Abstract
The current study describes synthesis of diindolylmethane (DIM) derivatives based-thiadiazole as a new class of urease inhibitors. Diindolylmethane is natural product alkaloid reported to use in medicinal chemistry extensively. Diindolylmethane-based-thiadiazole analogs (1–18) were synthesized and characterized by various spectroscopic techniques 1HNMR, 13C-NMR, EI-MS and evaluated for urease (jack bean urease) inhibitory potential. All compounds showed excellent to moderate inhibitory potential having IC50 value within the range of 0.50 ± 0.01 to 33.20 ± 1.20 µM compared with the standard thiourea (21.60 ± 0.70 µM). Compound 8 (IC50 = 0.50 ± 0.01 µM) was the most potent inhibitor amongst all derivatives. Structure-activity relationships have been established for all compounds. The key binding interactions of most active compounds with enzyme were confirmed through molecular docking studies.
Similar content being viewed by others
Introduction
Urease (EC 3.5.1.5) belongs to the family of amidohydrolase enzymes having two nickel atoms in their core structure. Urease involves the conversion of urea into ammonia and carbon dioxide or carboamate1. Among the superfamily of bi-nuclear metallohydrolases, urease is quite different from having Ni (II) ions in their active site. Urease is broadly found in nature and bio-synthesized by different organisms such as plants, fungi, bacteria, invertebrates, algae and are found in soil as soil enzyme2,3. Urease also plays a vital role in the germination process of plants and involved in nitrogen metabolism4. The fertilization of urea creates a major increase in the pH which is happened when the high amount is released resulting in increasing the alkalinity of soil which leads to high damage of plants by depriving them of their necessary nutrients5,6. Ureases plays an important role in most of the pathogenic processes in humans and animals. It plays a great role in peptic ulceration, pyelonephritis, arthritis, urolithiasis, kidney stone, urinary catheter and encephalopathy7,8,9.
The indole having great importance due to its unique chemical structure and a wide range of biological properties10. Mostly C-3-substituted indoles play an important role in many building blocks for the synthesis of different biologically active compounds having antimalarial11, inhibitors of HIV-112, antimicrobial13,14, antileishmanial15, Urease inhibitors16, cytotoxic17, inhibitors of hepatitis C virus18, anti-diabetic19 and neuroprotective activities20. N-1 and C-3 substituted analogs of indole also showed anti-inflammatory, anti-nociceptive, anti-cancer, antioxidant and anti-psychotic activities21,22,23,24,25,26,27,28. Marine indole alkaloids have appeared as an important structural class showing anti-microbial, antitumor and anti-viral activity29,30,31. Some bis-Indole alkaloids with great biological importance have been collected from invertebrates such as bryozoans, tunicates, coelenterates, and sponges32,33,34,35. Five-membered nitrogen-containing heterocyclic compounds such as triazole and thiadiazole have great importance in medicinal chemistry because of their wide range of biological activities like antimicrobial, antifungal, antibacterial, antitumor, antiurease and antilipase36,37,38,39,40,41. Among different heterocycles, 1,2,4-triazole-3-thiones are important pharmacophores for urease inhibition because of their structural similarity to urea. Some 1,2,4-triazole derivatives were reported as potential urease inhibitors42,43.
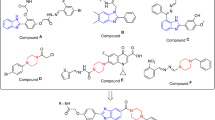
Our research group have already reported thiazole, flavones, triazinoindole, isatin, benzimidazole, biscoumarin, and oxadiazole analogs as potential α-glucosidase inhibitors44,45,46,47,48,49,50,51,52,53. We have also reported thiadiazole analogs as potent antileishmanial, α-glucosidase and urease inhibitors shown in Fig. 154,55,56. In this study, we have synthesized different substituted indole bearing thiadiazole analogs keeping in view their biological importance with the hope that it may show greater potential. Experimental results proved our previous hypothesis by obtaining good urease inhibitory potential of our synthesized molecules.
Results and discussion
Chemistry
The synthesis of diindolylmethane derivatives (1-18) was carried out in three steps. In the first step, 2 equivalents of indole were mixed with-4-formylbenzoic acid in acetic acid and reflux for 4–6 hours to afford intermediate product I. The Intermediate I was then treated with thiosemicarbazide in POCl3, then reflux for 3–4 hrs. to obtained intermediate I. The intermediate II was then mixed with different Aryl sulfonyl chloride to get the pure products (1–18) in high yield. All reactions completion was monitored by TLC.
