Abstract
Diffusion magnetic resonance imaging (dMRI) provides critical insights into the microstructural and connectional organization of the human brain. However, the availability of high-field, open-access datasets that include raw k-space data for advanced research remains limited. To address this gap, we introduce Diff5T, a first comprehensive 5.0 Tesla diffusion MRI dataset focusing on the human brain. This dataset includes raw k-space data and reconstructed diffusion images, acquired using a variety of imaging protocols. Diff5T is designed to support the development and benchmarking of innovative methods in artifact correction, image reconstruction, image preprocessing, diffusion modelling and tractography. The dataset features a wide range of diffusion parameters, including multiple b-values and gradient directions, allowing extensive research applications in studying human brain microstructure and connectivity. With its emphasis on open accessibility and detailed benchmarks, Diff5T serves as a valuable resource for advancing human brain mapping research using diffusion MRI, fostering reproducibility, and enabling collaboration across the neuroscience and medical imaging communities.
Similar content being viewed by others
Background & Summary
The exceptional soft tissue contrast and versatility of magnetic resonance imaging (MRI) render it an indispensable diagnostic modality for various disorders, including neurological, musculoskeletal, and oncological diseases1. Diffusion MRI (dMRI)2 is a unique and valuable modality for mapping brain tissue, microstructure and connectivity patterns from diffusion characteristics of endogenous water molecules.
Over the past few decades, the rapid advancement, availability, and diversity of large, open-source diffusion MRI datasets have significantly propelled human brain research. Notable datasets, such as the Human Connectome Project (HCP)3,4,5,6,7,8,9,10,11, the Cambridge Centre for Aging and Neuroscience (Cam-CAN)12, the UK Biobank13,14, the Adolescent Brain Cognitive Development (ABCD) Study7, the Amsterdam Ultra-high Field Adult Lifespan Database (AHEAD)15,16, and the Amsterdam Open MRI Collection (AOMIC)17, have provided critical resources for analyzing healthy and diseased human brain anatomical and connectional patterns in large scale. These datasets have enabled researchers to investigate white matter connectivity across the entire brain.
Despite these advancements, current open-access dMRI datasets were primarily acquired on 3.0 T or 7.0 T MRI systems, with their own. Especially, 3.0 T dMRI, while offering shorter acquisition times, is constrained by lower spatial resolution and signal-to-noise ratio (SNR). Conversely, 7.0 T dMRI has the potential to achieve superior spatial resolution and SNR but faces significant challenges, such as rapid transverse magnetization decay, increased B0 and B1 + inhomogeneity, and higher specific absorption rates (SAR)3,18,19,20,21. Therefore, it is important to investigate new ways for a better balance between spatial resolution, SNR, and acquisition time in multi-shell dMRI22.
5.0 T MRI systems are promising for bridging the gap between these two extremes. This intermediate field strength has already demonstrated application value in abdominal dMRI22,23, cerebral vascular TOF-MRA24 and cardiac imaging25,26,27. A 5.0 T MRI system is expected to offer higher spatial resolution and SNR than 3.0 T while reducing image artifacts compared to 7.0T21,28. The Diff5T dataset was created to provide the research community with a benchmark dataset supporting advancements in dMRI technology and applications.
In addition to images, Diff5T uniquely includes publicly accessible raw k-space data, allowing researchers to explore advanced reconstruction techniques. The complexity of the biophysical models used in dMRI often dictates the number of diffusion-weighted images (DWIs) required. Diffusion tensor imaging (DTI)29,30,31 requires at least six DWIs and one non-diffusion-weighted image. To accurately estimate fiber crossings and the properties of intra- and extra-cellular compartments, advanced methods32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47 typically require more DWIs. While this increases data volume, it presents an exciting opportunity to represent richer microstructural information. Efforts are ongoing to streamline acquisition and reconstruction processes.
Artificial intelligence (AI) empowered methods have emerged as powerful tools for accelerating acquisition48 and reconstruction49,50,51,52,53, as well as multi-parametric estimation54,55. AI-based joint reconstruction techniques leverage correlations across k-space, spatial-space, and q-space to allow for sparser sampling while compensating for missing data56,57,58. However, most of these methods rely on simulated k-space data synthesized from already-reconstructed magnitude images, rather than acquired k-space data. The use of raw k-space dMRI data is vital for training more robust and accurate AI models.
In this paper, we present Diff5T dataset, the first 5.0 T brain imaging dataset containing both k-space and image-space data. This dataset includes high-resolution T1-weighted (T1w) and T2-weighted (T2w) imaging, along with multi-shell, multi-direction, and multi-channel dMRI data from 50 subjects. Additionally, we provide fully documented reconstruction and preprocessing pipelines to support reproducible and further research. Diff5T represents a significant step forward in addressing the challenges of high-resolution dMRI, offering a critical resource for the imaging and neuroscientific communities.
Methods
Participants
Ethical approval for the study was obtained from the Institutional Review Board/Ethics Committee of the Shenzhen Institutes of Advanced Technology (Approval No. SIAT-IRB-230715-H0659). Written informed consent was obtained from all participants prior to data collection, in accordance with relevant guidelines and regulations. All participant records were de-identified before analysis and were reviewed by the institutional review boards to guarantee no potential risk to participants.
As part of the written consent process, all data were made publicly accessible. Participants were thoroughly informed about the significance of the study and provided written consent for the future public release of their data. The inclusion criteria for participation were as follows: (1) healthy adults with no history or diagnosis of brain disease, and (2) availability for an MRI examination encompassing all required imaging sequences.
