Abstract
Leptospirosis is a re-emergent worldwide zoonosis. It is endemic in Martinique where transmission conditions are favourable. Humans are usually infected through contact with water contaminated with urine of rodents. Recent human leptospirosis outbreaks in Martinique require today effective rodent management to prevent leptospirosis transmission. Nowadays, use of anticoagulant rodenticides (AR) is the main method implemented to control rodent populations. Nevertheless, intensive use of these AR has selected worldwide many VKORC1-based resistant rodent strains to AR. Our aim was to characterize the sensitivity of Martinique commensal rodents to AR to better prevent leptospirosis transmission. Resistance of house mice to first-generation and in rare cases even to second-generation ARs were clearly demonstrated in Martinique with the detection of the Y139C mutation with a very high allelic frequency of 40% and the A26T/Y139C double-mutation with an allelic frequency of 0.9%. In black rat, the most prevalent rodent in Martinique, 3 new Vkorc1 coding mutations were detected, the H68N, A115T and S149N mutations associated with moderate resistance to first generation AR. Therefore, rodent management in Martinique must be carried carefully to avoid resistance diffusion and maintain long-term effective rodent management, to be able to efficiently prevent leptospirosis transmission.
Similar content being viewed by others
Introduction
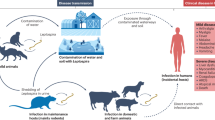
Leptospirosis, a bacterial disease, is one among the foremost widespread zoonoses within the world1,2. This disease has been reported to affect on more or less one million persons worldwide every year3 and may be accompanied by different clinical symptoms ranging from a flu-like syndrome with a moderate fever to a multi-organ failure4,5,6 which can lead to death7. Incidence of this disease is much higher in the tropics than in the temperate zones, the transmission conditions being more favorable1. In Martinique, an Island of the French West Indies, incidence was reported at 13.9 to 60.6 cases per 100 000 inhabitants per year8,9 with frequent outbreaks10,11. All preventive measures must be implemented to limit leptospirosis transmission in Martinique. Even if numerous wild and domestic animals are known as as hosts of pathogen Leptospira excreting them in their urine, the main reservoirs identified are rodents, hence the importance of managing their populations12,13.
Nowadays, use of anticoagulant rodenticides ARs is the main method implemented to control rodent populations. They provoke bleeding by the inhibition of the vitamin K epoxide reductase enzyme (VKORC1). This enzyme catalyses the reduction of vitamin K epoxide into vitamin K quinone permitting the vitamin K recycling. Vitamin K is useful as a cofactor of the gamma -glutamyl carboxylase enzyme that activates coagulation factors II, VII, IX and X by gamma-carboxylation. Anticoagulant rodenticides (AR), by inhibiting VKORC1, prevents the coagulation process14,15.
The massive use of ARs since the 1960s selected rodents resistant to the first-generation ARs (i.e., warfarin, chlorophacinone, diphacinone, coumatetralyl)16,17. Metabolic resistance caused by a faster elimination of ARs has been reported. Nevertheless, resistance to ARs is mainly due to Vkorc1 mutations or polymorphisms18,19,20,21 resulting in a decrease of the inhibition of VKORC1 enzyme by first-generation ARs. Such resistance has been first described in brown rats (Rattus norvegicus) and then in house mice (Mus musculus domesticus) in Europe, and then worldwide in the United States22, Canada23, Japan24 and in Australia25…. The selection of rodents with Vkorc1 mutations has led to an inefficiency of first-generation AR to control certain rodent populations. Second generation ARs -i.e., bromadiolone, difenacoum, difethialone, brodifacoum and flocoumafen) have been thus developed to overcome resistance issues in rodents. Because second generation ARs are persistent in rodent tissues and related to secondary exposure or poisoning of non-target wildlife, their use should be cautious and sometimes, in some countries, limited to professionals and/or after failure of a first-generation AR treatment and/or if resistance is demonstrated.
In Martinique where leptospirosis is so prevalent, rodent management must be optimal. This study was therefore intended to evaluate the presence and dispersion of Vkorc1 mutations in rodents in Martinique that could impair rodent management by ARs. To evaluate the impact of the detected Vkorc1 mutations and because all ARs can be used in Martinique as biocides and sometimes as plant protection products, exposition of rodents to ARs was characterized.
Results
Trapping success
A total of 512 traps were set in Martinique. The trap effort on the Martinique Island all along the study was 2048 trap-nights. The trap effort was different according to the site: 88 trap-nights for site 8, 100 for sites 1 to 6, 108 for site 7, 192 for site 9, 200 for sites 10 to 13, and 260 for site 14. A total of 206 rodents were captured (129 Rattus rattus, 59 Mus musculus domesticus and 18 Rattus norvegicus). Rodent species were confirmed based on matching of mtDNA cytochrome b sequences with more than 98% of homology with published sequences of the cytochrome b of the respective species. Trapping results per site are presented in Table 1. The trap success was 10.06 rodents per 100 trap-nights in Martinique Island including 6.30 Rattus rattus, 2.88 Mus musculus domesticus and 0.88 Rattus norvegicus. Results per site are presented in Table 2 with trap-success ranging from 0 rodent for site 4 to 23.5 rodents for the control site 14. The trap-success for Rattus rattus was comprised between 0 to 23.5 for site 14; for Mus musculus, between 0 to 12 (for site 5); for Rattus norvegicus, between 0 to 10 (for site 2).
