Abstract
Viruses exploit cellular machinery to complete their replication cycle. Furthering our understanding of this process provides insight into the mechanism of virus replication and potential targets for antiviral therapeutics. Genome-wide CRISPR screens have identified cellular pathways important in the SARS-COV-2 infection process, including vesicular traffic, lipid homeostasis and PI3K signalling. Functional genomics-driven analysis of host-encoded microRNAs (miRNAs) impacting SARS-CoV-2 infection would provide further unbiased and discovery-driven insight into the host-pathogen interface. Here we present findings from genome-wide complementary miRNA mimic and inhibitor screens performed in a bio-safety level (BSL)-4 laboratory using a combination of high-throughput robotics, high-content imaging and novel data analysis pipelines. This dataset has identified both miRNA promoters and inhibitors of SARS-CoV-2 replication which may be used by researchers to further explore therapeutic targets against SARS-CoV-2 and the host factors influencing COVID pathogenesis.
Similar content being viewed by others
Background & Summary
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a betacoronavirus belonging to the Coronaviridae family of RNA viruses. The positive-sense single stranded genome (~30 kB) of SARS-CoV-2 comprises 14 open reading frames (ORFs) encoding 26 proteins covering structural, non-structural and accessory functions1. Viruses from the Coronaviridae family contain important pathogens of mammals, amphibians, and birds. In humans, four coronaviruses are known to cause mild respiratory infections (HCoV-229E, HCoV-NL63, HCoV-OC43 and HCoV-HKU1), while three (SARS-CoV-2, Middle East respiratory syndrome [MERS]-CoV and SARS-CoV) can cause lethal disease (reviewed in2). The coronavirus disease-19 (COVID-19) pandemic caused by SARS-CoV-2 represents one of the largest public health crises in recent times.
Viruses are obligate pathogens that must co-opt host cell processes to complete their replication cycle. Knowledge of such factors furthers understanding of virus replication, immune evasion strategies employed by viruses to establish infection, and in the context of pandemic preparedness, potential targets for therapeutic intervention. Several genome-wide clustered regularly interspaced short palindromic repeats (CRISPR) screens have identified host factors required for SARS-CoV-2 infection3,4,5,6,7,8,9,10. In addition to validating entry receptors and proteases necessary for infection (angiotensin converting enzyme 2 [ACE2], transmembrane protease serine 2 and cathepsin L), meta-analysis of multiple screen datasets highlights vesicular trafficking, phosphoinositide 3-kinase (PI3K) signalling and lipid homeostasis as important regulators (reviewed in11).
MicroRNAs (miRNAs) are a class of single-stranded non-coding RNAs that mediate post-transcriptional regulation of protein-coding genes. miRNA genes are transcribed and processed within the cell nucleus to form primary miRNAs (pri-miRNAs) that are exported to the cytoplasm for further processing, resulting in the formation mature miRNA duplexes consisting of 5p and 3p arms (reviewed in12). miRNAs typically function by base-pairing with target mRNAs and acting as a guide for the RNA-induced silencing complex (RISC) to reduce gene expression13 and regulate most cellular processes, including immune development14, immune responses15 and viral infection16.
miRNA-based regulation of cellular pathways has been shown to influence infection outcomes. For example, miR-451a suppresses pro-inflammatory cytokine expression in dendritic cells and macrophages following influenza A virus infection17 and vaccination18. There is also evidence that viruses co-opt the host miRNA response to subvert antiviral immune responses. miR-30 family members are down-regulated during influenza infection, resulting in increased levels of multiple suppressor of cytokine signaling (SOCS) proteins and suppression of type I interferon (IFN) signaling19. Similarly, the upregulation of miR-132-3p in peripheral blood mononuclear cells of influenza A patients causes the suppression of type I IFN and IFN-stimulated gene production20. While studies investigating the roles of miRNAs in SARS-CoV-2 replication and COVID-19 pathogenesis are few, the SARS-CoV-2 genome is hypothesized to exhibit a miRNA sponging capability, prompting reduced miR-15a-5p and miR-17-5p binding to target mRNAs during infection21, highlighting the potential importance of modulating miRNA activity in SARS-CoV-2 infection.
Given the multitude of studies investigating protein-coding genes as proviral factors of SARS-CoV-2 infection, and the established role of miRNAs in regulating cellular pathways associated with infection, we sought to address the gap in knowledge of miRNAs impacting SARS-CoV-2 replication at a genome-wide level. We present findings from two high-throughput genome-wide screens of miRNA mimic and inhibitor libraries conducted at biosafety level (BSL-4). The miRNA mimics used in this study (Dharmacon miRIDIAN microRNA mimics) simulate mature miRNA duplexes that contain additional chemical modifications to provide bias to the desired guide strand (5p or 3p) for preferential incorporation into the RISC complex, essentially ‘overexpressing’ the desired miRNA. Conversely, the miRNA inhibitors used (Dharmacon miRIDIAN microRNA hairpin inhibitors) are RNA stem loop structures that are complementary to the miRNA of interest, inhibiting activity by blocking binding to their target mRNA via irreversible binding; these inhibitors also include additional chemical modifications to improve potency and longevity. In addition to investigating the impact of miRNAs on SARS-CoV-2 infection, our study describes the effect of miRNAs on cell number, cell cycle and morphology to begin to dissect the potential cellular effects of modulating miRNA expression and their impact on the viral lifecycle. The screens identify both miRNA inhibitors and promoters of SARS-CoV-2 infection for further study in the context of host factors influencing SARS-CoV-2 replication and COVID-19 pathogenesis.