A total of 50 healthy volunteers, aged 18–38 years, were recruited for the study, each providing written informed consent in accordance with ethical guidelines. These data have been subjected to quality control by data collectors and physicians with more than 20 years of extensive clinical experience.
Data acquisition
Hardware
Data were acquired using a 5.0 T MRI scanner (uMR Jupiter, United Imaging Healthcare, UIH, China) equipped with a maximum gradient strength of 120 mT/m and a slew rate of 200 T/m/s. Imaging was performed with a quadrature birdcage transmit coil and a 48-channel receiver coil (48/2 channel Rx/Tx head coil)28. To maintain consistency across imaging sessions, field shim settings were standardized to reduce image distortion differences. During the scans, three sponge pads were placed around the participants’ heads to minimize motion artifacts and ensure stability throughout the imaging session. The movement of participants was also recorded during the scans.
Protocols
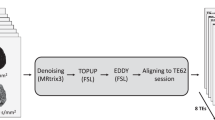
A standard scan session order is as follows: localization, three dMRI series, T1w and T2w series, as shown in Fig. 1.
Diff5T data processing pipelines. (a) The hardware and acquisition methods used to acquire the data. The Jupiter 5.0 T scanner, the 48-channel head coil, the EPI acquisition technique and the acquisition protocols. (b) The reconstruction pipeline. The dMRI data were pre-whitened and corrected for Nyquist ghosts, then reconstructed using slice-GRAPPA, GRAPPA, and partial Fourier reconstruction methods. The structural images were reconstructed on the scanner. (c) dMRI preprocessing pipeline. The preprocessed dMRI data were obtained following Gibbs ringing removal, susceptibility- and eddy current-induced distortion correction, bias field correction, and final registration to resampled T1-weighted image. (d) dMRI downstream tasks. Fitting the dMRI data to DTI and NODDI models and performing tractography.
dMRI data
To achieve 1.2-mm isotropic spatial resolution dMRI data with a balanced trade-off between SNR efficiency and scan time, the echo planar imaging (EPI) sequence was used in dMRI acquisition with the following parameters: TR/TE = 8277/67.9 ms; Echo spacing = 0.8 ms; Flip angle = 90°; In-plane FOV (RO × PE) = 212 × 212 mm2; Number of slices = 114; Bandwidth = 1850 Hz/Pixel; Multi-band acceleration factor = 2; In-plane acceleration factor = 2; Partial Fourier factor = 6/8; Acquisition matrix (RO × PE) = 176 × 176; Total acquisition time = 45.2 minutes. The dMRI images were obtained in the axial orientation.
To achieve high angular resolution, 291 volumes of dMRI data were acquired in a standard scan session, consisting of 90 DWIs at b = 1000 s/mm2, 90 DWIs at b = 2000 s/mm2 and 90 DWIs at b = 3000 s/mm2. The phase encoding was along posterior-to-anterior (PA) direction. A total of 21 b = 0 image volumes were acquired, with 6 of them acquired along anterior-to-posterior (AP) phase encoding direction and 15 of them acquired along PA phase encoding direction.
The uniform multi-shell diffusion gradient vectors were optimized by the spherical code method59 in DMRITool, which directly maximize the minimal separation angles between different diffusion samples from both within and across shells. More detailed imaging parameters are listed in Table 1.
Structural data
Two high resolution structural series (i.e., T1w and T2w) were also acquired following the dMRI series in each session.
The 0.5-mm isotropic T1w images were acquired with the Fast Spoiled Gradient Echo (GRE-FSP) sequence with the following parameters: TR/TE = 10.1/3.4 ms; TI = 1000 ms; ETL = 165; Flip angle = 9°; FOV = 256 × 256 × 150 mm3; Bandwidth = 160 Hz/Pixel; uCS (United Imaging Compressed Sensing) combined acceleration factor = 3; Total acquisition time = 13.6 minutes. The T1w images were obtained in the sagittal orientation.
The 0.5-mm isotropic T2w images were acquired with the 3D fast spin echo (FSE) using MATRIX (Modulated flip Angle Technique in Refocused Imaging with eXtended echo train) sequence, with the following parameters: TR/TE = 3000/421.68 ms; ETL = 200; Flip angle mode = T2; Flip angle (max/min) = 160/23°; Reference tissue T1/T2 = 1800/96 ms; FOV = 256 × 212 × 150 mm3; Bandwidth = 450 Hz/Pixel; uCS combined acceleration factor = 3; Total acquisition time = 13.7 minutes. The T2w images were obtained in the sagittal orientation.
Data reconstruction
The general pipeline to produce the Diff5T dataset is illustrated in Fig. 1.
The online reconstructed DICOM images were converted to compressed NIfTI format using dcm2niix60 tool. The online reconstructed data are hereafter referred to as ‘DICOM-to-NIfTI’ data, as shown in Fig. 2.
The offline reconstructed NIfTI images retain the same basic information as the DICOM-to-NIfTI images, such as the affine transformation matrix. The offline reconstructed data are hereafter referred to as ‘reconstructed’ data, as shown in Fig. 2. The details of offline reconstruction are as follows:
dMRI data
The raw files (.raw) were directly exported from the 5T scanner. Using the offline UIH toolbox (UIHRawdata2ISMRMRD) written in MATLAB (MathWorks, Natick, MA, USA), the k-space data were extracted from the raw files and saved in binary format (.bin). This process ensured the removal of all personal identifiers, including subject name, birthdate, height, and weight. The following steps were used to perform offline reconstruction of dMRI k-space data in MATLAB.
