Writing – review & editing. Adrienne Kooij: Investigation, Writing – review & editing. Anita Koolhaas: Data curation, Methodology, Project administration, Software, Validation, Writing – review & editing. Hidde Kressin: Investigation, Writing – review & editing. Felianne van Leersum: Investigation, Writing – review & editing. Simone Miguel: Investigation, Writing – review & editing. Luc G. G. de Monte: Investigation, Writing – review & editing. Dennis Mosk: Investigation, Project administration, Supervision, Validation, Writing – review & editing. Amin Niamir: Investigation, Software, Writing – review & editing. Dorien Oude Luttikhuis: Investigation, Writing – review & editing. Myron A. Peck: Funding acquisition, Supervision, Writing – review & editing. Theunis Piersma: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. Reyhaneh Roohi: Investigation, Writing – review & editing. Léon Serre-Fredj: Software, Writing – review & editing. Marten Tacoma: Data curation, Software, Validation, Writing – review & editing. Evaline van Weerlee: Investigation, Writing – review & editing. Bas de Wit: Investigation, Writing – review & editing. Roeland A. Bom: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bijleveld, A.I., de la Barra, P., Danielson-Owczynsky, H. et al. SIBES: Long-term and large-scale monitoring of intertidal macrozoobenthos and sediment in the Dutch Wadden Sea. Sci Data 12, 239 (2025). https://doi.org/10.1038/s41597-025-04540-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41597-025-04540-9