Methods
Cell culture
HelaACE2 (a kind gift from Damien Purcell of the Peter Doherty Institute) is an adherent human epithelial cervical carcinoma cell line that is efficiently transfected and stably expresses the ACE2 receptor and thus supports SARS-CoV-2 replication. Vero E6 (ATCC CRL-1586) is an adherent epithelial kidney cell line isolated from Circopithecus aethiops that supports the replication of a broad range of viruses allowing for viral stock preparation and the determination of viral titres post-miRNA transfection. Both cell lines were cultured in GibcoTM minimal essential medium (MEM) supplemented with Earle’s salts, GlutaMAX, 10 mM HEPES, non-essential amino acids, 1 mM sodium pyruvate 10% foetal calf serum (FCS), 100 U/ml penicillin, and 100 μg/mL streptomycin (Life Technologies). Transfections were performed using cells in medium without antibiotics. All cells were kept at 37 °C in a humidified incubator (5% CO2). Cell lines were cultured under the same conditions and experiments were completed with cells at the same passage number.
Virus
All virology work was conducted at the CSIRO Australian Centre for Disease Preparedness (ACDP) at Biosafety Level 4 (BSL-4). The isolate of SARS-CoV-2 (BetaCoV/Australia/VIC01/2020) used was received from the Victorian Infectious Disease Reference Laboratory (VIDRL; Melbourne, Australia) and passaged in Vero E6 cells for isolation and stock generation. All virus stocks were aliquoted and stored at −80 °C.
High-throughput microRNA screening
Screen workflow is shown in Fig. 1. High throughput miRNA mimic and inhibitor screens were performed largely as described in22. Libraries were screened at the Victorian Centre for Functional Genomics (VCFG, Peter MacCallum Cancer Centre, Melbourne, Australia). The screen was performed as a single experiment in one week. All transfection lipid and miRNA reagents were procured from Dharmacon RNAi technologies (Revvity). The miRIDIAN miRNA libraries (Version 16) used in the screen contain 879 mimics (CS-001010) and 885 inhibitors (IH-001010) and were designed to miR Base 16. HeLaACE2 cells (800 per well) were reverse transfected in 384 well plates in duplicate using DharmaFECT 1 lipid transfection reagent as previously described23. The final concentration of miRNA mimic and inhibitor was 25 nM.
Workflow for genome-wide complementary mimic and inhibitor screens of host-encoded miRNAs impacting SARS-CoV-2 infection. (A) The miRNA mimic (1,239 targets) and inhibitor (1,255 targets) libraries were screened in duplicate in HeLa-ACE2 cells. Using high throughput liquid handling automation (Janus G3 robot), cells (800 per well) were reverse transfected using DharmaFECT 1 in 384 well plates at a final concentration of 25 nM. Using an EL406 liquid handling workstation, media was changed after 24 h. (B) At 72 h post transfection, plates were transferred to BSL-4, infected with SARS-CoV-2 (MOI 0.5, 24 h), and fixed for 1 h using 4% PFA. Plates were placed in polypropylene bags filled with 4% PFA and heat sealed prior to removal from the BSL-4 laboratory via a dunk tank containing MicroChemPlus (diluted 1/64). Plates were then transferred to a fume hood at BSL-3 and washed extensively with distilled water followed by PBS. Cells were stained with a rabbit polyclonal antibody targeting the SARS-CoV-2 N protein (Sino Biological, 40588-T62, Lot#HD14AP2604), and counter-stained with DAPI (nucleus), CellMask Deep Red (whole cell) and Phalloidin (actin cytoskeleton). (C) Plates were imaged using the CellInsight CX7 LZR High-Content Screening (HCS) Platform (16 fields/well) at the VCFG. High content image analysis was carried out using CellProfiler 3.0.0 image analysis software. Screen readouts included quantification of cell number, SARS-CoV-2 infection, cell cycle and morphology. Output data was analysed in R.
Various controls were included on each 384-well plate to normalise for data collection and reproducibility between plates. An ON-TARGETplus non-targeting control (siOTP-NT (D-001810-10), 10 wells/plate, final concentration 40 nM), a lethal control (siTOX (D-001500-01), final concentration 40 nM, 4 wells/plate, siPLK1 (transfection efficiency control, 6 wells/plate, final concentration 40 nM) and a mock control (lipid/Opti-MEM alone, 4 wells/plate). Both siPLK1 and siTOX induce cell death following transfection into cells, allowing for quantitative confirmation of robust transfection efficiency. siPLK1 targets polo-like kinase 1, knockdown of which triggers apoptosis23; the target of siTOX is proprietary (per Dharmacon RNAi Technologies). Each plate also contained uninfected controls (2 wells/plate). The assay plate layout is shown in Supplementary Fig. 1. Controls were plated at the VCFG on the day of screening. Transfection was performed using the Janus G3 liquid handling robot (Revvity, Waltham, MA, USA) and cells added using the EL406 liquid handling workstation (BioTek Instruments, Agilent, Winooski, VT, USA). Transfected cells were incubated under cell culture conditions for 24 h before the medium was changed to a final volume of 40 µL.
At 72 h following transfection, cells were transferred to BSL-4 at CSIRO ACDP and infected with SARS-CoV-2. Virus was diluted to provide an MOI of 0.5 and added to all wells of the plates, excluding rows O and P of column 24. The infection of virus was performed using an EL406 liquid handling workstation housed within a class II biosafety cabinet. At 24 hours post infection, the media was removed and replaced with 4% paraformaldehyde (PFA) to fix the infected cells and allow transfer from BSL-4 to BSL-3.
Specialised consumable reagents required for miRNA mimic/inhibitor screens:
-
Dharmacon miRIDIAN miRNA mimic (CS-001010) and inhibitor (IH-001010) libraries
-
DharmaFECT 1 Transfection Reagent (Dharmacon RNAi Technologies, Revvity, #T-2001-04).
-
Opti-MEM (Gibco, #31985062).