-
Pre-whitening
Pre-scan noise data were used to pre-whiten the k-space data, which helped remove complex correlations and structures in its statistical properties. This process made the noise more predictable and easier to model61,62.
-
Ghost correction
Nyquist Ghost Corrected (NGC) or Ghost Elimination via Spatial and Temporal Encoding (GESTE)63 was used to suppress Nyquist ghosts by a fusion of reported temporal64 and spatial65 encoding methods. Generally, GeneRalized Partially Parallel Acquisitions (GRAPPA) methods estimate missing points in accelerated measurement data using kernels trained from auto-calibration signal (ACS) data. However, different sampling patterns and readout polarity shifts due to eddy currents cause kernel estimation errors, leading to ghosting artifacts66. Thus, these two methods were used to adjust the reference and accelerated k-space data to match the same pattern. Empirically, NGC was used to correct raw k-space data and muti-band ACS data, while GESTE was used to correct in-plane ACS data.
-
Inter-slice unaliasing
Slice-GRAPPA67 was used to disassemble the slice-aliased images and reconstruct multi-band accelerations in parallel imaging. Slice-GRAPPA has been shown to significantly reduce image artifacts in in-vivo acquisitions67.
-
In-plane unaliasing
1D GRAPPA68 was used to unaliased the in-plane data by synthesizing the missing data points directly in k-space. To estimate the full k-space data, Projection Onto Convex Set (POCS)69 was implemented for Partial Fourier reconstruction to recover the k-space data by utilizing the conjugate symmetry of k-space.
-
Muti-channel combination
To obtain the complex-valued images from multi-channel data, an adaptive combination method70 was implemented to combine the multi-channel images with lower noise floor than sum-of-squares method71.
-
PCA denoising
The eigen spectrum of random covariance matrices of dMRI data can be priorly described by a Marchenko-Pastur distribution72,73. Moeller et al.74,75 proposed the NOise Reduction with DIstribution Corrected (NORDIC) method as a noise reduction framework for diffusion MRI, which leverages low-rank modelling of gfactor-corrected complex dMRI reconstruction and non-asymptotic random matrix distributions to remove noise signal. Finally, the magnitude is taken as the reconstructed image.
After these steps, the reconstructed complex-valued k-space data and the reconstructed magnitude-valued dMRI images were obtained.
Structural data
The reconstruction of the T1w and T2w images were performed using vendor’s commercial UIH online reconstruction software (including vendor’s implementation of parallel imaging reconstruction, fast Fourier transformation, coil combination, root mean square combination of different echo images and calculation of field maps) and were exported as DICOM format from scanner.
Data preprocessing
The following preprocessing steps were applied to both the DICOM-to-NIfTI images and the reconstructed images.
dMRI data
The preprocessing pipeline for dMRI data is shown in Fig. 1. Three dMRI series were collected, each corresponding to a distinct q-space shell, with five b0 images included in each series. After preprocessing each series individually, all three were concatenated in their original scanning order as the final step. The preprocessed DICOM-to-NIfTI images are shown in Fig. 3a described in detail as below:
-
Degibbs
Gibbs ringing appears in MRI images as spurious oscillations around sharp tissue boundaries, resulting from the truncation of k-space sampling. Depending on the location of the sharp edge relative to the sampling grid, this can lead to attenuation of the Gibbs ringing artifact. The used method mrdegibbs in MRtrix3 toolbox (version 3.0.4)76 tries to estimate the subvoxel-shift in pixels necessary to minimize this distance and interpolates the image accordingly77.
-
Distortion correction
The susceptibility-induced and the eddy current-induced off-resonance field distortions were estimated and corrected using topup78,79 and eddy80,81,82 (GPU version eddy_cuda10.2) methods in FSL toolbox (version 6.0.7.14)79,83. The field maps were estimated from one b = 0 image pairs acquired with reversed PE blips using topup method. The topup outputs were used to perform the eddy method. The eddy method effectively corrected for movement of subject, eddy current-induced and susceptibility-induced distortion. The brain mask for eddy correction was generated from the mean b = 0 image volume after topup correction, using bet284 with a fractional intensity threshold of 0.12. To account for head motion, eddy adjusted the diffusion directions, leading to small variations for each participant.
-
Filed bias correction
The N4 bias field correction algorithm is a popular method for correcting low frequency intensity non-uniformity present in MRI image data. The bias field, caused by magnetic field inhomogeneity during acquisition, negatively impacts any intensity-based segmentation. Thus, the N4 bias field correction algorithm in ANTs was implemented by using MRtrix3 command dwibiascorrect ants76,85.
-
Registration to structural
Finally, the undistorted mean b0 image obtained from topup was registered to the T1w structural image using a boundary-based registration (BBR)86. The dMRI data (b ≠ 0) were then transformed according to structural volume space by the estimated displacement field.
Structural data
Refer to HCP minimal pipelines87, the 0.5-mm isotropic T1w and T2w volumes were aligned to the standard MNI space template (0.7-mm isotropic) with rigid body registration. Then, cerebral cortical surface reconstruction and volumetric segmentation were performed using the “recon-all” function of the FreeSurfer software (version v6.0.0)88,89,90. Finally, T2w were registered to T1w using BBR to ensure alignment across tissue boundaries. Facial features were obscured in each image volume by face masking method mideface in FreeSurfer software (version v7.4.1).