Exposition of trapped rodent populations to ARs
Rodent exposure to ARs was assessed by analyzing residues of ARs in liver. The rodents trapped in control sites 9 and 14 did not have hepatic residues of ARs. In the other sites, 61 rodents presented liver ARs residues of less than 10 ng/g (54% of rodents trapped in ARs sites); 26 rodents, concentrations between 10 and 100 ng/g (or 23% of rodents trapped in ARs sites); 23, concentrations between 100 and 1000 ng/g (or 20% of rodents trapped in ARs sites); and 3, concentrations greater than 1000 ng/g (i.e., 2.6% of rodents trapped in ARs sites). The majority of ARs residues were molecules belonging to second-generation ARs with difenacoum residues significantly higher than other second-generation molecules (Figs 1, 2B).
AR concentration in liver of trapped rodents in Martinique Island. Concentrations of individual (A) or grouped in first or second generation (B) AR molecules are presented. AR concentrations were determined by LC-MS/MS as previously described47. Results are presented as median with interquartile range. First-generation AR = CTTL, coumatetralyl; WARF, warfarin; CHL, chlorophacinone. Second-generation AR = BROMA, bromadiolone; DFC, difenacoum; BFC, brodifacoum; FLO, flocoumafen; DFT, difethialone. The dashed line corresponds to the concentration of 1000 ng/g.
AR concentrations in liver of rodents trapped in the different trapping sites. Concentrations of total (A) or individual (B) ARs are presented. AR concentrations were determined by LC-MS/MS as previously described47. Results are presented in (A) as median with a box extending from the 25th to 75th percentiles, in (B) as median with interquartile range. Sites 9 and 14 corresponded to control sites. First-generation AR = CTTL, coumatetralyl; WARF, warfarin; CHL, chlorophacinone. Second-generation AR = BROMA, bromadiolone; DFC, difenacoum; BFC, brodifacoum; FLO, flocoumafen; DFT, difethialone. The dashed line corresponds to the concentration of 1000 ng/g. RR for Rattus rattus, MM for Mus musculus and RN for Rattus norvegicus.
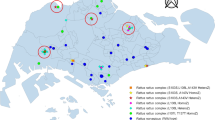
A Generalized Linear Model as a smoothing model was used to analyze the geographical pattern of ARs exposition. The best model selected by its AIC (Akaike information criterion) used the latitude, the longitude, an interaction between latitude and longitude and the nature of the site (natural area or not) as variables. All these parameters were highly statistically significant. Spatial variables (Ylat, Xlong and interaction between Ylat and Xlong) revealed a strong spatial pattern with low exposure in South-west to a high exposure to Northeast (Fig. 3A). Furthermore, as expected, sites in natural area showed significant lower AVK exposure.
Estimation of the proportion of individuals exposed to ARs (A), the proportion of mutants in rat population (B), the proportion of mutants in mouse population (C). A Generalized Linear Model as a smoothing model was used to analyze the geographical pattern. Binomial distribution for the response variable (proportion of rodent with ARs or proportion of mutated rodents) and logit link were used. The coordinates X and Y was used as explanatory variables. Proportion of rodent exposed to ARs increases from yellow to red. Proportion of mutant rodents increases from grey to black. White areas represent natural area where AVK treatments are unexpected. “0” symbols represent locations of sampled sites in natural area (control sites), “ + ” symbols, those in other locations. Note that map of mouse model (C) is adjusted on a smallest number of sites than (A) and (B) and that estimated coefficients (Xlong, Ylat and interaction between Xlong and Ylat) are less accurate.
Whether it was agricultural site or industrial site, residues of ARs were similar (result not shown), even if there were noticeable differences between agricultural sites, with highly treated sites such as sites 1 and 8 (significantly different from the control sites) and weakly treated sites such as site 13 (not significantly different from the control sites) (Fig. 2A).
ARs residues were detected in Rattus rattus, Rattus norvegicus and Mus musculus (Fig. 4). First generation ARs were detected in concentration higher than 5 ng/g only in liver of Mus musculus. Second generation AR were detected in all species but were significantly higher in liver of Rattus rattus than Mus musculus. No difference in concentration was observed between mutated and non-mutated rodents (data not shown) even when only one mutation was considered to perform this correlation. On the other hand, a positive correlation was observed between the occurrence of mutants in rats [0.17372, +/− 0.01346] and mice [0.11303 +/− 0.03465] population and the occurrence of ARs.
AR concentrations in liver of trapped Rattus rattus (RR), Rattus norvegicus (RN) and Mus musculus (MM). Concentrations of total (A), first generation (B), and second generation (C) ARs are presented. AR concentrations were determined by LC-MS/MS as previously described47 First-generation AR = coumatetralyl + warfarin + chlorophacinone. Second-generation AR = bromadiolone + difenacoum + brodifacoum + flocoumafen + difethialone. The dashed line corresponds to the concentration of 1000 ng/g.
