Abstract
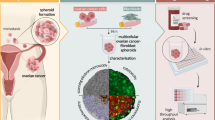
Spheroids are widely used in oncology for testing drugs, but models composed of a single cell line do not fully capture the complexity of the in vivo tumours targeted by chemotherapy. Developing 3D in vitro models that better mimic tumour architecture is a crucial step for the scientific community. To enable more reliable drug testing, we generated multiculture spheroids and analysed cell morphology and distribution over time. This dataset is the first publicly available single-cell light-sheet fluorescence microscopy image collection of 3D multiculture tumour models comprising of three different cell lines analysed at different time points. Specifically, we created models composed of one cancer cell line (melanoma, breast cancer, or osteosarcoma) alongside two stromal cell lines (fibroblasts and endothelial cells). Then, we acquired single-cell resolution light-sheet fluorescence 3D images of the spheroids to analyse spheroid morphology after 24, 48, and 96 hours. The image collection, whole spheroid annotations, and extracted features are publicly available for further research and can support the development of automated analysis models.
Similar content being viewed by others
Background & Summary
Three-dimensional (3D) cell cultures are of increasing interest in research as they provide an intermediate model system between 2D cultures and animal models1. They overcome the limitations of 2D cell cultures, such as (i) the lack of natural structures or cell-to-cell and cell-matrix interactions of native tissues, (ii) the unrestricted access to oxygen, nutrients, metabolites, and signal molecules, and (iii) altered cell morphology, polarity and functions, e.g., differentiation, proliferation, vitality, gene expression patterns and responsiveness to stimuli2. As 3D cultures closely resemble native tissues, they have become an essential model system in a wide range of research areas, including regenerative medicine3 and cancer research4.
In particular, the so-called multiculture 3D tumour spheroids mimic solid tumours in many aspects, such as the heterogeneous architecture, growth kinetics, physical interactions or complex communication5. Unlike 2D mono-cultures, they also represent the tumour microenvironment (TME), which is an active participant of all stages of cancer development6,7. However, today, even the classic 3D single-culture spheroids seem too simple for reliable high-content screening (HCS) analysis for predicting in vivo effects of chemotherapy treatments8. Current technology allows co-culturing tumour cells with healthy stromal cells, e.g., fibroblasts, mesenchymal stem cells, endothelial cells or immune cells, for obtaining advanced but reproducible preclinical tumour models for a wide variety of uses, ranging from basic research for the development of therapeutic approaches to advanced HCSs of chemotherapy drugs9,10. In this scenario, 3D multiculture spheroids seem to offer the current best trade-off for drug HCS, providing advantages over both classical 3D single-culture spheroids6,7 and the more complex yet less reproducible patient-derived tumour organoids11. While organoids possess self-renewal and self-organization capacities recapitulating the original tumour characteristics, their clinical application is hampered by technical difficulties11,12.
Once the model has been defined, comprehending the complex nature of the tumour microenvironment is essential in order to gain an in-depth knowledge of cancer biology at a single-cell level13. Current high-resolution imaging systems can be used to understand cell-cell interactions within spheroids. For instance, light-sheet fluorescence microscopes (LSFMs) provide better imaging depth, faster imaging, less phototoxicity, and less photobleaching compared to wide-field or confocal microscopes. Furthermore, the optical sectioning results in less light-scattering and provides high-resolution plane-by-plane imaging, allowing the visualisation of single cells even in a dense 3D microenvironment14.