-
384-well plate, polystyrene, tissue-culture treated, clear flat bottom wells, sterile, with lid, black (Corning, #3764).
-
SARS-CoV-2 anti-nucleocapsid (N) protein (Sino Biological, 40588-T62, Lot#HD14AP2604), 1:2,000
-
Anti-rabbit AlexaFluor 488 (Invitrogen, A11008), 1:1000.
-
DAPI (4’,6-Diamidino-2-Phenylindole, Dihydrochloride) (Life Technologies, #D1306); (1:500).
-
CellMask Deep Red (Life Technologies, # C10046); (1:20,000).
-
Rhodamine Phalloidin CF540 (Thermo Fisher Scientific, #R415); 20 nM (1:500).
Staining and imaging
Fixed plates were stained with rabbit polyclonal antibody targeting the SARS-CoV-2 nucleocapsid (N) protein at a dilution of 1:2,000 for 1 h. Cells were subsequently stained with an anti-rabbit AlexaFluor 488 secondary antibody at a dilution of 1:1,000 for 1 h. The nuclei were counterstained with DAPI to allow for the enumeration of viable cells for 1 h. Cells were incubated with CellMask Deep Red (diluted 1:20,000) and Rhodamine Phalloidin (diluted 1:500) in 50 mM Tris-HCl pH 7.6, 0.2% Triton X-100 for 20 min. Plates were imaged at the VCFG using the CellInsight CX7 LZR High-Content Screening (HCS) Platform (Thermo Fisher Scientific, Waltham, MA, USA) at 20 x magnification (2 × 2 binning), 16 fields/well in widefield. The following channels were used: (1) DAPI (405 nm, 0.2 sec exposure), (2) AlexaFluor 488 (488 nm, 0.04 sec exposure), (3) Rhodamine Phalloidin (561 nm, 0.2 sec exposure), (4) CellMask Deep Red (647 nm, 0.1 sec exposure).
Image analysis
Image analysis was carried out using a custom-built pipeline in CellProfiler image analysis software (Broad Institute of MIT and Harvard, Cambridge, MA, USA, version 3.0.0). Briefly, the raw images were loaded directly into CellProfiler. Illumination correction was performed on the raw images by calculating the overall illumination pattern common across all images on each plate for each of the three channels. The illumination patterns for each plate and channel were output as NumPy array files, which store the image data in a binary format compatible with Python. These files were imported into the analysis pipeline and applied to the raw images to correct for uneven illumination. The primary object (nuclei) segmentation was then performed based on the DAPI channel using two-class Otsu thresholding (correction factor 0.84) and the intensity method to split clumped objects. Using the Watershed–Image identification method, the Minimum Cross-Entropy thresholding method (correction factor 0.67) and the primary objects mask as seeds, the secondary objects (cell bodies) were then identified based on the CellMask Deep Red channel. The cell boundary was then expanded based on the Rhodamine Phalloidin channel using Propagation method and a manual intensity threshold of 0.0085. The CellProfiler image analysis pipeline (cppipe file extension) is available in the BioImage Archive (http://www.ebi.ac.uk/bioimage-archive) under the accession number S-BIAD877 along with all associated Numpy array files (.npy file extension) and raw image files (.C01 file extension). The raw images and NumPy array files can be loaded into CellProfiler in their current format. The image analysis pipeline file can then be directly imported into CellProfiler to repeat the image analysis as described.
Data analysis
All raw data derived from image analysis was processed and analysed in RStudio using R version 4.0.3. The R project file and repository was created with the workflowr package (version 1.6.2) for reproducibility and tracked with Git for version control. All raw per-well cell viability, infection, cell cycle and morphology feature values were normalised for batch effects by fold changing to the median of the siOTP-NT control wells on a per-plate basis before replicate plates were averaged. There was an automated seeding issue, which resulted in lower cell counts in columns 02 and 03 of replicate A plates 19009, 19013 and 19055. For these plates, replicate B fold change (FC) values were used for impacted wells rather than the replicate average. For all other plates, the averaged FC values for all sample wells were then robust Z-scored to the siOTP-NT control wells using the following equation:
Robust Z-score = (sample value - control median) / control median absolute deviation).
Cell viability
Cell viability was assessed using cell count (total number of DAPI-stained nuclei in each well). miRNAs were classified into the following viability bins based on FC to siOTP-NT: high viability (>1.15), normal viability (0.8–1.15), moderate viability (0.5–0.8) and low viability (<0.5). Figure 4A. shows an example of a miRNA with low viability in the mimic screen (hsa-miR-3140) compared to a miRNA with normal viability in both screens (hsa-miR-614).
Virus infection
Cells were classified as infected if the per-cell mean SARS-CoV-2 N protein intensity was above the defined cut-off of 0.005 (or 0.007 for plate 19055 replicate B, which had higher intensity values overall). This cut-off was calculated as median + (2 x interquartile range (IQR)) of the uninfected cell population intensity values and resulted in <2% of cells being classified as infected in the uninfected control wells on all plates, except for 19055 B, which had a slightly higher percentage of cells being classified as infected in the uninfected control wells (4.5%) due to higher intensity values overall, as mentioned previously. Approximately 40% of cells were classified as infected in both the mock and siOTP-NT control wells and variability was low, with average percent coefficient of variation (%CV) of ~7.5% across all plates in both screens (see Table 2). Figure 4B. shows an example of a miRNA with no significant impact on infection in the mimic or inhibitor screens (hsa-miR-100) compared to a miRNA with significant impacts in both screens (hsa-miR-92a-1*).