Vkorc1 genotyping of trapped rodent populations
From the sampling, Vkorc1 of 81 Rattus rattus, 55 Mus musculus and 16 Rattus norvegicus have been sequenced. From Mus musculus, all the sequences were exploitable. From Rattus sp., only Vkorc1 sequences of 61 Rattus rattus and 15 Rattus norvegicus were exploitable. 34 Rattus rattus presented no silent or missense Vkorc1 mutation in the coding sequence (i.e., 55.7% of the sequenced samples from Rattus rattus species) and 4 presented missense mutations in the coding sequence (i.e., 6.6% of the sequenced samples from Rattus rattus species). One Rattus norvegicus presented no silent or missense Vkorc1 mutation in the coding sequence, the others presented silent mutations. Only 21 Mus musculus presented no silent or missense Vkorc1 mutation in the coding sequence (i.e., 38.2% of the sequenced samples from Mus musculus species) and 34 presented missense mutations (i.e., 61.8% of the sequenced samples from Mus musculus species).
Among sequenced Rattus rattus, 6 different SNPs or mutations found either alone or in combination were detected including 3 silent mutations L94L (g.1087 C > T), S120S (g.2005A > T), A143A (g.2083 A > G) and 3 missense mutations H68N (g.1009 C > A), A115T (g. 1997G > A), Y129N (g.2039 T > A). Allelic frequencies of the different genotypes are presented in Table 3 and locations of genotypes leading to protein mutations are presented in Fig. 5.
Among sequenced Rattus norvegicus, only 1 silent mutation H68H (g.1140 C > T) was detected with an allelic frequency of 93.3%. This silent mutation was found in sites 2, 3, 8, 9, 10, 11 and 13. A Generalized Linear Model as a smoothing model was used to analyze the geographical pattern of the proportion of mutations in rats. The best model selected by its AIC used the latitude, the longitude, an interaction between latitude and longitude and the nature of the site (natural area or not) as variables. All these parameters were highly statistically significant. Spatial variables (Ylat, Xlong and interaction between Ylat and Xlong) revealed a strong spatial pattern with a proportion of rats with Vkorc1 mutations increasing highly increased to North and South and less in West (Fig. 3B). Furthermore, as expected, sites in natural area showed significant lower proportion of mutants.
Among sequenced Mus musculus, 5 different SNPs or mutations found either alone or in combination were detected. All these mutations were missense mutations A26T (g.76 G > A), A48T (g.142 G > A), R61L(g.976 G > T), Y139C(g.2223 A > G) and S149N(g.2253 G > A). Allelic frequencies of the different genotypes are presented in Table 3 and locations of genotypes leading to protein mutations are presented in Fig. 5. A spatial analysis was also carried out but due to the small number of samples and sites, the criteria used were not statistically significant.
Functional consequences of VKORC1 mutations
To evaluate the consequences of VKORC1 mutations on the susceptibility of VKORC1 enzyme towards AR, the unknown VKORC1 mutants were overexpressed as c-myc-fused proteins in P. pastoris; wild type VKORC1 of Rattus sp. or Mus musculus and some mutants (i.e., MmVKORC1-A26T and MmVKORC1-Y139C) having been previously characterized (Goulois et al., 2016, 2017, Hodroge et al., 2011). P. pastoris were able to efficiently expressed all the VKORC1 mutants with the same molecular weight of approximately 20-kDa. Respective inhibition constants (Ki) towards AR of first generation (i.e., warfarin, chlorophacinone) or second generation (i.e., bromadiolone, difenacoum, difethialone or brodifacoum) were determined. Resistance factors of the mutated VKORC1 are presented in Fig. 6. This resistance factor corresponds to the ratio between obtained Ki for the mutated VKORC1 and obtained Ki for the wild type VKORC1. Previous results obtained for MmVKORC1-A26T and MmVKORC1-Y139C in previous studies have been added to allow the comparison.
Resistance factors towards various ARs molecules of mutated VKORC1 detected in trapped rodents on Martinique Island. Resistance factor correspond to the ratio between Ki for mutated VKORC1 and wild type VKORC. Ki was the inhibition constant determined by expressing and characterising the susceptibility to AR of the recombinant mutated or non mutated VKORC1 enzyme. First-generation AR = WARF, warfarin; CHL, chlorophacinone. Second-generation AR = BROMA, bromadiolone; DFC, difenacoum; BFC, brodifacoum; DFT, difethialone. The dashed lines correspond to the resistance factor of 1, 5 and 10.
Discussion
This study is the first study reporting rodent resistance to ARs in Martinique. This finding is crucial to improve their management in this island where leptospirosis is an endemic disease9. Rodents are central in the transmission of leptospirosis. This portage has been widely described in brown rats (Rattus norvegicus) in the French West Indies, but it has been also reported in black rats (Rattus rattus) and domestic mice (Mus musculus)26,27. The management of these 3 species of rodents is therefore vital to limit leptospirosis in the French West Indies. This study aimed therefore to explore practices and effectiveness of rodent chemical treatments in Martinique.