In this work, we created 3D tumour multiculture spheroids composed of three different fluorescently labelled cell lines analysed at different time points. In particular, we created three different tumour models composed of one cancer cell line (i.e., melanoma, breast cancer, or osteosarcoma), labelled with CellTracker (CT) Orange CMTMR dye, and two stromal cell lines, i.e., fibroblasts and endothelial cells, stained with CT DeepRed and CT Green CMFDA, respectively. To mimic chemotherapeutic stress, we applied low-dose doxorubicin (dox), which is a widely used antineoplastic drug in a myriad of malignancies15. Then, using the Leica True Confocal Scanning (TCS) SP8 Digital LightSheet (DLS) microscope, we acquired single-cell light-sheet fluorescence 3D images of the generated spheroids to analyse the cellular distribution after 24, 48, and 96 hours. While tracking structural changes in spheroids over an extended period can be informative, the high level of cell-to-cell communication led to the exchange of cytoplasmic dyes between different cell types, making it challenging to distinguish them. Finally, an expert microscope operator performed the segmentation of the entire spheroids and extracted morphological features using publicly available tools, i.e. AnaSP16,17 and ReViSP18.
The acquired LSFM 3D images of multiculture spheroids, the obtained segmentation masks and the extracted morphological features are freely available at figshare19. They can be used for further biological investigations on the cell distribution in 3D environments, for instance to investigate the position of the cancer cells over time when co-cultured with healthy stromal cells20. In addition, the 3D data can be exploited by the research community to compare various computational metrics quantitatively to assess image quality21, or to generate training sets for deep learning and machine learning techniques22, as well as to validate other segmentation approaches23.
Methods
Cell lines description
In this work, we used five different commercial human cell lines obtained from the American Type Culture Collection (ATCC, Manassas, Virginia, USA); three cancer cell lines, i.e. T-47D ductal carcinoma (ATCC, HTB-133, Lot: 63542749), A375 melanoma (ATCC, CRL-1619, Lot: 63905420), MG-63 osteosarcoma (ATCC, CRL1427, Lot: 70054704), and two stromal cell lines, i.e. MRC-5 fibroblasts (ATCC, CCL-171, Lot: 63405646), and EA.hy926 endothelial cells (ATCC, CRL-2922, Lot: 70030244). They were maintained following the corresponding ATCC guidelines.
Spheroid generation and staining
Multiculture tumour spheroids were generated by co-culturing three cell lines, specifically MRC-5, EA.hy926, and one type of tumour cell, either T-47D, A375, or MG-63. They were co-cultured in 384-well ultra-low attachment U-bottom plates (Greiner Bio-One, Kremsmünster, Austria), in DMEM supplemented with 10% Opticlone FBS (EuroClone, Milan, Italy), 2 mM L-glutamine and 1% Penicillin-Streptomycin-Amphotericin B mixture (all from Lonza, Basel, Switzerland). Before seeding, cells were stained with CellTracker dyes based on the manufacturer’s instructions (Invitrogen, Thermo Fisher Scientific, Waltham, Massachusetts, USA). Precisely, MRC-5, EA.hy926, and the tumour cells were stained with 1 µM Deep Red, 25 µM Green CMFDA, and 25 µM Orange CMTMR dyes, respectively. Then, the cells were mixed to generate tri-culture spheroids; we used 40% MRC-5, 40% EA.hy926, and 20% tumour cells. Spheroids were incubated for 24, 48, or 96 hours at 37 °C and 5% CO2 in the control medium or 0.6 µM dox-containing medium. Before live-cell imaging nuclei were stained with 1.5 µM Hoechst 33342 in DMEM for 60 min.
Image acquisition
The imaging setup and all imaging parameters were previously described in Diosdi et al.24,25. Briefly, for each spheroid, fluorescent 16-bit images were produced using a Leica TCS SP8 DLS microscope with a sCMOS DFC9000 camera (Leica Microsystems, Wetzlar, Germany). The images were acquired at three different time points (i.e., 24, 48, and 96 hours after seeding) using a 200 ms exposure time and a 25x/0.95 detection objective with a 2.5 mm mirror device on the objective. The laser intensity was adjusted for each channel at 405, 488, 552, and 638 nm (maximum laser intensity 350 mW). For imaging, DPBS mounting medium was used for every spheroid. The resolution of the images is 2048 × 2048 pixels, with a pixel size of 0.14370117 µm and a distance of 3.7 µm between each image in z-stack. The Leica Application Suite X (LAS X) software produced Maximum Intensity Projection (MIP) 8-bit images with a pixel resolution of 1683 × 1683 and a pixel size of 0.17486631 µm.