Cell cycle
Cell cycle analysis was performed using the per-cell total DAPI intensity value to infer DNA content. A density plot of total DAPI intensity was generated for each negative control cell population (all siOTP-NT wells) on a per-plate basis. The maximum peak was used to approximately identify the G0/G1 peak. This value was then doubled to approximately identify the G2/M peak. The lower and upper intensity limits if each phase of the cell cycle were then defined as 30% of the intensity range between the two peaks. An example of this type of plot is shown in Fig. 4D. Using the calculated cut-offs, cells in the sample wells were classified as below G0/G1, G0/G1, S, G2/M or above G2/M based on their own total DAPI intensity values and the per-well % cells in each phase of the cycle was calculated. Figure 4D shows an example of a miRNA with no significant impacts on cell cycle in either the mimic or inhibitor screens (hsa-miR-19b) compared to a miRNA with an extreme impact in both screens (hsa-miR-492).
Hit selection
A tiered approach was taken when selecting the final hit list of miRNAs (see Fig. 5B). First, miRNAs were selected based on % cells infected (robust Z-score ≥ 2 or ≤ -2). Figure 5A shows all miRNAs in both screens ranked in order of robust Z-score. Next, miRNAs which did not meet a minimum level of cell viability (FC ≥ 0.5) were removed, as were miRNAs which resulted in extreme impacts to cell cycle (robust Z-score ≥ 5 or ≤ -5). Finally, miRNAs which did not have opposing effects between mimic/inhibitor screens were also removed, leaving the final hit list of 33 miRNAs (~1% of combined libraries) shown in Fig. 5C. These miRNAs were then analysed for alterations in cell morphology.
Cell morphology
The PowerLogLog slope image quality feature extracted from CellProfiler was used to remove out of focus images based on the DAPI channel. Poor quality images were defined as having PowerLogLog slope values < median – (2.5 x IQR) of all images, calculated on a per-plate basis. The per-image nuclei and cell area/shape, intensity and texture features for the DAPI (DNA), AlexaFluor 488 (SARS-CoV-2) and Rhodamine Phalloidin (F-actin) channels were aggregated into per-well median values (the CellMask channel was used for object segmentation only). The feature selection process consisted of removing features with Inf, NA or NaN values, low variance across samples, low correlation (Pearson’s correlation coefficient < 0.5) between technical replicates and redundant features (Pearson’s correlation coefficient > 0.9), leaving a final set of 46 morphology features remaining.
Unbiased hierarchical clustering was then performed using the average method and Euclidean distance metric in the pheatmap (version 1.0.12) package in R to group the miRNA mimics and inhibitors based on morphological similarity. The results are displayed in clustered heatmaps (see Fig. 6).
Screen workflow
Day 1: Reverse transfection
Cells were washed in PBS and incubated with 0.25% Trypsin-EDTA (Gibco, #25200-072) for 5 min at 37 °C for harvesting. Cells were spun at 500 × g for 5 min to remove trypsin and the cell pellet was resuspended in growth media without antibiotics. Cells were counted and diluted to 320,000 cells/mL, equating to 800 cells/well in 25 μL medium. miRNA library stock plates were thawed at room temperature 1 h prior to use.
For the mimic screen, transfection lipid was prepared in Opti-MEM (Life Technologies) at a ratio of 172.80 μL:66.43 mL, equating to a final concentration of 0.03 μL lipid in 11.56 μL per well. The transfection lipid-Opti-MEM mix was incubated for 5 min, before 23 μL was dispensed into each well of the flat bottom 384 well replicate A assay plates (Corning #353988) using an EL406 liquid handling workstation. Next, 1.875 μL of miRNA mimic (stock concentration 1 μM) was transferred from the library plates to the replicate A assay plates using the Janus G3 liquid handling robot, mixed, and incubated for 20 min at room temperature. For the inhibitor screen, transfection lipid was prepared in Opti-MEM (Life Technologies) at a ratio of 126.72 μL:44.75 mL, equating to a final concentration of 0.03 μL lipid in 10.625 μL per well. The transfection lipid-Opti-MEM mix was incubated for 5 min, before 21 μL was dispensed into each well of the replicate A assay plates using an EL406 liquid handling workstation. Next, 3.75 μL of miRNA inhibitor (stock concentration 0.5 μM) was transferred from the library plates to the replicate A assay plates using the Janus G3 liquid handling robot, mixed, and incubated for 20 min at room temperature. For both mimic and inhibitor screens, after 20 min incubation, 12.5 μL of lipid/miRNA complexes was transferred from each replicate A assay plate to the corresponding replicate B assay plate. Plates were then centrifuged at 500 × g for 30 sec. Prepared cells were robotically seeded into every well. Plates were again briefly centrifuged (500 × g for 30 sec) and incubated for 10 min at room temperature (RT), then incubated overnight at 37 °C in a LiCONiC STX220 automated microplate humidified incubator (LiCONiC, Lichtenstein) (37 °C with 5% CO2). All plates were handled in the exact same consecutive order at each step of the screen workflow.
Day 2: Media change
Medium was changed on all assay plates 24 h post transfection using an EL406 liquid handling workstation. Aspiration steps were performed using a z-height of 36 (4.57 mm above carrier, leaving ~6 µL residual), to ensure that the cell monolayer was not disturbed, and 40 μL of pre-warmed growth medium was dispensed into each well. Assay plates were briefly centrifuged (500 × g for 30 sec), then returned to the incubator.
Day 4: SARS-CoV-2 infection
Cells were transported from the VCFG to the ACDP and transferred to the BSL-4 laboratory. SARS-CoV-2 viral stock was diluted in growth medium and, using an EL406 liquid handling workstation housed in a class II biosafety cabinet, SARS-CoV-2 virus (25 µL) was added to each well for an MOI of 0.5. Plates were centrifuged (500 × g for 30 sec) and incubated for 24 h under cell culture conditions.