Urease activity
New series of diindolylmethane-based-thiadiazole analogs (1–18) synthesised and evaluated for their in vitro urease (jack bean urease) inhibitory activity. All the derivatives exhibited urease potential with IC50 value ranging between0.50 ± 0.01 to 33.20 ± 1.20 µM as compared to the standard thiourea (21.60 ± 0.70 µM). Among the synthesised compounds, fifteen derivatives 8, 6, 3, 9, 10, 2, 4, 7, 15, 13, 12, 1, 14 and 18 with IC50 values 0.50 ± 0.01, 0.70 ± 0.01, 1.10 ± 0.01, 1.10 ± 0.01, 1.6 ± 0.01, 1.80 ± 0.01, 2.20 ± 0.10, 2.30 ± 0.10, 3.90 ± 0.10, 5.10 ± 0.10, 8.50 ± 0.30, 13.20 ± 0.30, 19.80 ± 0.80 and 20.40 ± 1.20 respectively, which is better than the standard thiourea.
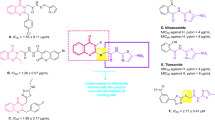
The most active compound among the series is analog 8 (IC50 = 0.50 ± 0.01 µM) having two-nitro groups at ortho and para position on phenyl ring. The greater inhibition shown by this compound is seemed due to electron-withdrawing group on phenyl ring. The second most active analog among the series is compound 6 (IC50 = 0.70 ± 0.01 µM) having three chloro groups on phenyl ring at 2,4,5-position. The greater potential of this analog is also seeming due to having EWG at phenyl ring.
If we compare analog 8 (IC50 = 0.50 ± 0.01 µM) with other nitro group containing analogs like 2, a para nitro analog (IC50 = 1.80 ± 0.01 µM) 3, a ortho nitro analog 13, (IC50 = 1.10 ± 0.01 µM) ortho nitro meta methyl analog (IC50 = 5.10 ± 0.10 µM) and 15, a ortho nitro meta methoxy analog (IC50 = 3.90 ± 0.10 µM), the compound 8 is superior. The difference in the inhibitory potential of all these analogs are seemed due to the difference in number and nature of substituents on phenyl ring.
The analog 7 (IC50 = 2.30 ± 0.10 µM) and analog 10 (IC50 = 1.6 ± 0.01 µM) having di-chloro groups at 2,4 and 3,4 position respectively when compare with analog 6 (IC50 = 0.70 ± 0.01 µM) having tri-chloro groups shows more potency as compared to analog 7 and 10. The difference in their activity seems due to substituents different numbers and positions on phenyl ring.
Similarly the decline in inhibition was observed when EWG is replaced by methyl group as shown in analog 16 (IC50 = 23.80 ± 1.00) having methyl at the ortho position of phenyl ring with analog 17 (IC50 = 28.60 ± 1.20) having methyl at meta position and analog 18 (IC50 = 20.40 ± 1.20) having methyl at the para position. All of the three analogs contain methyl groups attached at different positions showed a different kind of inhibition, which might be due to attachment of substituents at a different position on phenyl ring. In the current study, we have found that inhibitory potential was greatly affected by the nature, position, and number of substituents. All those analogs having electron-withdrawing groups (EWG) on phenyl ring showed greater potential as compared to those analogs having electron-donating groups (EDG). The binding interaction was confirmed through molecular docking studies.
Molecular docking
The IC50 values diindolylmethane bearing thiadiazol derivatives as a potent urease inhibitor are presented in Table 1. The urease inhibition by the synthesized derivatives may strongly related to the type, number, positions of the functional group in the aromatic ring of basic skeleton of diindolylmethane bearing thiadiazol derivatives and to the strength of the intermolecular interaction that may have formed these functional groups and the residues of the active of urease (Table 1). To understand the urease inhibition by the synthesized derivatives, a molecular docking study has been carried out to determine the binding modes of all synthesized derivatives 1–18 from one side and the active residues of the urease from another side. These compounds differ by the number and position of the substituted functional groups in the aromatic ring (Table 1). For instance, compounds 2, 3 and 10 are substituted by a mono nitro in the group in para and ortho positions, and di-nitro groups in ortho and para positions, respectively (Table 1). Compounds 6, 7 and 10 also differ by the number and positions of substituted chloro groups (Table 1). 16–18 are monosubstituted by a methyl group at ortho, meta and para positions respectively (Table 1). Table 2 summarized the calculated binding energies of the stable complexes ligand-urease, the number of established intermolecular hydrogen bonding between the synthesized compounds (1–18) and active site residues of urease.