Microstructural modelling
To demonstrate the range of analyses possible with this comprehensive dataset, several diffusion models were applied to estimate the microstructural complexity of axons in vivo. These models, commonly used in clinical and neuroscience research, are supported by publicly available software and codes.
-
DTI
To estimate the fiber orientation, the diffusion tensor imaging (DTI)29,30,31 was fitted to the b = 1000 s/mm2 data using the dtifit method from FSL.
-
NODDI
The neurite orientation dispersion and density imaging (NODDI)42 parameters were estimated using Accelerated Microstructure Imaging via Convex Optimization (AMICO)91 method on the b = 1000, 2000, 3000 s/mm2 data.
-
MSMT-CSD
The multi-shell multi-tissue constrained spherical deconvolution (MSMT-CSD)92,93 was conducted on the b = 1000, 2000, 3000 s/mm2 data using the dwi2response dhollander method. Fiber Orientation Distribution (FOD) maps for white matter, grey matter, and cerebrospinal fluid, represented in the Spherical Harmonic (SH) basis, were obtained by dwi2fod msmt_csd. The fod2dec method was used to generate the FOD-based DEC, which was scaled according to the integral of the FOD. The above methods are implemented using the MRtrix3 tool.
-
Tractography
Before generating streamlines, we first used 5ttgen fsl and 5tt2gmwmi methods to segment the tissue to delineate the grey-white matter interface and place seed points along this boundary. This strategy keeps the streamlines within anatomically plausible paths and prevents them from terminating in unrealistic locations. Streamlines tractography was performed using the tckgen method with the probabilistic Second-order Integration over Fiber Orientation Distributions (iFOD2) algorithm, which integrates second-order FODs. The white matter FOD map, derived from dwi2fod, was used as the input to tckgen. The tissue-segmentation output generated by 5ttgen was provided as the input for the Anatomically Constrained Tractography (“-act” option). The “-step” was set to 0.6., while the “-minlength” and the “-maxlength” were set to 20 and 120 respectively. The desired number of streamlines “-select” was set to 10 million. To reduce the hard-ware burden during visualization, we used tckedit to extract a 200 k-streamline subset from 10 million streamlines. The above methods are implemented using the MRtrix3 tool.
Data Records
The dataset is publicly accessible via the https://doi.org/10.57760/sciencedb.2512294.
The four types of data records listed in this section are publicly available in Diff5T dataset. The k-space data are in binary MAT-format (.mat) and the image data are in compressed NIfTI format (.nii.gz). The overall folder hierarchy and organization of the dataset are described in detail and visualized in Fig. S1, which outlines the structure for anatomical, diffusion, and k-space data across all subjects.
Raw k-space data
This includes raw k-space data for each diffusion direction, as well as associated reference and navigator lines used for reconstruction and ghost correction. Files are stored in.mat format. Specifically, ‘raw_dirx’ files represent diffusion direction-specific k-space data, while ‘mbref’, ‘pparef’, and ‘*_nav’ files provide auto-calibration signals (ACS) and navigator lines. The ‘*_rpflag’ files indicate the phase-encoding polarity during acquisition.
Reconstructed k-space data
The data include processed k-space data. After performing pre-whitening, ghost correction, and estimation of undersampled k-space (including slice-GRAPPA, in-plane GRAPPA and POCS) on the raw k-space as shown in Fig. 1b, the reconstructed multi-channel k-space data was obtained. Specifically, the ‘recon_dirx’ files correspond to the reconstructed k-space data for specific diffusion direction.
Reconstructed images
The reconstructed images include both un-preprocessed and preprocessed data. The un-preprocessed images were obtained from the reconstructed k-space data using Fourier transformation, adaptive multi-channel coil combination, and NORDIC denoising. The preprocessed images were subsequently obtained by applying the preprocessing pipeline illustrated in Fig. 1 to the un-preprocessed images.
DICOM-to-NIfTI images
The DICOM data consists of spatially resolved images, with the raw data discarded during the acquisition process. DICOM files contain a greater variety of scanning settings than the raw k-space data. To standardize the image format, DICOM files were converted to NIfTI format. This conversion process anonymized the images while retaining essential acquisition parameters in.json files, which include the field of view (FOV), acquisition matrix, number of slices, slice thickness, number of coils, TR/TE, flip angle, down-sampling factor, and other relevant details.
Technical Validation
Assessment of diffusion data quality
To assess the feasibility of using the provided k-space data for the image reconstruction task, a series of parallel reconstruction methods (Fig. 1b) were employed as benchmark examples. The reconstructed images for all participants were visually inspected for quality control. Fig. 2 shows the DICOM-to-NIfTI images on the left, and the reconstructed images using the pipeline in Fig. 1b on the right. In comparison, the signal-to-noise ratio (SNR) of DICOM-to-NIfTI images is relatively higher, potentially because the vendor’s pipeline applied some sort of denoising. The reconstructed images exhibited slightly higher background noise levels compared to DICOM-to-NIfTI images. However, the reconstructed images appeared smoother, with clearer edge details, particularly for b = 3000 images. It is worthy to note that the pipeline adopted in this work is not optimal. Researchers are encouraged to refine this pipeline or employ their own to achieve higher-quality results.