Day 5: Fixing and staining for virus infection and cell counts
Using the same EL406 liquid handling workstation housed in a class II biosafety cabinet and a dedicated tubing cassette, media was aspirated and cells washed with 80 µL PBS per well. 100 µL of 4% PFA was added to each well and plates incubated for 1 h at RT. Plates were placed in polypropylene bags filled with 4% PFA and heat sealed prior to removal from the BSL-4 laboratory via a dunk tank containing MicroChemPlus (National Chemical Laboratories of PA) diluted 1/64. Entire plates were thoroughly washed with water and subsequently PBS and all wells were rinsed with PBS before staining. Cells were stained with a polyclonal rabbit antibody targeting the SARS-CoV-2 N protein diluted to 1:2,000 for 1 h at RT (25 µL/well). Cells were subsequently stained with an anti-rabbit AF488 antibody diluted to 1:1,000 for 1 h at RT (25 µL/well). Nuclei were counterstained with DAPI diluted to 1:500 for 10 min at RT (25 µL/well). All pipetting work was performed using a Finnpipette Novus electronic pipette (Thermo Fisher Scientific).
Day 6: Staining for cell morphology, image and data capture
Cells were stained with CellMask Deep Red (diluted 1:20,000) and Rhodamine Phalloidin (diluted 1:500) in 50 mM Tris-HCl pH 7.6, 0.2% Triton X-100 for 20 min at RT. Cells were then washed with 50 µL PBS per well and left in fresh PBS (50 µL/well) for imaging. Plates were imaged using the CellInsight CX7 LZR High-Content Screening (HCS) Platform at 20 × widefield magnification capturing 16 fields/well.
Data Records
Data record 1
Fluorescent images of DAPI (DNA), AlexaFluor 488 (SARS-CoV-2 N protein), Rhodamine Phalloidin (F-actin) and CellMask Deep Red stained cells taken in widefield at 20x magnification for the entire screen are available in the Bioimage Archive (http://www.ebi.ac.uk/bioimage-archive) under the accession number S-BIAD87724. The images are provided, as analysed, in their original raw format (greyscale, 16 separate fields per-well). Image files are labelled using the following nomenclature A50363_210920150001_I21f04d0 where this reflects: VCFG internal unique plate identifier, the well ID, field number and channel (d0 = DAPI, d1 = AlexaFluor 488, d2 = Rhodamine Phalloidin, d3 = CellMask Deep Red). Plate and well annotations, including miRNA name and sequence, are included as image metadata.
Data record 2
Mimic and inhibitor screen data for cell counts and virus infection are available with the images at the BioImage Archive under the accession number S-BIAD87724 or alternatively as downloadable CSV files via Figshare25. Samples are defined by miRNA name, Entrez Gene ID, Entrez Gene Symbol, miRNA sequence and vendor catalogue number (Dharmacon RNAi Technologies, Revvity). Screen-wide raw and normalised data, robust Z-scores and the results of viability binning strategies are provided.
Data record 3
Mimic and inhibitor screen data for cell cycle are available at the BioImage Archive under the accession number S-BIAD87724 or alternatively as downloadable CSV files via Figshare25. Raw and normalised data and robust Z-scores are provided for the entire dataset. Samples are defined as described in Data record 224,25.
Data record 4
The final hit list of miRNAs analysed for cell morphology for the mimic and inhibitor dataset is available at the BioImage Archive under the accession number S-BIAD87724 or alternatively as downloadable CSV files via Figshare25. Raw and normalised data and robust Z-scores are provided for the final set of imaging features. Samples are defined as described in Data record 224,25. Definitions and further information regarding each imaging feature can be found in the CellProfiler Manual (http://cellprofiler-manual.s3.amazonaws.com/CellProfiler-3.0.0/index.html).
Technical Validation
Control performance and plate quality control (QC)
The quality of screen data was assessed based on the performance of negative and positive controls for cell viability (total cell count) and % cells infected. The following controls were included on each plate to ensure quality data collection and reproducibility: a non-targeting control (siOTP-NT, 10 wells/plate), two lethal transfection controls (siTOX, 4 wells/plate) and (siPLK1, 6 wells/plate) and a mock control (lipid-Opti-MEM alone, 4 well/plate). Uninfected controls (2 wells/plate) were also included for QC of the image analysis pipeline.
Cell viability
The screen QC metrics for cell viability are presented in Table 1. Initially, control %CVs were higher than acceptable (<24%) on replicate A of plates 19009, 19013 and 19055 due to an automated seeding issue which resulted in lower cell counts in columns 02 and 03 of those plates (in subsequent analyses, the B replicate alone was used for impacted sample wells). Once these wells were excluded, %CVs averaged < 15% for mock, siOTP-NT and the uninfected controls across all plates in both the mimic and inhibitor screens. Cell numbers between mock and siOTP-NT groups were consistent, suggesting no siOTP-NT toxicity was observed. The siPLK1 lethal control was extremely effective, resulting in near complete cell death in both the mimic and inhibitor screens. The experimental robustness was evaluated for each plate using the Z’ factor calculation26 and Z’ Factor values for siPLK1 indicated a robust difference in cell viability to siOTP-NT for every plate in the screen (Z’ > 0.5 for 15/18 plates, Z’ > 0.1 for 3/18 plates). A higher degree of variability was seen in the siTOX lethal control, with viability impacts ranging from ~10 - 40% of cells remaining and failing Z’ Factor values (Z’ < 0) for 7/10 plates in the mimic screen and 2/8 plates in the inhibitor screen. We suspect there may have been a batch issue with the tube of siTOX as the same tube was used in several other screens running concurrently in the lab and a similar level of variability was observed. Due to the performance of the siPLK1 and the low variability for all negative controls, we can be confident that this issue was isolated to the siTOX control and had no impact on the sample wells in the mimic and inhibitor screens. The box plots included in Fig. 2C (left panel) also indicate low variability in cell counts across all plates the screen, with only a small number of outlier wells present.