The formed complexes between diindolylmethane bearing thiadiazol derivatives (1–18) and the active residues of urease displayed negative bending energies, which indicates that the inhibition of urease by the selected diindolylmethane bearing thiadiazol derivatives is thermodynamically favorable (Table 2). From the docking results in Table 2, binding energies of the stable complexes vary slightly from −10.55 to −8.20 kcal/mol. Such variation is low enough to be considered as a potent descriptor in rationalizing the observed inhibition of urease by the selected derivatives. However, the number of hydrogen bonding, its distances and intermolecular interactions between the substitute groups of the selected derivatives and the active residues may strongly help in understanding the observed urease inhibition by these selected compounds. For instance, the higher urease inhibition of 8 compared with 2 and 3 may refer to the number of hydrogen bonding formed between the nitro groups in the former and latter (Fig. 2). Indeed, in the 8-urease complex two hydrogen bonds are formed between the nitro groups at ortho and para positions with ARG 366 and VAL 367 amino acids of distances 2.46 and 2.93 Å, respectively. While in 2-urease and 3-urease complexes, the hydrogen bonds are formed between the nitro group at para (2) and ortho (3) positions with ARG 336 amino acid of distances 2.76 and 2.67 Å, respectively. The higher urease inhibition of 3 compared with 2 may also refer to the stronger hydrogen bond formed with the former (2.76Å) compared with the latter (2.67 Å).
Similarly, the higher urease inhibition of 6 compared with 7 and 10 may refer to the number of residues that interact with chloro groups in the former and to the strength of these interactions (Table 2).
The diindolylmethane bearing thiadiazol derivatives monosubstituted with chlorine (6–7,10), nitro (2–3,8), or disubstituted with functional groups (chlorine, nitro, hydroxyl, methoxy, and bromine) showed higher urease inhibition than those monosubstituted with methyl (16–18) and benzene ring (11). The significant decrease of urease inhibition in 16–18 and 11 may refer to the fact that these groups are not involved in intermolecular interactions with the closest residues of urease (16–18) or too weak interactions in case of 11 (Fig. 3).
Conclusion
We synthesized eighteen analogs (1–18) of diindolylmethane-based-thiadiazole (1–18) and evaluated against urease inhibitory potential. All analogs showed excellent to a good inhibitory potential having IC50 ranging from IC50 = 0.50 ± 0.01 to 33.20 ± 1.20 µM) as compared to the standard thiourea (21.60 ± 0.70 µM). Analog 8 (IC50 value 0.50 ± 0.01 µM) is the most potent among the series. The structure-activity relationship was mainly based upon by bringing about the difference of substituents on phenyl rings. For binding interaction of the most active analogs molecular docking study was performed.
Experimental
Material and methods
NMR experiment was carried out on Avance Bruker AM 500 MHz (Wissembourg, Switzerland). TLC was done on pre-coated silica gel aluminum plates (Kieselgel 60, 254, E. Merck, Darmstadt, Germany). Chromatograms were envisioned through UV at 254 and 365 nm.
Synthesis of 4-(bis(5-bromo-1H-indol-3-yl)methyl)benzoic acid (I)
A mixture of 5-bromo-1H-indole (9.75 g, 50 mmol), 4-formylbenzoic acid (3.75 g, 25 mmol) and a catalytic amount of acetic acid in methanol (50 mL) was heated under reflux for 6 hours.