The study aimed to be as representative as possible of the island with an important number of study sites (i.e., 14 sites) distributed homogeneously on the island (i.e., 5 sites in the North, 4 sites in the Center and 5 sites in the South), representing all the sectors of activity (i.e., 2 industrial / commercial sites, 2 intensive breeding sites, 2 forest/natural areas, 8 agricultural areas of sugar cane, cassava, banana, sweet potatoes or chayotes), since rodents present and practices may be different depending on the sector. 206 rodents were captured in this study, this must be analyzed considering to the trapping effort. For this, the trap-success index, which reflects the population levels, has been calculated. This success trap, including all the sites and the 3 species of rodents is 10.06 rodents/100 trap nights. A recent study conducted in a Parisian park in which rats were known to be abundant as many visitors were reporting rat sightings at day time, pointed out a trap success between 0.32 and 1.5828. The population of rodents in Martinique would therefore be quite abundant, especially since all the sites except the control ones are sites on which rodent management is organized. In our study, brown rats, black rats, and house mice were trapped. These 3 species are therefore present in Martinique, nevertheless the black rat seems most abundant (i.e., trap success of 6.3), then the domestic mouse (i.e., trap success of 2.9), the brown rat population (i.e., trap success of 0.9), being rather limited. Nevertheless, this distribution varies according to the site.
The management of brown rats, black rats and house mice in Martinique, as in the rest of the French West Indies, is largely based on the use of rodenticide anticoagulants. In our study, 54% of rodents were exposed to at least one AR molecule, 29% of which were exposed to more than 2 molecules, confirming this preferential use of ARs in rodent management and suggesting the use of several types of active ingredients on the same site. First- and second-generation AR were detected in rodents trapped in urban as well as agricultural sites. Second-generation molecules were most frequently detected (in the liver of 50% of rodents) while first-generation molecules were very rarely detected (in the liver of only 6% of rodents). Difenacoum was the most frequently detected molecule in 38% of rodents; nevertheless, hepatic concentrations were higher than 100 ng/g, threshold compatible with the death of the animal in only 7% of the cases. Bromadiolone, brodifacoum and difethialone were detected with similar respective frequencies of 15.5, 20 and 14.5%. This intense use of second-generation molecules in agricultural areas is surprising because French regulations provide for restricted use of ARs. According to the French regulation, ARs can be used as biocides - in and around the buildings for the control of the populations of commensal rodents - or as plant protection products - in field for the control of voles. Currently, 8 active ingredients are authorized as biocides (i.e., warfarin, coumatetralyl, chlorophacinone, bromadiolone, difenacoum, brodifacoum, difethialone, flocoumafen), whereas only bromadiolone is authorized for the control of voles. Nevertheless, because of the high leptospirosis risk, a collective management of brown rats, black rats and mice, subjected to a preliminary prefectural authorization, is organized every year by the FREDON in Martinique and foresees a wider use of the ARs in the cultures and Border Fields (www.martinique.pref.gouv.fr). This may explain this important detection of ARs in agricultural areas. Until now, ecotoxicity issues associated to the use of ARs have not been reported in Martinique.
Among the 14 trapping sites, some sites seem to use few or no rodenticide treatment (i.e.,, sites 2, 5, 6, 7, 9, 10, 11, 13, 14) with a median for total ARs contents less than 10 ng/g in liver of trapped rodents. Two of these are the control sites (i.e., sites 9 and 14) confirming the non-use of ARs in forest/natural areas; six are agricultural sites (i.e.,, sites 2, 5, 6, 7, 10 and 13) and correspond to sites in which rodent populations appear, not surprisingly, to be rather abundant with a trap success greater than 8 rodents per 100 trap-nights, except for site 10; the last is an industrial/commercial site. Sites 1 and 3 seem to use frequently ARs treatment with a median hepatic concentration for total ARs greater than 600 ng/g. However, the rodent population on site 1 still seems abundant with a trap-success of 5 rodents for 100 trap-nights.
The intense use of ARs in Martinique is a factor that may favor the selection of resistant strains due to mutations of the Vkorc1 gene. This resistance has been widely reported in Europe20,29,30,31,32 but also worldwide, in brown rats and house mice and also more recently in black rats24,33,34,35. This study demonstrates that resistance of rodents to ARs is present in Martinique.
Despite completely independent analyses, the same global geographical pattern for mutant distribution in rat and mouse population was observed (Fig. 3B,C) with an increasing occurrence toward the north and the south of the island. A positive correlation was observed between occurrence of AR residues and occurrence of mutant in rats and mice populations suggesting an influence of ARs use. Nevertheless, the geographical pattern for ARs exposition was not similar with a low exposure in South-west to a high exposure to Northeast. (Fig. 3A) This analysis should be performed considering each AR molecule individually. Unfortunately, the limited number of samples did not allow this analysis.
This resistance is evident for the house mouse with the detection of the Y139C mutation in Martinique. This mutation has been reported to induce a very strong resistance to first generation molecules and some second generation molecules such as bromadiolone29 and to some extent to difenacoum based on results obtained in rats36. The allelic frequency of this mutation is 40% in our sampling with 10 homozygous and 21 heterozygous mice. This allelic frequency is much greater than that reported during the resistance study conducted recently in France29. As this mutation is present on all mice trapping sites, the use of appropriate second-generation molecules (i.e., difethialone, brodifacoum or flocoumafen) is required to control mouse populations in Martinique. Nevertheless, these molecules must be used in a carefully way and by professionals informed of the risks related to their use. Moreover, their use could be conditioned by a preliminary genotyping to minimize any ecotoxicity problem.