Gating strategy for defining infected cells and screen performance. (A) SARS-CoV-2 N protein average intensity was used to classify each cell as infected or uninfected. The maximum intensity cut-off for uninfected cells was calculated as the median + (2 x IQR) of the uninfected cell population. Cells with a SARS-CoV-2 mean intensity above this value were classified as infected. The per-cell SARS-CoV-2 mean intensities for negative control conditions (uninfected, mock, siOTP-NT) are shown in the plot. The calculated cut-off is indicated by the vertical dotted line (>0.005). (B) Mimic and inhibitor screen replicate plate reproducibility was assessed for cell count and virus infection using correlation analysis. Cell count (left panels) and % cells infected (right panels) are both represented as fold change (FC) to siOTP-NT and replicate plates for all miRNA samples are plotted. The Pearson Correlation Co-efficient is displayed for each comparison. (C) Box and whisker plots showing distribution of positive (siPLK1, siTOX) and negative (mock, siOTP-NT, uninfected) controls for cell count (left panels) and % cells infected (right panels) for all wells on all plates in both screens. Outlier wells are defined as having values > 1.5 x IQR above the 75th percentile or < 1.5 x IQR below the 25th percentile and are represented by black dots in the plot. Note, knock down of genes that induce extensive cell death, such as the viability positive control PLK1, are associated with higher variability in terms of infection due to the limited number of cells remaining in the well.
Infection
The screen QC metrics for infection are presented in Table 2. The % cells infected were very consistent across all plates in the screen and %CVs were low (~7%) for both mock and siOTP-NT. In the absence of a biological control for infection, the experimental robustness and quality of the imaging and analysis pipeline was evaluated for each plate using the uninfected control wells. Acceptable Z’ Factor values indicated a robust difference in % cells infected between the uninfected wells and siOTP-NT for every plate in the screen (Z’ > 0.5 for 17/18 plates, Z’ > 0.1 for 1/18 plates). Knockdown of genes that induce extensive cell death, such as lethal control siPLK1, are associated with higher variability in terms of infection. This can be seen in the high %CVs and in the box plots shown in Fig. 2C (right panel). Higher variability in the siTOX lethal control could also be due to the viability impacts and/or the batch issue described previously Fig. 3.
Analysis of inter-plate variation across mimic and inhibitor screen plates. Cell count (upper panels) and % cells infected (lower panels) are represented as FC to siOTP-NT, across all screen plates (10 mimic and 8 inhibitor). Data is shown for miRNAs in addition to screen controls (uninfected, mock, siOTP-NT, siTOX, and siPLK1) and noting the last mimic plate had a portion of the plate with no targets (defined as empty). Screen outcomes were highly concordant with the exception of the replicates of plate 19055 from the inhibitor library which showed an increase in % cells infected in replicate B. As mentioned in the Technical Validation section, these wells were flagged as discordant and should be treated with caution.
Quantitation of miRNA impact on cell viability, infection and cell cycle. (A) hsa-miR-614 mimic and inhibitor both had no impact on cell viability when normalised to siOTP-NT and as shown in a field of DAPI-stained nuclei. In contrast, hsa-miR-3140 mimic significantly impacted viability while it’s inhibitor counterpart did not. A target with a viability < 0.5, as shown in italics for hsa-miR-3140 was excluded from further analysis. A single field of DAPI stained nuclei shows a clear reduction in the number of nuclei in the hsa-miR-3140 mimic compared to the inhibitor and siOTP-NT control. (B) An example of a miRNA mimic and inhibitor that had an opposing impact on the % of cells infected as measured by Z-score to siOTP-NT. hsa-miR-92a-1* mimic shows a highly significant decrease in infection versus the hsa-miR-92a-1* inhibitor which is significantly increased. In contrast, hsa-miR-100 showed no difference in infectivity, as indicated by a non-significant Z- score and staining that looks similar to siOTP-NT with no change in cell number via DAPI stain (grey nuclei) and infection (SARS-CoV-2 N protein, green). Targets that generated a non-significant Z-score as shown in the table in italics were excluded from further analysis. (C) Cell cycle analysis indicated that hsa-miR-492 had an extreme impact on cell cycle in both the mimic and inhibitor screens, while hsa-miR-19b resulted in no impact, except for a slight reduction in the % cells in G0/G1. This change is difficult to discern by eye in the images of DAPI-stained nuclei shown. (D) The per-cell total DAPI intensity values for the hsa-miR-492 mimic show a dramatic shift in distribution to G2/M compared to hsa-miR-19b, which is much more similar to siOTP-NT. The same field is shown for all siOTP-NT images (mimic screen image is taken from plate 19009 well D23, inhibitor screen image is taken from plate 19055 well C02). Scale bar for all images = 50 um.
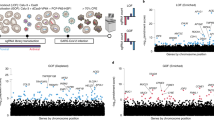
Hit identification strategy. (A) The entire miRNA mimic and inhibitor screens were ranked using a robust Z-score as illustrated in a waterfall plot from lowest (decreased virus infection) to highest (increased virus infection). Dotted horizontal lines represent the threshold of hit identification (Z ≥ 2 or ≤ −2). The number of miRNA hits above this threshold is shown for miRNAs that resulted in increased (red dots) or decreased (blue dots) SARS-CoV-2 infection. (B) The hit selection strategy involved four steps that iteratively refine the number of targets: (1) miRNAs were selected based on % cells infected (Z ≥ 2 or ≤ −2), (2) miRNAs which did not meet a minimum level of cell viability (FC ≥ 0.5) were removed, (3) miRNAs which resulted in extreme impacts to cell cycle phases G0/G1, S or G2/M (Z ≥ 5 or ≤ −5) were removed, (4) only miRNAs that had opposing effects between the mimic and inhibitor were retained. This final hit list was then subjected to morphology analysis (outlined in Fig. 6). (C) The final 33 miRNAs are represented by robust Z-scores showing the opposing outcomes for increased and decreased infection.