The mixture was dried and the crude product (I) was washed with diethyl ether, then crystallized from methanol and gives pinkish solid, (11.2 g, 90.0%); Rƒ. 0.46 (ethylecetate/hexane 4:6); m.p. 288–289 °C; IR (KBr): 3530–2570 cm−1 br. (COOH-str), 1680 cm−1 (C=0 str), 1617 cm−1 (Ar C=C), 1146 cm−1 (C-O str), 630 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ 11.90 (s, 2H, NH), 7.92–7.86 (m, 4H), 7.72 (t, J = 7.8 Hz, 2H), 7.44 (d, J = 7.7 Hz, 2H), 7.40 (s, 2H), 7.32 (td, J = 7.5, 2.0 Hz, 2H), 5.73 (s, 1H), 4.08 (s, 1H, OH); 13C-NMR (150 MHz, DMSO-d6,): δ 169.3, 143.1, 135.2, 135.2, 130.1, 130.1, 129.5, 129.5, 128.9, 128.9, 126.9, 122.7, 122.7, 121.0, 121.0, 120.9, 120.9, 120.7, 120.7, 117.1, 117.1, 113.2, 113.2, 55.1; HREI-MS: m/z calcd for C24H16Br2N2O2 [M + 4]+ 525.9520, [M + 3]+ 524.9580, [M + 2]+ 523.9548, [M + 1]+ 522.9605, [M]+ 521.9560.
Synthesis of 5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-amine
The 4-(bis(5-bromo-1H-indol-3-yl)methyl)benzoic acid (20 mmol) was heated under reflux with thiosemicarbazide (21mmole) in POCl3 for 6 hours. The completion of reaction was monitored by TLC. The mixture of reaction was poured in cold water. The precipitate formed was washed with dilute sodium bicarbonate solutions and recrystallized in ethanol to get pure compound (II).
Yellow solid (11.2 g, 90.0%); Rƒ. 0.60 (ethylecetate/hexane 4:6); m.p. 288–289 °C; IR (KBr): 3420 cm−1 (NH-str), 3230 cm−1 (2°amine N-H Str), 1615 cm−1 (Ar C=C), 1351 cm−1 (N-S=O), 626 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ 11.96 (s, 2H, NH), 7.90–7.85 (m, 4H), 7.71 (t, J = 7.6 Hz, 2H), 7.43 (d, J = 7.5 Hz, 2H), 7.42 (s, 2H), 7.31 (t, J = 7.7 Hz, 2H), 5.72 (s, 1H), 4.26 (s, 2H, NH2); 13C-NMR (150 MHz, DMSO-d6,): δ 175.3, 161.2, 143.2, 135.3, 135.3, 130.2, 130.2, 129.4, 129.4, 128.8, 128.8, 126.8, 122.6, 122.6, 121.2, 121.2, 120.8, 120.8, 120.6, 120.6, 117.2, 117.2, 113.1, 113.1, 55.3; HREI-MS: m/z calcd for C25H17Br2N5S [M + 4]+ 580.9520, [M + 3]+ 579.9575, [M + 2]+ 578.9542, [M + 1]+ 577.9601, [M]+ 576.9553.
General procedure for the synthesis of diindolylmethane-based-thiadiazole analogs Characterization (1–18)
The intermediate (II) was treated with different aryl sulfonyl chloride in the presence of pyridine under stirring for overnight. After completion of reaction the solvent was removed and the crude product was washed with cold water, filtered and dried. The crude product was recrystallized from ethanol. NMR spectra of all 1-18 compounds are provided in supplementary data.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)benzenesulfonamide (1)