Other mutations were detected in mice during this study. Some have already been described previously in Europe such as the mutation A26T29, which confers moderate resistance to first-generation ARs and no resistance to second-generation ARs. Others are detected for the first time such as the S149N mutation, which also confers very little resistance to all ARs. These mutations were found with low allelic frequencies. Nevertheless, their presence on sites where the Y139C mutation is very frequently present is responsible for the emergence of a double mutant A26T/Y139C, which is certainly due to genetic recombination between mutated alleles. The emergence of double mutants has been described in France29, but this specific double mutant is described for the first time. The emergence of double mutation confers an evident benefit for mice carrier of these double mutations compared to mice carrier of the corresponding single mutations. While the single A26T or Y139C mutations lead to moderate or severe resistance to first generation ARs, the double mutations lead to resistance to all ARs currently available with resistance factors reaching levels higher than 10 towards difenacoum, brodifacoum and difethialone. Such levels of resistance to such molecules will certainly complicate mice management if double-mutants frequency becomes greater in a species largely associated with leptospirosis transmission (L. interrogans, L. kirschneri, L. borgpetersenii) to humans and domestic animals37,38.
Interestingly, another group of A48T/R61L mutations was also detected in an individual in the homozygous state. These mutations have been described in the introgression of the Spretus mouse Vkorc1 gene into the domestic mouse genome that has occurred in Europe39. This introgression led to the introduction into the genome of the domestic mouse of a group of 4 R12W/A26S/A48T/R61L coding mutations resulting in a very strong resistance to first generation AR. In Europe, mice with this group of 4 mutations are very frequently detected, but also, mice with either one, two, or three of these mutations29,39. Surprisingly only 2 of these 4 mutations were detected on the island of Martinique. The presence of these mutations in Martinique may be due either to point mutations of the Vkorc1 gene or to the importation of introgressed domestic mice from Europe. This last hypothesis should be accompanied by the presence in Martinique of domestic mice with the group of 4 characteristic mutations of the Spretus mouse genome associated with a strong resistance to first generation AR. Any additional conclusions should be based on the search in Martinique for additional traces of the spretus genome
For brown rats, no mutation of Vkorc1 was found in our sample. As the brown rat population appeared to be limited in Martinique, our sampling included only 16 brown rats for which Vkorc1 was sequenced and might not be sufficiently representative. Nevertheless, if mutations of Vkorc1 are present in the brown rat, their frequency might be rather limited contrary to what has been described in Europe20,30. The use of second-generation molecules that are certainly consumed in sufficient quantities by brown rats, in urban as in agricultural, has certainly allowed to avoid the selection of resistant alleles.
For black rats, only 3 coding mutations were detected in the Vkorc1 gene in our sample, the H68N, A115T and S149N mutations. They had never been described in previous studies24,33,34,35. These new mutations seem to induce moderate (resistance factor less than 4) or no resistance to neither the first-generation nor the second-generation molecules. Moreover, these mutations have been found only in the heterozygous state with very low allelic frequencies and never together on a single site. The black rats of Martinique therefore do not seem to present target resistance such as that described in the brown rat or the house mouse. Nevertheless, the black rat being frugivorous, they consume certainly baits in a very limited way. The low degree of resistance induced by the H68N, A115T and Y129N mutations could thus induce an inefficiency or limited efficiency of baits if present in the homozygous state. It is therefore essential to have correct treatment practices to avoid selecting these mutations and thus increasing their allelic frequency.
In conclusion, this study enables the first-time description of rodent resistance to ARs in French West Indies where leptospirosis is an endemic disease so prevalent. This resistance is clearly present with high frequency in house mouse, species which are more and more associated with leptospirosis transmission (L. interrogans, L. kirshneri, L. borgpeterseni) to humans and domestic animals37,38. Mice management in Martinique should be carried out carefully to prevent diffusion of resistance to first and second generation ARs and to avoid selection/emergence of new Vkorc1 mutants or double-mutants. In black rats, even if two new Vkorc1 mutations have been reported in this study, target resistance to ARs does not currently seem an important issue for the management of this species in Martinique. Nevertheless, other resistance mechanisms to ARs due to metabolism modification40,41,42 could exist and this study did not explore the presence and frequency of such mechanisms in rodents of Martinique.
To efficiently control rodents in Martinique in the future, it could be of interest to establish partnerships with AR users in order to build efficient strategies adapted for each population and compatible with biodiversity issues. The use of AR should be reasoned and included in an Integrated Pest Management approach combining mechanical, biological and chemical controls. First-generation ARs should be favored for the management of brown or even black rats to minimize the risk of secondary ecotoxicity because of the absence of Vkorc1-based resistance. On the other hand, the management of domestic mice must be based on a precautionary use of the second-generation ARs because of the high prevalence of the Y139C mutation.
Methods
Ethics statement
This study is part of the integrated program of surveillance, prevention and control of leptospirosis developed by the Regional Health Agencies of Guadeloupe, Martinique and Guyana and the Interregional Epidemiology Unit Antilles-Guyana. Trappings were performed by FREDON Martinique. This study was not an “experimental procedure” as defined by the French legislation (Rural Code, Article R214–89). Therefore, this study was not subject to an ethical committee approval in France. This study complied with the European Directive 2010/63/EU governing the care and use of animals in research. Wild rodents trapped in this study did not belong to endangered or protected species.