Morphology analysis of hits. Clustered heat maps show the results of hierarchical clustering for the mimic and inhibitor screens analysed separately, based on Z-score to siOTP-NT for the final set of morphology features as shown on the X axis. On the Y axis, % cells infected is indicated by increased infection in pink (Z ≥ 2) or decreased infection in blue (Z ≤ −2), (A) miRNA mimics were grouped into 13 clusters based on morphological similarity. The heat map shows that there are several clusters with extreme changes in cell morphology, particularly nucleus shape and texture and intensity in the DAPI channel. (B) miRNA inhibitors were grouped into 12 clusters based on morphological similarity. The heatmap shows that the morphological changes are less extreme than in the mimic screen, but there are still a number of inhibitors which have significant impact, particularly on nucleus shape, texture and intensity in the DAPI channel and cell shape and texture in the Phalloidin channel.
Replicate plate reproducibility
Replicate plate reproducibility was assessed for cell viability and virus infection by plotting the fold change to siOTP-NT for all miRNA samples from replicate assay plates. The Pearson Correlation co-efficient showed that there is a higher degree of discordance between replicate plates for % cells infected in the inhibitor screen. Sample wells with a difference of > 0.25 in FC for cell count and/or % cells infected have been flagged as discordant in all data records24,25. We don’t exclude these data points from subsequent analysis but prefer to draw their attention as potential false positives that can be assessed at a secondary screen stage.
Impact of mimics/inhibitors on cell growth
We evaluated the impact of select mimics and inhibitors on cell growth and viability over time spans beyond that used in the screen. We investigated mimics, and their inhibitor counterparts, that had the greatest impacts on SARS-CoV-2 replication; these included proviral mimics hsa-miR-19b and hsa-miR-3617 (robust Z-score 5.01 and 4.94, respectively), and antiviral mimics hsa-miR-421 and hsa-miR-92a-1* (robust Z-score −13.94 and −8.4, respectively) effects on SARS-CoV-2. Transfected HeLa-ACE2 cells were imaged 14 days post-transfection and confluency measured as an indicator of cell growth and viability (Supplementary Figure 2, Supplementary Methods). No impact on cell growth was observed compared to the negative control, siOTP-NT, except for the positive cell death control, siPLK1, and slightly slower growth induced by the hsa-miR-92a-1* mimic.
Target validation
Next, we sought to validate the effects of these top proviral and antiviral mimics on SARS-CoV-2 replication using qPCR. In addition, we assessed the temporal kinetics and stability of transfected mimics by evaluating two additional timepoints, 24 h and 120 h post transfection, alongside the 72 h duration performed for the screen. Levels of SARS-CoV-2 N gene expression in infected cells transfected with hsa-miR-19b and hsa-miR-3617 mimics trended toward increased levels compared to the negative control (siOTP-NT) following 24 and 72 h transfection duration, in particular hsa-miR-3617 was significantly increased at 72 h (Supplementary Figure 3). In contrast, following 120 h transfection, N gene expression was significantly reduced by hsa-miR-19b, while hsa-miR-3617 had no effect, although trended downwards. hsa-miR-421 and hsa-miR-92a-1* had the expected opposite effect on N gene expression, with an increasing downward trend following 24 and 72 h transfection compared to the negative control; significantly decreased levels were observed following 120 h transfection. Together, these data support the results of the screen and further expand knowledge of the kinetics of the use of miRNA mimics and inhibitors to modulate virus infection.
Code availability
All R code used to analyse the data is publicly available in the following Bitbucket repository: https://atlassian.petermac.org.au/bitbucket/projects/VCFG/repos/rootes_et_al_2025_sars-cov-2_mirna_screen.
To download the repository as a zipped folder:
1. Visit the link above.
2. On the repository page, click the “•••” (more options) button next to the “master” branch selector.
3. Choose “Download repository” from the drop-down menu.
The main R script, Rootes_et_al_2025_analysis.Rmd, is located in the “analysis” subdirectory.
References
Naqvi, A. A. T. et al. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim Biophys Acta Mol Basis Dis. 1866, 165878, https://doi.org/10.1016/j.bbadis.2020.165878 (2020).
Cui, J., Li, F. & Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 17, 181–192, https://doi.org/10.1038/s41579-018-0118-9 (2019).
Wang, R. et al. Genetic Screens Identify Host Factors for SARS-CoV-2 and Common Cold Coronaviruses. Cell. 184, 106–119.e14, https://doi.org/10.1016/j.cell.2020.12.004 (2021).
Daniloski, Z. et al. Identification of Required Host Factors for SARS-CoV-2 Infection in Human Cells. Cell. 184, 92–105.e16, https://doi.org/10.1016/j.cell.2020.10.030 (2021).
Zhu, Y. et al. A genome-wide CRISPR screen identifies host factors that regulate SARS-CoV-2 entry. Nat Commun. 12, 961, https://doi.org/10.1038/s41467-021-21213-4 (2021).
Baggen, J. et al. Genome-wide CRISPR screening identifies TMEM106B as a proviral host factor for SARS-CoV-2. Nat Genet. 53, 435–444, https://doi.org/10.1038/s41588-021-00805-2 (2021).
Rebendenne, A. et al. Bidirectional genome-wide CRISPR screens reveal host factors regulating SARS-CoV-2, MERS-CoV and seasonal coronaviruses. bioRxiv. https://doi.org/10.1101/2021.05.19.444823 (2021).