Orange solid. Yield: 81.40%; Rƒ. 0.70 (ethylecetate/hexane 3:7); m.p.: 301–302 °C; IR (KBr): 3255 cm−1 (2°amine N-H Str), 1612 cm−1 (Ar C=C), 1360 cm−1 (N-S=O), 638 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ 11.98 (s, 1H, NH), 11.52 (s, 1H, NH), 11.17 (s, 1H, NH), 7.81 (d, J = 7.4 Hz, 2H), 7.50 (t, J = 7.2 Hz, 2H), 7.41 (d, J = 7.2 Hz, 2H), 7.40 (d, J = 8.4 Hz, 2H), 7.31–7.27 (m, 3H), 7.11 (t, J = 7.2 Hz, 2H), 6.94 (t, J = 7.2 Hz, 2H), 6.85 (s, 2H), 5.98 (s, 1H, CH);13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 139.7, 138.1, 135.5, 135.5, 131.9, 130.5, 129.6, 129.6, 129.0, 129.0, 129.5, 129.5, 127.4, 127.4, 127.3, 127.3, 124.7, 124.7, 123.0, 123.0, 121.0, 121.0, 116.4, 116.4, 113.2, 113.2, 112.1, 112.1, 54.6: HREI-MS: m/z Calcd for C31H21Br2N5O2S2 [M + 4]+ 720.9455, [M + 3]+ 719.9511, [M + 2]+ 718.9477, [M + 1]+ 717.9530, [M]+ 716.9496.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-4-nitrobenzenesulfonamide (2)
Yellow. Yield: 80.0%; Rƒ. 0.56 (ethylecetate/hexane 3:7); m.p.: 312–313 °C; IR (KBr): 3242 cm−1 (2°amine N-H Str), 1625 cm−1 (Ar C=C), 1556 cm−1 (N-O str), 1355 cm−1 (N-S=O), 630 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ12.42 (s, 1H, NH), 12.01 (s, 1H, NH), 10.98 (s, 1H, NH), 8.01 (d, J = 7.7 Hz, 2H), 7.93 (d, J = 7.7 Hz, 2H), 7.52 (d, J = 8.2 Hz, 2H), 7.41 (d, J = 8.2 Hz, 2H), 7.32 (d, J = 7.6 Hz, 2H), 7.13 (t, J = 7.4 Hz, 2H), 6.91 (t, J = 7.4 Hz, 2H), 6.86 (s, 2H), 5.98 (s, 1H); 13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 151.1, 146.3, 145.8, 142.3, 136.8, 136.5, 135.2, 131.8, 130.5, 130.3, 129.5, 129.5, 128.2, 128.2, 127.4, 127.4, 127.4, 124.2, 124.2, 121.7, 121.7, 120.0, 119.8, 118.0, 111.2, 111.1, 47.3, 13.3: HREI-MS: m/z Calcd for C31H20Br2N6O4S2 [M + 4]+ 765.9302, [M + 3]+ 764.9352, [M + 2]+ 763.9321, [M + 1]+ 762.9379, [M]+ 761.9342.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-2-nitrobenzenesulfonamide (3)
Light yellow, Yield: 78.0%; Rƒ. 0.61 (ethylecetate/hexane 3:7); m.p.: 314–315 °C; IR (KBr): 3246 cm−1 (2°amine N-H Str), 1621 cm−1 (Ar C=C), 1552 cm−1 (N-O str), 1349 cm−1 (N-S=O), 632 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ12.09 (s, 1H, NH), 11.32 (s, 1H, NH), 8.72 (s, 1H, NH), 8.11 (d, J = 7.6 Hz, 2H), 7.92 (t, J = 8.2 Hz, 2H), 7.82 (t, J = 7.5 Hz, 2H), 7.68–7.64 (m, 2H), 7.51 (d, J = 8.3 Hz, 2H), 7.38 (d, J = 8.3 Hz, 2H), 7.32 (d, J = 7.7 Hz, 2H), 6.94 (t, J = 7.1 Hz, 2H), 6.87 (s, 2H), 5.98 (s, 1H);13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 147.2, 138.1, 135.5, 135.5, 135.1, 134.4, 132.8, 130.5, 129.6, 129.6, 129.5, 129.5, 128.2, 127.4, 127.4, 124.7, 124.7, 124.2, 123.0, 123.0, 121.0, 121.0, 116.4, 116.4, 113.2, 113.2, 112.1, 112.1, 54.6: HREI-MS: m/z Calcd for C31H20Br2N6O4S2 [M + 4]+ 765.9306, [M + 3]+ 764.9355, [M + 2]+ 763.9324, [M + 1]+ 762.9373, [M]+ 761.9347.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-2-hydroxy-5-methoxybenzenesulfonamide (4)