Assessment of participant head motion
Participant head motion during the scan was evaluated, as shown in Fig. 3b.
This motion was quantified in each shell using FSL eddy, specifically using restricted movement root mean squared (RMS) outputs80 which show RMS movement in each volume relative to the previous volume.
Preprocessed dMRI. (a) The preprocessed DICOM-to-NIfTI images were obtained following the preprocessing steps illustrated in Fig. 1c. (b) The root mean squared (RMS) voxel-wise displacement relative to the previous diffusion weighted volume, calculated using FSL’s eddy.
Assessment of microstructural modelling and tractography results
The quality of the diffusion MRI data is also assessed by evaluating the microstructural modelling results using different methods, shown in Fig. 4.
Microstructural modelling. (a) The MD, FA, and DEC derived from the tensor were fitted to a single-shell (b = 1000 s/mm2) DTI model using FSL’s dtifit. (b) The OD, Vic, and Viso parameters were obtained by fitting a multi-shell (b = 1000, 2000, 3000 s/mm2) NODDI model using the AMICO toolbox. (c) The WM-VF, GM-VF, DEC were estimated by using the MRTrix3 toolbox. (d) The DTI tensor and FOD of the left centrum semiovale and the coronal radiata.
Different microstructural metrics were derived from two distinct diffusion-based models of DTI and NODDI. Specifically, maps of mean diffusivity (MD), fractional anisotropy (FA) and directionally encoded colour (DEC) FA maps are displayed for DTI, as shown in Fig. 4a. Maps of intra-cellular volume fraction (Vic), isotropic cerebrospinal fluid volume fraction (Viso), and orientation dispersion (OD) are displayed for NODDI, as shown in Fig. 4b. The fiber orientation distribution (FOD) was fitted using the MSMT-CSD algorithm. Figure 4c shows the volume fraction maps of white matter (WM-VF) and gray matter (GM-VF), as well as the DEC FA maps weighted by the integral of the FOD. Figure 4d shows DTI tensor and FOD within the left centrum semiovale and the coronal radiata of a representative participant. The results in these two regions are visually consistent with the anatomical structure.
To support the biological plausibility of the reconstructed fiber pathways, we generated a whole-brain white matter tractogram using the probabilistic iFOD2 algorithm, as shown in Fig. 5. These methods have different data requirements for estimation, which are all met by this dataset. The resulting estimates are consistent with those reported in the relevant literature and visually align with human brain’s anatomy.
Code availability
The MATLAB code for the dMRI reconstruction described above are available. The ismrm_sunrise_parallel tools used in the noise pre-whitening are available in (https://hansenms.github.io/sunrise/sunrise2013/). The NC-IGT Fast Imaging Library used to achieved EPI Nyquist Ghost correction is publicly available (https://www.nitrc.org/projects/igt_fil/). The grappa-tools used in slice-GRAPPA are publicly available in (https://mchiew.github.io/Tools.html). The in-plane GRAPPA code is publicly available in (https://github.com/dakodako/MBEPIRecon/blob/main/grappa.m). The tool of Partial Fourier reconstruction with POCS is publicly available in (https://www.mathworks.com/matlabcentral/fileexchange/39350-mri-partial-fourier-reconstruction-with-pocs). The code of NORDIC method for PCA denoising is available in (https://github.com/SteenMoeller/NORDIC_Raw). The tool used in muti-channel adaptive combination is publicly available in (https://github.com/fluese/reconstructionPipeline/blob/master/processingPipeline/adaptiveCombine.m).
The preprocessing tool described above are available. The HCPpipelines and the Connectome Workbench for co-registration are publicly available (https://github.com/Washington-University/HCPpipelines; https://github.com/Washington-University/workbench). The FMRIB Software Library used in the diffusion data preprocessing and DTI model fitting is publicly available (https://fsl.fmrib.ox.ac.uk/fsl/docs/#/install/index). The MRtrix3 software for the diffusion data preprocessing, constrained spherical deconvolution and tractography generation is publicly available (https://www.mrtrix.org/). The Advanced Normalization Tools (https://github.com/ANTsX/ANTs). The FreeSurfer software, versions V6.0.0 and V7.4.1, are publicly available (https://surfer.nmr.mgh.harvard.edu/fswiki/DownloadAndInstall). The Accelerated Microstructure Imaging via Convex Optimization for fitting NODDI model is publicly available (https://github.com/daducci/AMICO). The DMRITool for optimizing uniform multi-shell gradient vectors is publicly available (https://diffusionmritool.github.io/).
References
Lerch, J. P. et al. Studying neuroanatomy using MRI. Nat Neurosci 20, 314–326 (2017).
Le Bihan, D. & Breton, E. Imagerie de diffusion in-vivo par résonance magnétique nucléaire. Comptes-Rendus de l’Académie des Sciences 93, 27–34 (1985).
Vu, A. T. et al. High resolution whole brain diffusion imaging at 7 T for the Human Connectome Project. NeuroImage 122, 318–331 (2015).
Fan, Q. et al. MGH–USC Human Connectome Project datasets with ultra-high b-value diffusion MRI. NeuroImage 124, 1108–1114 (2016).
Glasser, M. F. et al. The Human Connectome Project’s neuroimaging approach. Nat Neurosci 19, 1175–1187 (2016).
Harms, M. P. et al. Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. NeuroImage 183, 972–984 (2018).
Hagler, D. J. et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage 202, 116091 (2019).