Image analysis
Morphological features have been extracted by analysing the MIP of each spheroid reported in the collection using AnaSP version 3.017. Briefly, MIP images were obtained by merging all the fluorescent channels and these were manually segmented by an expert microscope operator for obtaining binary masks, with the foreground in white over a black background. Several morphological features (e.g., Diameter, Perimeter, Area, Circularity, and Sphericity) have been extracted, including the Volume, which was reconstructed using ReViSP. The mathematical equations describing the features are available in the AnaSP’s user manual and Supplementary File 1.
Data Records
The dataset is available at figshare19, with this section being the primary source of information on the availability and content of the data being described. The collection includes images of spheroids acquired at three time points from the generation of the multiculture 3D tumour models. The images are single-cell light-sheet fluorescence 3D data saved in a multi-tiff file format. Each spheroid was stained with the same four fluorescent dyes. For each staining, a 3D stack is provided for each spheroid. Figure 1 reports the MIP for the four channels (Nuclei - ch001, EA.hy926 - ch002, tumour - ch003, and MRC-5 - ch004) available in the collection for a random spheroid shown as an example. All spheroids are acquired from top to down and each layer of the tiff file corresponds to a different z-section. The number of layers ranges from 38 to 90 sections, and the z-step between subsequent layers is the same for all the spheroids. In a separate folder MIP images are also provided for each spheroid and all of the four channels and a merged one.
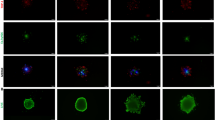
Representative Maximum Intensity Projection (MIP) images of a 3D tumour multiculture spheroid of the A375 cell line imaged 24 hours after seeding. Four channels were acquired for visualisation, precisely Blue - Nucleus (Hoechst 33342), White - MRC-5 (Deep Red), Green - EA.hy926 (Green CMFDA), and Red – A375 (Orange CMTMR). The scale bar represents 50 µm for all the images. Images were taken and visualised using Leica LAS X software.
For each tumour cell line and time point, images of 5 spheroids have been acquired for both the control and the drug-treated condition. The three different tumour types were labelled as A - T-47D, B - A375, and C - MG-63, and dox-treated samples were differentiated with “+” symbol. In total, 90 spheroids are included in the collection according to the scheme reported in Table 1. All the data are freely available at figshare19. The uploaded files follow the naming convention: “TumourType_ + _TimePoint_SpheroidID_channelID.tif” where (+) labels doxorubicin treated samples. Figure 2 shows a random spheroid for each condition obtained using the different fluorescence channels, excluding the nuclei signal for a better understanding of the cell distribution.
Representative Maximum Intensity Projection (MIP) images of the 3D tumour multiculture spheroid dataset. The three different tumour types were labelled as A - T-47D, B - A375, and C - MG-63, and dox- treated samples were differentiated with “+” symbol. For visualisation, three channels were merged, precisely White - MRC-5 (Deep Red), Green - EA.hy926 (Green CMFDA), and Red - Tumour (Orange CMTMR dyes). The scale bar represents 50 µm. The images were taken and visualised using Leica LAS X software.
For each spheroid included in the collection, several morphological features have been extracted using merged MIP images. The MIP images and related binary masks are freely available at figshare19. For each spheroid analysed, Supplementary Table 1 reports values for selected features, Also, a table reporting all the values for the different extracted features is available in a .csv and .xls format at figshare19. The average values are reported in Table 2. The single values are reported in µm in the case of Diameter and Perimeter, in µm2 for Area, in µm3 for Volume, and dimensionless (i.e. [−]) for Circularity and Sphericity.
Technical Validation
All images were collected as part of routine wet-lab analyses using a commercial microscope. Accordingly, quality assurance was performed just with daily calibrations done on the system. The raw images were uploaded without editing to figshare. Two expert microscopists carefully checked the uploaded data in blind.