Biering, S. B. et al. Genome-wide bidirectional CRISPR screens identify mucins as host factors modulating SARS-CoV-2 infection. Nat Genet. 54, 1078–1089, https://doi.org/10.1038/s41588-022-01131-x (2022).
Schneider, W. M. et al. Genome-Scale Identification of SARS-CoV-2 and Pan-coronavirus Host Factor Networks. Cell. 184, 120–132.e14, https://doi.org/10.1016/j.cell.2020.12.006 (2021).
Wei, J. et al. Genome-wide CRISPR Screens Reveal Host Factors Critical for SARS-CoV-2 Infection. Cell. 184, 76–91.e13, https://doi.org/10.1016/j.cell.2020.10.028 (2021).
Baggen, J., Vanstreels, E., Jansen, S. & Daelemans, D. Cellular host factors for SARS-CoV-2 infection. Nat Microbiol. 6, 1219–1232, https://doi.org/10.1038/s41564-021-00958-0 (2021).
Macfarlane, L.-A. & Murphy, P. R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr Genomics. 11, 537–61, https://doi.org/10.2174/138920210793175895 (2010).
Kawamata, T., Seitz, H. & Tomari, Y. Structural determinants of miRNAs for RISC loading and slicer-independent unwinding. Nat Struct Mol Biol. 16, 953–60, https://doi.org/10.1038/nsmb.1630 (2009).
Mehta, A. & Baltimore, D. MicroRNAs as regulatory elements in immune system logic. Nat Rev Immunol. 16, 279–94, https://doi.org/10.1038/nri.2016.40 (2016).
Tsitsiou, E. & Lindsay, M. A. microRNAs and the immune response. Curr Opin Pharmacol. 9, 514–20, https://doi.org/10.1016/j.coph.2009.05.003 (2009).
Tribolet, L. et al. MicroRNA Biomarkers for Infectious Diseases: From Basic Research to Biosensing. Front Microbiol. 11, 1197, https://doi.org/10.3389/fmicb.2020.01197 (2020).
Rosenberger, C. M. et al. miR-451 regulates dendritic cell cytokine responses to influenza infection. J Immunol. 189, 5965–75, https://doi.org/10.4049/jimmunol.1201437 (2012).
Okamoto, M. et al. MicroRNA-451a in extracellular, blood-resident vesicles attenuates macrophage and dendritic cell responses to influenza whole-virus vaccine. J Biol Chem. 293, 18585–18600, https://doi.org/10.1074/jbc.RA118.003862 (2018).
Lin, X., Yu, S., Ren, P., Sun, X. & Jin, M. Human microRNA-30 inhibits influenza virus infection by suppressing the expression of SOCS1, SOCS3, and NEDD4. Cell Microbiol. 22, e13150, https://doi.org/10.1111/cmi.13150 (2020).
Zhang, F., et al. MicroRNA-132-3p suppresses type I IFN response through targeting IRF1 to facilitate H1N1 influenza A virus infection. Biosci Rep. 39. https://doi.org/10.1042/BSR20192769 (2019).
Fossat, N. et al. Identification of the viral and cellular microRNA interactomes during SARS-CoV-2 infection. Cell Rep. 42, 112282, https://doi.org/10.1016/j.celrep.2023.112282 (2023).
Foo, C. H. et al. Dual microRNA Screens Reveal That the Immune-Responsive miR-181 Promotes Henipavirus Entry and Cell-Cell Fusion. PLoS Pathog. 12, e1005974, https://doi.org/10.1371/journal.ppat.1005974 (2016).
Deffrasnes, C. et al. Genome-wide siRNA Screening at Biosafety Level 4 Reveals a Crucial Role for Fibrillarin in Henipavirus Infection. PLoS Pathog. 12, e1005478, https://doi.org/10.1371/journal.ppat.1005478 (2016).
Rootes, C. L. et al. Biostudies https://identifiers.org/biostudies:S-BIAD877 (2025).
Rootes, C. L. et al. Figshare https://doi.org/10.6084/m9.figshare.29095802 (2025).
Birmingham, A. et al. Statistical methods for analysis of high-throughput RNA interference screens. Nat Methods. 6, 569–75, https://doi.org/10.1038/nmeth.1351 (2009).
Acknowledgements
This work was funded by the CSIRO and Therapeutics Innovation Australia (grant awarded to C.R.S. and K.J.S.). A.M.B. was a recipient of a CSIRO Early Career Research (CERC) post-doctoral fellowship. We acknowledge support from colleagues at the Australian Centre for Disease Preparedness (https://www.grid.ac/institutes/grid.413322.5) for maintaining BSL-4 laboratory space used in this study. The Victorian Centre for Functional Genomics (K.J.S.) is funded by the Australian Cancer Research Foundation (ACRF), Phenomics Australia, through funding from the Australian Government’s National Collaborative Research Infrastructure Strategy (NCRIS) program, the Peter MacCallum Cancer Centre Foundation and the University of Melbourne Collaborative Research Infrastructure Program. We thank Professor Damien Purcell (The University of Melbourne) for providing the HeLaACE2 cell line.
Author information
Authors and Affiliations
Contributions
C.L.R., K.J.C., A.M.B., H.G.B. and R.M.S. designed and performed experiments and analysed the data. C.L.R., K.J.C., A.M.B. and H.G.B. contributed to manuscript writing. K.J.S. and C.R.S. conceived the study, supervised the work and co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rootes, C.L., Cowley, K.J., Brice, A.M. et al. Genome-wide analysis of host-encoded microRNAs modulating SARS-CoV-2 infection. Sci Data 12, 1330 (2025). https://doi.org/10.1038/s41597-025-05669-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41597-025-05669-3