Study area
This study took place in Martinique, a French island located in the Caribbean. Thirty percent of this island is cultivated. The north is composed of hills and a volcano and different types of crops (bananas, sugar cane, cassava, pineapple…) are present. The center of the island is more urbanized. The south is tourist and composed of hills, savannas and different types of crops (Fig. 7). Field investigations conducted by FREDON Martinique have identified areas where ARs have been intensively used (ARs sites) to control rodents and in which decreases or losses of efficiency have been reported. Eight agricultural areas (sugar cane, sweet potato, cassava, banana, christophine), 2 intensive breeding sites and 2 industrial and commercial zones were selected based on these investigations for this study. Furthermore, 2 control areas without any AR use (control sites) have been also selected (Table 1 and Fig. 7). All of these sites were homogeneously distributed on Martinique Island (Central, South and North of Martinique).
Trapping methods
Trapping in Martinique began on April 13, 2015 and ended on November 27, 2015 (Table 1). For each site, the trapping time was systematically 5 consecutive days. Depending on the size of the sites, 22 to 65 trapping stations have been set up (Table 1). These stations were homogeneously and consistently distributed on each site. The trapping stations were 10 meters apart and located in places likely to be frequented by rodents (border plots, ecotones, rivers, around livestock buildings, forest trails…). Each station included a Manufrance rat trap and an INRA trap for mice. Manufrance rat trap were baited with peanut butter, oat flakes and sardine oil, checked daily and re-baited, if necessary.
The captured rodents were killed at the trapping site by cervical dislocation, morphologically identified (i.e., Rattus rattus, Rattus norvegicus or Mus musculus), housed in an individual referenced bag and placed in a cooler with ice buns for the transport. Rodents were then kept at −20 °C until the day before their autopsy. For each rodent, a piece of tail for sequencing (1 cm maximum) and the liver to quantify ARs residues were taken and stored respectively in 70% ethanol at 4 °C and at −20 °C until sent to the laboratory.
Rodent species identification
Species of trapped rodents were identified by morphologic aspects during autopsy. Species of trapped animals were molecularly confirmed by sequencing a portion of the mtDNA cytochrome b gene as described by43 by using specific cytb-S (5′-TCTCCATTTCTGGTTTACAAGAC-3′) and cytb-AS (5′-AACAATGACATGAAAAATCATCG TT-3′) primers. The amplified product was sequenced on both strands; the resulting sequence was submitted to blast analysis.
Vkorc1 genotyping
Genomic DNA extracted from tail was amplified using specific primers of vkorc1 gene as described by29,44 to sequence Vkorc1 gene and detect mutations comparatively to the published Vkorc1 sequences for Mus Musculus domesticus (Genbank No. GQ905710.1), Rattus norvegicus (Genbank No. CM000231.2) and Rattus rattus (Genbank No. AB702679.1).
Heterologous expression of new VKORC1 mutants
Construction of Mm VKorc1 or RrVKORC1 mutants was carried out using pPICZ-RrVKORC144 or pPICZ-MmVKORC129 as template with the Quickchange site directed mutagenesis kit (Stratagene) according to the manufacturer’s recommendations. Recombinant VKORC1 proteins were produced by Pichia pastoris as described previously19,29,33,38,44,45. Yeast microsomes were obtained by differential centrifugation, as described previously19,29,33,38,44,45 and frozen at −80 °C until use. Protein concentrations in micrososomal fractions were determined by the method of Bradford46.
Assays of VKORC1 mutant activities and kinetics
Microsomal VKOR activity was assayed as described previously19,29,33,38,44,45. Vitamin K production was determined by liquid chromatography-mass spectrometry.
Determination of AR concentrations in liver
AR concentrations in liver samples were determined by the method described by47. The ARs detected and quantified with this method were warfarin, coumatetralyl, chlorophacinone, bromadiolone, difenacoum, brodifacoum, flocoumafen and difethialone. Limit of quantification was between 1 and 2 ng/g wet weight.
Data analysis
A night trap effort (i.e., number of traps used × number of nights of trapping) was calculated for each site. Trap success (number of trapped rodents/trap effort × 100) was calculated to evaluate relative rodent abundance as described by48.
Ki values were determined in triplicate performed on two different batches of yeasts expressing the recombinant VKORC1 mutant after addition of increasing concentrations of AR (from 0.05 to 20Ki) in the presence of different concentrations of vit K > 0 (from 0.003 to 0.2 mmol/L), as described previously19,29,33,38,44,45. Results were fitted by nonlinear regression to the noncompetitive inhibition model v = (Vmax/(1 + (I/Ki))) * (S/(Km + S)) using the R-fit program.
Statistical analyses were performed using GraphPad Prism 6 software using a Mann-Whitney or a Kruskal-Wallis test.
A Generalized Linear Model as a smoothing model was used to analyze the geographical pattern of ARs exposition and mutant rats/mice distribution. Binomial distribution for the response variable (proportion of rodent with ARs or proportion of mutated rodents) and logit link were used. The coordinates X and Y was used as explanatory variables. When possible, we took into account that 2 plots (sites 9 and 14) was in natural area where AR treatment was unexpected (used as control site) by adding a variable with 2 factors (variable named “Type”, levels; “natural area”, “other”). Because of the limited number of data, it was impossible to accurate the spatial trends with unlinear smoothing methods like Generalized Linear Model, however, we tested if interactions between X coordinates and Y coordinates was relevant. Variable selection was made by AIC procedure.