References
Jensen, C. & Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Frontiers in molecular biosciences. 7, 33, https://doi.org/10.3389/fmolb.2020.00033 (2020).
Kapałczyńska, M. et al. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci. 14(4), 910–919, https://doi.org/10.5114/aoms.2016.63743 (2018).
Kim, S. J., Kim, E. M., Yamamoto, M., Park, H. & Shin, H. Engineering multi‐cellular spheroids for tissue engineering and regenerative medicine. Advanced Healthcare Materials. 9(23), 2000608, https://doi.org/10.1002/adhm.202000608 (2020).
Peirsman, A. et al. MISpheroID: a knowledgebase and transparency tool for minimum information in spheroid identity. Nature methods. 18(11), 1294–1303, https://doi.org/10.1038/s41592-021-01291-4 (2021).
Zanoni, M. et al. 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Scientific reports. 6(1), 19103, https://doi.org/10.1038/srep19103 (2016).
Senrung, A. et al. 3D tumor spheroids: morphological alterations a yardstick to anti-cancer drug response. In vitro models. 2, 219–248, https://doi.org/10.1007/s44164-023-00059-8 (2023).
Valente, R. et al. Doxorubicin-sensitive and -resistant colorectal cancer spheroid models: assessing tumor microenvironment features for therapeutic modulation. Front Cell Dev Biol. 11, 1310397, https://doi.org/10.3389/fcell.2023.1310397 (2023).
Lampart, F. L., Iber, D. & Doumpas, N. Organoids in high-throughput and high-content screenings. Frontiers in Chemical Engineering. 5, 1120348, https://doi.org/10.3389/fceng.2023.1120348 (2023).
Han, S. J., Kwon, S. & Kim, K. S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 21, 152, https://doi.org/10.1186/s12935-021-01853-8 (2021).
Manduca, N., Maccafeo, E., De Maria, R., Sistigu, A. & Musella, M. 3D cancer models: One step closer to in vitro human studies. Front Immunol. 11(14), 1175503, https://doi.org/10.3389/fimmu.2023.1175503 (2023).
Sakalem, M. E., De Sibio, M. T., da Costa, F. A. D. S. & de Oliveira, M. Historical evolution of spheroids and organoids, and possibilities of use in life sciences and medicine. Biotechnology Journal. 16(5), 2000463, https://doi.org/10.1002/biot.202000463 (2021).
Yang, Y. et al. Advances and Applications of Cancer Organoids in Drug Screening and Personalized Medicine. Stem Cell Rev and Rep 20, 1213–1226, https://doi.org/10.1007/s12015-024-10714-6 (2024).
Janesick, A. et al. High resolution mapping of the tumor microenvironment using integrated single-cell, spatial and in situ analysis. Nature Commun. 14(1), 8353, https://doi.org/10.1038/s41467-023-43458-x (2023).
Shi, Y. et al. Smart lattice light-sheet microscopy for imaging rare and complex cellular events. Nature Methods. 21, 301–310, https://doi.org/10.1038/s41592-023-02126-0 (2024).
Sritharan, S. & Sivalingam, N. A comprehensive review on time-tested anticancer drug doxorubicin. Life sciences 278, 119527, https://doi.org/10.1016/j.lfs.2021.119527 (2021).
Piccinini, F. AnaSP: a software suite for automatic image analysis of multicellular spheroids. Computer Methods and Programs in Biomedicine. 119(1), 43–52, https://doi.org/10.1016/j.cmpb.2015.02.006 (2015).
Stellato, M. et al. Radiomic analysis of 3D spheroids using 2D brightfield images. Biomedical Signal Processing and Control 103, 107366, https://doi.org/10.1016/j.bspc.2024.107366 (2025).
Piccinini, F., Tesei, A., Arienti, C. & Bevilacqua, A. Cancer multicellular spheroids: Volume assessment from a single 2D projection. Computer Methods and Programs in Biomedicine. 118(2), 95–106, https://doi.org/10.1016/j.cmpb.2014.12.003 (2015).