Howell, B. R. et al. The UNC/UMN Baby Connectome Project (BCP): An overview of the study design and protocol development. NeuroImage 185, 891–905 (2019).
Bookheimer, S. Y. et al. The Lifespan Human Connectome Project in Aging: An overview. NeuroImage 185, 335–348 (2019).
Wang, F. et al. In vivo human whole-brain Connectom diffusion MRI dataset at 760 µm isotropic resolution. Sci Data 8, 122 (2021).
Tian, Q. et al. Comprehensive diffusion MRI dataset for in vivo human brain microstructure mapping using 300 mT/m gradients. Sci Data 9, 7 (2022).
Taylor, J. R. et al. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: Structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. NeuroImage 144, 262–269 (2017).
Miller, K. L. et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci 19, 1523–1536 (2016).
Littlejohns, T. J. et al. The UK Biobank imaging enhancement of 100,000 participants: rationale, data collection, management and future directions. Nat Commun 11, 2624 (2020).
Alkemade, A. et al. The Amsterdam Ultra-high field adult lifespan database (AHEAD): A freely available multimodal 7 Tesla submillimeter magnetic resonance imaging database. NeuroImage 221, 117200 (2020).
Keuken, M. C. et al. A high-resolution multi-shell 3T diffusion magnetic resonance imaging dataset as part of the Amsterdam Ultra-high field adult lifespan database (AHEAD). Data Brief 42, 108086 (2022).
Snoek, L. et al. The Amsterdam Open MRI Collection, a set of multimodal MRI datasets for individual difference analyses. Sci Data 8, 85 (2021).
Setsompop, K. et al. Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. NeuroImage 80, 220–233 (2013).
Reischauer, C., Vorburger, R. S., Wilm, B. J., Jaermann, T. & Boesiger, P. Optimizing signal-to-noise ratio of high-resolution parallel single-shot diffusion-weighted echo-planar imaging at ultrahigh field strengths. Magnetic Resonance in Medicine 67, 679–690 (2012).
Uğurbil, K. Magnetic Resonance Imaging at Ultrahigh Fields. IEEE Transactions on Biomedical Engineering 61, 1364–1379 (2014).
Gallichan, D. Diffusion MRI of the human brain at ultra-high field (UHF): A review. NeuroImage 168, 172–180 (2018).
Zhang, Y. et al. Preliminary Experience of 5.0 T Higher Field Abdominal Diffusion-Weighted MRI: Agreement of Apparent Diffusion Coefficient With 3.0 T Imaging. Journal of Magnetic Resonance Imaging 56, 1009–1017 (2022).
Zheng, L., Yang, C., Sheng, R., Dai, Y. & Zeng, M. Renal imaging at 5 T versus 3 T: a comparison study. Insights into Imaging 13, 155 (2022).
Shi, Z. et al. Time-of-Flight Intracranial MRA at 3 T versus 5 T versus 7 T: Visualization of Distal Small Cerebral Arteries. Radiology 306, 207–217 (2023).
Czum, J. M. Venturing Further into Ultrahigh-Field-Strength MRI: Myocardial Late Gadolinium Enhancement at 5 T. Radiology 313, e242935 (2024).
Guo, Y. et al. Myocardial Fibrosis Assessment at 3-T versus 5-T Myocardial Late Gadolinium Enhancement MRI: Early Results. Radiology 313, e233424 (2024).
Lu, H. et al. Feasibility and Clinical Application of 5-T Noncontrast Dixon Whole-Heart Coronary MR Angiography: A Prospective Study. Radiology 313, e240389 (2024).
Wei, Z. et al. 5T magnetic resonance imaging: radio frequency hardware and initial brain imaging. Quant Imaging Med Surg 13, 3222–3240 (2023).
Basser, P. J., Mattiello, J. & LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophysical journal 66, 259–267 (1994).
Pierpaoli, C., Jezzard, P., Basser, P. J., Barnett, A. & Di Chiro, G. Diffusion tensor MR imaging of the human brain. Radiology 201, 637–648 (1996).
Le Bihan, D. et al. Diffusion tensor imaging: Concepts and applications. Journal of Magnetic Resonance Imaging 13, 534–546 (2001).
Assaf, Y., Blumenfeld-Katzir, T., Yovel, Y. & Basser, P. J. Axcaliber: A method for measuring axon diameter distribution from diffusion MRI. Magnetic Resonance in Medicine 59, 1347–1354 (2008).
Tuch, D. S. Q-ball imaging. Magnetic Resonance in Medicine 52, 1358–1372 (2004).
Assaf, Y. & Basser, P. J. Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain. NeuroImage 27, 48–58 (2005).
Jensen, J. H., Helpern, J. A., Ramani, A., Lu, H. & Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magnetic Resonance in Medicine 53, 1432–1440 (2005).
Descoteaux, M., Angelino, E., Fitzgibbons, S. & Deriche, R. Regularized, fast, and robust analytical Q-ball imaging. Magnetic Resonance in Medicine 58, 497–510 (2007).
Behrens, T. E. J., Berg, H. J., Jbabdi, S., Rushworth, M. F. S. & Woolrich, M. W. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage 34, 144–155 (2007).
Wedeen, V. J. et al. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. NeuroImage 41, 1267–1277 (2008).