References
Levett, P. N. Leptospirosis. Clin. Microbiol. Rev. 14, 296–326 (2001).
Adler, B. & de la Peña Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 140, 287–296 (2010).
Costa, F. et al. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl. Trop. Dis. 9, e0003898 (2015).
Hochedez, P. et al. Factors Associated with Severe Leptospirosis, Martinique, 2010–2013. Emerg. Infect. Dis. 21, 2221–2224 (2015).
Herrmann-Storck, C. et al. Severe Leptospirosis in Hospitalized Patients, Guadeloupe. Emerg. Infect. Dis. 16, 331–334 (2010).
Haake, D. A. & Levett, P. N. Leptospirosis in Humans. In: Adler, B., editor. Leptospira and Leptospirosis [Internet]. Berlin, Heidelberg: Springer Berlin Heidelberg; p. 65–97. (Current Topics in Microbiology and Immunology). Available from, https://doi.org/10.1007/978-3-662-45059-8_5 (2015).
Gravekamp, C. et al. Detection of seven species of pathogenic leptospires by PCR using two sets of primers. J. Gen. Microbiol. 139, 1691–1700 (1993).
Rapports d’activité du CNR de la Leptospirose [Internet]. Institut Pasteur. Available from: https://www.pasteur.fr/fr/sante-publique/CNR/les-cnr/leptospirose/rapports-d-activite (2016)
Cassadou, S. et al. Underestimation of Leptospirosis Incidence in the French West Indies. PLoS Negl. Trop. Dis. 10, e0004668 (2016).
Hochedez, P. et al. Outbreak of Leptospirosis after a Race in the Tropical Forest of Martinique. Am. J. Trop. Med. Hyg. 84, 621–626 (2011).
Hochedez, P. et al. Outbreak of leptospirosis among canyoning participants, Martinique, 2011. Eurosurveillance. 18, 20472 (2013).
Stritof Majetic, Z. et al. Epizootiological survey of small mammals as Leptospira spp. reservoirs in Eastern Croatia. Acta. Trop. 131, 111–116 (2014).
Ayral, F. Vers une surveillance des zoonoses associées aux rats (Rattus norvegicus) [Internet] [thesis]. Grenoble Alpes; Available from: http://www.theses.fr/2015GREAS004 (2015).
Suttie, J. W. Vitamin K-dependent carboxylase. Annu. Rev. Biochem. 54, 459–477 (1985).
Furie, B. & Furie, B. C. The molecular basis of blood coagulation. Cell. 53, 505–518 (1988).
Boyle, C. M. Case of Apparent Resistance of Rattus norvegicus Berkenhout to Anticoagulant Poisons. Nature. 188, 517 (1960).
Dodsworth, E. Mice are spreading despite such poisons as warfarin. Minic. Engin. Lond. 3746, 1668 (1961).
Grandemange, A. et al. Consequences of the Y139F Vkorc1 mutation on resistance to AVKs: in-vivo investigation in a 7th generation of congenic Y139F strain of rats. Pharmacogenet. Genomics. 19, 742–750 (2009).
Hodroge, A., Longin-Sauvageon, C., Fourel, I., Benoit, E. & Lattard, V. Biochemical characterization of spontaneous mutants of rat VKORC1 involved in the resistance to antivitamin K anticoagulants. Arch. Biochem. Biophys. 515, 14–20 (2011).
Pelz, H.-J. The Genetic Basis of Resistance to Anticoagulants in Rodents. Genetics. 170, 1839–1847 (2005).
Rost, S. et al. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 427, 537–541 (2004).
Jackson, W. B. & Kaukeinen, D. Resistance of wild Norway rats in North Carolina to warfarin rodenticide. Science. 176, 1343–1344 (1972).
Siddiq, Z. & Blaine, W. D. Anticoagulant resistance in house mice in Toronto, Canada. Environ. Health. Rev. 2, 49–51 (1982).
Tanaka, K. D. et al. The genetic mechanisms of warfarin resistance in Rattus rattus found in the wild in Japan. Pestic. Biochem. Physiol. 103, 144–151 (2012).
Saunders, G. R. Resistance to warfarin in the roof rat in Sydney, NSW. Search. 9, 39–40 (1978).
Desvars, A., Cardinale, E. & Michault, A. Animal leptospirosis in small tropical areas. Epidemiol. Infect. 139, 167 (2011).
Bourhy, P. et al. Serovar Diversity of Pathogenic Leptospira Circulating in the French West Indies. PLoS. Negl. Trop. Dis. 14, 7 (2013).
Desvars-Larrive, A. et al. Population genetics, community of parasites, and resistance to rodenticides in an urban brown rat (Rattus norvegicus) population. PloS One. 12, e0184015 (2017).
Goulois, J., Lambert, V., Legros, L., Benoit, E. & Lattard, V. Adaptative evolution of the Vkorc1 gene in Mus musculus domesticus is influenced by the selective pressure of anticoagulant rodenticides. Ecol. Evol. 7, 2767–2776 (2017).