Piccinini, F. & Diosdi, A. 2024_Diosdi_ tumour-stroma_spheroid_multicultures_dataset. figshare https://doi.org/10.6084/m9.figshare.c.7357135 (2024).
Shishido, A. et al. Mesothelial cells facilitate cancer stem-like properties in spheroids of ovarian cancer cells. Oncology reports. 40(4), 2105–2114, https://doi.org/10.3892/or.2018.6605 (2018).
Diosdi, A. et al. A quantitative metric for the comparative evaluation of optical clearing protocols for 3D multicellular spheroids. Computational and Structural Biotechnology Journal. 19, 1233–1243, https://doi.org/10.1016/j.csbj.2021.01.040 (2021).
Mukashyaka, P. et al. High-throughput deconvolution of 3D organoid dynamics at cellular resolution for cancer pharmacology with Cellos. Nature Commun. 14(1), 8406, https://doi.org/10.1038/s41467-023-44162-6 (2023).
Hollandi, R. et al. Nucleus segmentation: towards automated solutions. Trends in Cell Biology. 32(4), 295–310, https://doi.org/10.1016/j.tcb.2021.12.004 (2022).
Diosdi, A. et al. HCS-3DX, a next-generation AI-driven automated 3D high-content screening system. BioRxiv. 2024.07.15.603536 https://doi.org/10.1101/2024.07.15.603536 (2024).
Diosdi, A. et al. Cell lines and clearing approaches: a single-cell level 3D light-sheet fluorescence microscopy dataset of multicellular spheroids. Data in Brief. 36, 107090, https://doi.org/10.1016/j.dib.2021.107090 (2021).
Acknowledgements
We acknowledge support from the HUNRENTECH (TECH-2024-34), Lendület BIOMAG grant (no. 2018–342), TKP2021-EGA09, Horizon-BIALYMPH, Horizon-SYMMETRY, Horizon-SWEEPICS, H2020-Fair-CHARM, CZI Deep Visual Proteomics, HAS-NAP3, the ELKH-Excellence grant from OTKA-SNN no. 139455/ARRS, the FIMM High Content Imaging and Analysis Unit (FIMM-HCA; HiLIFE-HELMI), and Finnish Cancer Society. FP and GC acknowledge support from the MAECI Science and Technology Cooperation Italy-South Korea Grant Years 2023–2025 by the Italian Ministry of Foreign Affairs and International Cooperation (CUP project: J53C23000300003); MH acknowledges support from the ÚNKP-23-4-SZTE-639 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund and the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. TB acknowledges support from the EKÖP-24-3-SZTE-318. We would like to thank Lilla Pinter (HUN-REN BRC, Szeged, Hungary) for technical assistance in preparing the spheroids; Mariachiara Stellato (University of Bologna, Italy) for checking all the files uploaded to figshare.
Funding
Open access funding provided by HUN-REN Biological Research Centre, Szeged.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.H., A.D., G.D.; Data curation: M.H., A.D., G.C., F.P.; Formal analysis: M.H., A.D., F.P.; Funding acquisition: P.H., K.B., M.H.; Investigation: M.H., A.D., T.B.; Methodology: M.H., A.D., G.D.; Project administration: M.H., A.D., F.P.; Resources: P.H., K.B.; Software: A.D.; Supervision: P.H., K.B.; Validation: M.H.; Visualization: G.C., F.P.; Writing - original draft: M.H., A.D., F.P.; Writing - review & editing: G.C., P.H., K.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Diosdi, A., Piccinini, F., Boroczky, T. et al. Single-cell light-sheet fluorescence 3D images of tumour-stroma spheroid multicultures. Sci Data 12, 492 (2025). https://doi.org/10.1038/s41597-025-04832-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41597-025-04832-0
This article is cited by
-
HCS-3DX, a next-generation AI-driven automated 3D-oid high-content screening system
Nature Communications (2025)