Cheng, J., Ghosh, A., Jiang, T. & Deriche, R. Model-Free and Analytical EAP Reconstruction via Spherical Polar Fourier Diffusion MRI. in Medical Image Computing and Computer-Assisted Intervention – MICCAI 2010 (eds. Jiang T., Navab N., Pluim J. P. W. & Viergever M. A.) 590–597, https://doi.org/10.1007/978-3-642-15705-9_72 (Springer, Berlin, Heidelberg, 2010).
Fieremans, E., Jensen, J. H. & Helpern, J. A. White matter characterization with diffusional kurtosis imaging. NeuroImage 58, 177–188 (2011).
Wang, Y. et al. Noninvasively Diffusion Basis Spectrum Imaging (DBSI): A Phantom Study (2011).
Zhang, H., Schneider, T., Wheeler-Kingshott, C. A. & Alexander, D. C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 61, 1000–1016 (2012).
White, N. S., Leergaard, T. B., D’Arceuil, H., Bjaalie, J. G. & Dale, A. M. Probing tissue microstructure with restriction spectrum imaging: Histological and theoretical validation. Human Brain Mapping 34, 327–346 (2013).
Özarslan, E. et al. Mean apparent propagator (MAP) MRI: A novel diffusion imaging method for mapping tissue microstructure. NeuroImage 78, 16–32 (2013).
Kaden, E., Kelm, N. D., Carson, R. P., Does, M. D. & Alexander, D. C. Multi-compartment microscopic diffusion imaging. NeuroImage 139, 346–359 (2016).
Novikov, D. S., Kiselev, V. G. & Jespersen, S. N. On modeling. Magnetic Resonance in Medicine 79, 3172–3193 (2018).
Diakite, A. et al. Dual-Uncertainty Guided Multimodal MRI-Based Visual Pathway Extraction. IEEE Transactions on Biomedical Engineering 72, 1993–2000 (2025).
Yang, H. et al. Artificial Intelligence for Neuro MRI Acquisition: A Review. (2024).
Wang, S. et al. Accelerating magnetic resonance imaging via deep learning. in 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI) 514–517, https://doi.org/10.1109/ISBI.2016.7493320 (IEEE, Prague, Czech Republic, 2016).
Yang, J. et al. Simultaneous Q-Space Sampling Optimization and Reconstruction for Fast and High-Fidelity Diffusion Magnetic Resonance Imaging. IEEE International Symposium on Biomedical Imaging (ISBI), pp. 1-4, (Athens, Greece, 2024).
Qin, C. et al. Convolutional Recurrent Neural Networks for Dynamic MR Image Reconstruction. IEEE Transactions on Medical Imaging 38, 280–290 (2019).
Chandra, S. S. et al. Deep learning in magnetic resonance image reconstruction. Journal of Medical Imaging and Radiation Oncology 65, 564–577 (2021).
Wang, S. et al. Knowledge‐driven deep learning for fast MR imaging: Undersampled MR image reconstruction from supervised to un‐supervised learning. Magnetic Resonance in Medicine 92, 496–518 (2024).
Li, C. et al. Artificial intelligence in multiparametric magnetic resonance imaging: A review. Medical Physics 49 (2022).
Faiyaz, A., Doyley, M. M., Schifitto, G. & Uddin, M. N. Artificial intelligence for diffusion MRI-based tissue microstructure estimation in the human brain: an overview. Front. Neurol. 14, 1168833 (2023).
Cheng, J., Shen, D., Basser, P. J. & Yap, P.-T. Joint 6D k-q Space Compressed Sensing for Accelerated High Angular Resolution Diffusion MRI. in Information Processing in Medical Imaging (eds. Ourselin, S., Alexander, D. C., Westin, C.-F. & Cardoso, M. J.) vol. 9123, 782–793 (Springer International Publishing, Cham, 2015).
Wu, R. et al. CSR-dMRI: Continuous Super-Resolution of Diffusion MRI with Anatomical Structure-Assisted Implicit Neural Representation Learning. in Machine Learning in Medical Imaging (eds. Xu, X., Cui, Z., Rekik, I., Ouyang, X., Sun, K.) vol. 15241, 114–123 (Springer International Publishing, Cham, 2025).
Chen, G. et al. XQ-SR: Joint x-q space super-resolution with application to infant diffusion MRI. Medical Image Analysis 57, 44–55 (2019).
Cheng, J., Shen, D., Yap, P.-T. & Basser, P. J. Single- and Multiple-Shell Uniform Sampling Schemes for Diffusion MRI Using Spherical Codes. IEEE Trans. Med. Imaging 37, 185–199 (2018).
rordenlab/dcm2niix. Chris Rorden’s Lab (2024).
Kellman, P. & McVeigh, E. R. Image reconstruction in SNR units: A general method for SNR measurement. Magnetic Resonance in Medicine 54, 1439–1447 (2005).
Kellman, P. Erratum to Kellman P, McVeigh ER. Image reconstruction in SNR units: a general method for SNR measurement. Magn Reson Med. 2005;54:1439–1447. Magnetic Resonance in Medicine 58, 211–212 (2007).
Hoge, W. S., Tan, H. & Kraft, R. A. Robust EPI Nyquist ghost elimination via spatial and temporal encoding. Magnetic Resonance in Medicine 64, 1781–1791 (2010).
Xiang, Q.-S. & Ye, F. Q. Correction for geometric distortion and N/2 ghosting in EPI by phase labeling for additional coordinate encoding (PLACE). Magnetic Resonance in Medicine 57, 731–741 (2007).