Grandemange, A., Lasseur, R., Longin-Sauvageon, C., Benoit, E. & Berny, P. Distribution of VKORC1 single nucleotide polymorphism in wild Rattus norvegicus in France. Pest. Manag. Sci. 66, 270–276 (2010).
Buckle, A. P., Klemann, N. & Prescott, C. V. Brodifacoum is effective against Norway rats (Rattus norvegicus) in a tyrosine139cysteine focus of anticoagulant resistance in Westphalia, Germany. Pest. Manag. Sci. 68, 1579–1585 (2012).
Meerburg, B. G., van Gent-Pelzer, M. P., Schoelitsz, B. & Van der Lee, T. A. Distribution of anticoagulant rodenticide resistance in Rattus norvegicus in the Netherlands according to Vkorc1 mutations. Pest. Manag. Sci. 70, 1761–1766 (2014).
Goulois, J. et al. Study of the efficiency of anticoagulant rodenticides to control Mus musculus domesticus introgressed with Mus spretus Vkorc1. Pest. Manag. Sci. 73, 325–331 (2017).
Díaz, J. C., Song, Y., Moore, A., Borchert, J. N. & Kohn, M. H. Analysis of vkorc1 polymorphisms in Norway rats using the roof rat as outgroup. BMC Genet. 11, 43 (2010).
Cowan, P. E. et al. Vkorc1 sequencing suggests anticoagulant resistance in rats in New Zealand. Pest. Manag. Sci. 73, 262–266 (2017).
Buckle, A., Endepols, S., Klemann, N. & Jacob, J. Resistance testing and the effectiveness of difenacoum against Norway rats (Rattus norvegicus) in a tyrosine139cysteine focus of anticoagulant resistance, Westphalia, Germany. Pest. Manag. Sci. 69, 233–239 (2013).
Moseley, M. et al. Mixed leptospira infections in a diverse reservoir host community, Madagascar, 2013–2015. Emerg. Infect. Dis. 24, 1138–1140 (2018).
Marquez, A., Ulivieri, T., Benoit, E., Kodjo, A. & Lattard, V. House mice as a real sanitary threat for human and animal leptospirosis: proposal for integrated management. Biomed. Res. Intern. (In Press).
Song, Y. et al. Adaptive introgression of anticoagulant rodent poison resistance by hybridization between old world mice., Adaptive introgression of anticoagulant rodent poison resistance by hybridization between old world mice. Curr. Biol. 21, R581–583 (2011).
Sugano, S. et al. Suppression of CYP3A2 mRNA expression in the warfarin-resistant roof rat, Rattus rattus: possible involvement of cytochrome P450 in the warfarin resistance mechanism. Xenobiotica. 31, 399–407 (2001).
Ishizuka, M. et al. Elevated Warfarin Metabolism in Warfarin-Resistant Roof Rats (Rattus rattus) in Tokyo. Drug. Metab. Dispos. 35, 62–66 (2007).
Boitet, M. et al. Elevated difenacoum metabolism involved in the difenacoum-resistant phenotype observed in Berkshire rats homozygous for the L120Q mutation in Vkorc1 gene. Pest. Manag. Sci. 74, 1328–1334 (2017).
Pagès, M. et al. Revisiting the taxonomy of the Rattini tribe: a phylogeny-based delimitation of species boundaries. BMC Evol. Biol. 10, 184 (2010).
Goulois, J. et al. Evidence of a target resistance to antivitamin K rodenticides in the roof rat Rattus rattus: identification and characterization of a novel Y25F mutation in the Vkorc1 gene Pest. Manag. Sci. 72, 544–550 (2016).
Hodroge, A. et al. VKORC1 mutations detected in patients resistant to vitamin K antagonists are not all associated with a resistant VKOR activity. J. Thromb. Haemost. 10, 2535–2543 (2012).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Fourel, I., Damin-Pernik, M., Benoit, E. & Lattard, V. Core-shell LC-MS/MS method for quantification of second-generation anticoagulant rodenticides diastereoisomers in rat liver in relationship with exposure of wild rats. J Chromatogr B Analyt Technol Biomed Life Sci. 1041-1042, 120–132 (2017).
Theuerkauf, J., Rouys, S., Jourdan, H. & Gula, R. Efficiency of a New Reverse-Bait Trigger Snap Trap for Invasive Rats and a New Standardised Abundance Index. Ann. Zool. Fenn. 48, 308–318 (2011).
Author information
Authors and Affiliations
Contributions
A.M., T.O., V.L. participated in research design; A.M., R.A.K., I.F., T.O., E.B., V.L. conducted experiments; A.M., R.A.K., I.F., T.O., A.P., E.B., V.L. performed data analysis; A.M., R.A.K., I.F., T.O., A.P., R.A., G.T., G.J., A.K., E.B., V.L. wrote or contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marquez, A., Khalil, R.A., Fourel, I. et al. Resistance to anticoagulant rodenticides in Martinique could lead to inefficient rodent control in a context of endemic leptospirosis. Sci Rep 9, 13491 (2019). https://doi.org/10.1038/s41598-019-49661-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49661-5
This article is cited by
-
A One Health approach to the prevention, control, and management of leptospirosis: a scoping review
Discover Public Health (2025)
-
Widespread anticoagulant resistance in house mice (Mus musculus musculus) linked to the Tyr139Phe mutation in the Czech Republic
Scientific Reports (2025)