Kellman, P. & McVeigh, E. R. Phased array ghost elimination. NMR Biomed 19, 352–361 (2006).
Koopmans, P. J. Two-dimensional-NGC-SENSE-GRAPPA for fast, ghosting-robust reconstruction of in-plane and slice-accelerated blipped-CAIPI echo planar imaging. Magnetic Resonance in Medicine 77, 998–1009 (2017).
Cauley, S. F., Polimeni, J. R., Bhat, H., Wald, L. L. & Setsompop, K. Interslice leakage artifact reduction technique for simultaneous multislice acquisitions. Magnetic Resonance in Medicine 72, 93–102 (2014).
Griswold, M. A. et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic Resonance in Med 47, 1202–1210 (2002).
Haacke, E. M., Lindskogj, E. D. & Lin, W. A fast, iterative, partial-fourier technique capable of local phase recovery. Journal of Magnetic Resonance (1969) 92, 126–145 (1991).
Walsh, D. O., Gmitro, A. F. & Marcellin, M. W. Adaptive reconstruction of phased array MR imagery. Magnetic Resonance in Medicine 43, 682–690 (2000).
Roemer, P. B., Edelstein, W. A., Hayes, C. E., Souza, S. P. & Mueller, O. M. The NMR phased array. Magnetic Resonance in Medicine 16, 192–225 (1990).
Veraart, J., Fieremans, E. & Novikov, D. S. Diffusion MRI noise mapping using random matrix theory. Magnetic Resonance in Medicine 76, 1582–1593 (2016).
Cordero-Grande, L., Christiaens, D., Hutter, J., Price, A. N. & Hajnal, J. V. Complex diffusion-weighted image estimation via matrix recovery under general noise models. NeuroImage 200, 391–404 (2019).
Moeller S. et al. Feasibility of very high b-value diffusion imaging using a clinical scanner. in International Society for Magnetic Resonance in Medicine vol. 3515 (2017).
Moeller, S. et al. NOise reduction with DIstribution Corrected (NORDIC) PCA in dMRI with complex-valued parameter-free locally low-rank processing. NeuroImage 226, 117539 (2021).
Tournier, J.-D. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 202, 116137 (2019).
Kellner, E., Dhital, B., Kiselev, V. G. & Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magnetic Resonance in Medicine 76, 1574–1581 (2016).
Andersson, J. L. R., Skare, S. & Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20, 870–888 (2003).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23, S208–S219 (2004).
Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 125, 1063–1078 (2016).
Andersson, J. L. R., Graham, M. S., Zsoldos, E. & Sotiropoulos, S. N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage 141 (2016).
Andersson, J. L. R., Graham, M. S., Drobnjak, I., Zhang, H. & Campbell, J. Susceptibility-induced distortion that varies due to motion: Correction in diffusion MR without acquiring additional data. NeuroImage 171, 277–295 (2018).
Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. FSL. NeuroImage 62, 782–790 (2012).
Smith, S. M. Fast robust automated brain extraction. Hum Brain Mapp 17, 143–155 (2002).
Tustison, N. J. et al. N4ITK: Improved N3 Bias Correction. IEEE Transactions on Medical Imaging 29, 1310–1320 (2010).
Greve, D. N. & Fischl, B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage 48, 63–72 (2009).
Glasser, M. F. et al. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage 80, 105–124 (2013).
Dale, A. M., Fischl, B. & Sereno, M. I. Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. NeuroImage 9, 179–194 (1999).
Fischl, B., Sereno, M. I. & Dale, A. M. Cortical Surface-Based Analysis: II: Inflation, Flattening, and a Surface-Based Coordinate System. NeuroImage 9, 195–207 (1999).
Fischl, B. FreeSurfer. NeuroImage 62, 774–781 (2012).
Daducci, A. et al. Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data. NeuroImage 105, 32–44 (2015).
Dhollander, T., Mito, R., Raffelt, D. & Connelly, A. Improved white matter response function estimation for 3-tissue constrained spherical deconvolution. in Proc. Intl. Soc. Mag. Reson. Med vol. 555 (2019).
Dhollander, T., Raffelt, D. & Connelly, A. Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. (2016).
Shanshan, W. et al. Diff5T. https://doi.org/10.57760/sciencedb.25122 (2025).
Acknowledgements
This research was partly supported by the National Natural Science Foundation of China (No. 62222118, No. U22A2040), Shenzhen Medical Research Fund (No. B2402047), State Key Laboratory of Biomedical Imaging Science and System, and Youth lnnovation Promotion Association CAS.
Author information
Authors and Affiliations
Contributions
S.W. proposed the idea and initialized the project and the collaboration. S.W. and S.Y. developed and implemented the framework. S.W., Y.X., J.C., C.T. and Y.D. provided the protocols. S.Y. and S.W. collected the brain MRI data. S.Y., S.W., S.J., J.Z. and H.P. developed the reconstruction workflow. S.Y., S.W., J.C., S.J., J.H., F.Z., Q.T., H.G. and G.L. analysed the results and plotted the figures. S.Y., S.W. and J.C. wrote the manuscript. S.W. and H.Z supervised the project. All authors read and contributed to revision and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, S., Yu, S., Cheng, J. et al. Diff5T: Benchmarking human brain diffusion MRI with an extensive 5.0 Tesla k-space and spatial dataset. Sci Data 12, 1352 (2025). https://doi.org/10.1038/s41597-025-05640-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41597-025-05640-2