Orange solid. Yield: 82.0%; Rƒ. 0.55 (ethylecetate/hexane 3:7); m.p.: 298–297 °C; IR (KBr): 3470 cm−1 (OH-str), 3210 cm−1 (2°amine N-H Str), 1345 cm−1 (N-S=O), 1148 cm−1 (C-O str), 624 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): 11.88 (s, 1H, NH), 10.52 (s, 1H, NH), 9.58 (s, 1H, NH), 9.21(s, 1H, OH), 7.80 (d, J = 8.1 Hz, 2H), 7.44 (d, J = 8.1 Hz, 2H), 7.37 (d, J = 8.1 Hz, 2H), 7.31 (d, J = 7.4 Hz, 2H), 7.11 (t, J = 7.4 Hz, 2H), 6.93–6.90 (m, 3H), 6.78 (d, J = 8.3 Hz, 2H), 5.96 (s, 1H), 3.80 (s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 152.9, 151.9, 138.1, 135.5, 135.5, 130.5, 129.6, 129.6, 129.5, 129.5, 127.4, 127.4, 124.7, 124.7, 123.0, 123.0, 122.2, 121.0, 121.0, 121.0, 118.9, 116.4, 116.4, 113.2, 113.2, 112.6, 112.1, 112.1, 55.8, 54.6: HREI-MS: m/z Calcd for C32H23Br2N5O4S2 [M + 4]+ 766.9510, [M + 3]+ 765.9562, [M + 2]+ 764.9530, [M + 1]+ 763.9582, [M]+ 762.952.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-5-chloro-2-methoxybenzenesulfonamide (5)
Organge solid. Yield: 80.0%; Rƒ. 0.68 (ethylecetate/hexane 3:7); m.p.: 315–316 °C; IR (KBr): 3235 cm−1 (2°amine N-H Str), 1619 cm−1 (Ar C=C), 1350 cm−1 (N-S=O), 1122 cm−1 (C-O str), 843 cm−1 (C-Cl str), 616 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ11.84(s, 2H, NH), 10.98 (s, 1H, NH), 7.80 (d, J = 7.5 Hz, 2H), 7.63 (d, J = 7.6 Hz, 1H), 7.51–7.45 (m, 2H), 7.38 (d, J = 8.3 Hz, 2H), 7.31(d, J = 7.7 Hz, 2H), 7.13 (t, J = 7.4 Hz, 2H), 6.93 (t, J = 7.4 Hz, 2H), 6.86 (s, 2H), 5.97 (s, 1H), 3.75 (s, 3H, OCH3); 13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 155.8, 138.1, 135.5, 135.5, 134.1, 130.5, 129.6, 129.6, 129.5, 129.5, 127.6, 127.4, 127.4, 126.9, 124.7, 124.7, 123.0, 123.0, 121.0, 121.0, 121.0, 116.4, 116.4, 116.0, 113.2, 113.2, 112.1, 112.1, 55.8, 54.6: HREI-MS: m/z Calcd for C32H22Br2ClN5O3S2 [M + 4]+ 784.9165, [M + 3]+ 783.9225, [M + 2]+ 782.9186, [M + 1]+ 781.9242, [M]+ 780.9205.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-2,4,5-trichlorobenzenesulfonamide (6)
Brown. Yield: 78.0%; Rƒ. 0.70 (ethylecetate/hexane 3:7); m.p.: 299–300 °C; IR (KBr): 3231 cm−1 (2°amine N-H Str), 1630 cm−1 (Ar C=C), 1356 cm−1 (N-S=O), 820 cm−1 (C-Cl str), 621 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ11.71(s, 1H, NH), 10.68 (s, 1H, NH), 9.53 (s, 1H, NH),7.80 (d, J = 8.4 Hz, 2H), 7.50 (d, J = 8.4 Hz, 2H), 7.40 (d, J = 8.2 Hz, 2H), 7.33 (d, J = 7.4 Hz, 2H), 6.91 (t, J = 7.2 Hz, 2H), 6.85 (s, 2H), 6.81 (s, 1H), 6.30 (s, 1H), 5.96 (s, 1H); 13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 139.2, 138.1, 138.0, 135.5, 135.5, 132.1, 131.8, 131.0, 130.5, 129.6, 129.6, 129.5, 129.5, 129.4, 127.4, 127.4, 124.7, 124.7, 123.0, 123.0, 121.0, 121.0, 116.4, 116.4, 113.2, 113.2, 112.1, 112.1, 54.6: HREI-MS: m/z Calcd for C31H18Br2Cl3N5O2S2 [M + 6]+ 824.8246, [M + 5]+ 823.8316, [M + 4]+ 822.8288, [M + 3]+ 821.8332, [M + 2]+ 820.8306,[M + 1]+ 819.8358 [M + ]+ 818.8320.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-2,4-dichlorobenzenesulfonamide (7)
Brown solid. Yield: 76.0%; Rƒ. 0.71 (ethylecetate/hexane 3:7); m.p.: 296–295 °C IR (KBr): 3224 cm−1 (2°amine N-H Str), 1624 cm−1 (Ar C=C), 1364 cm−1 (N-S=O), 790 cm−1 (C-Cl str), 643 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ 11.79(s, 1H, NH), 11.52 (s, 1H, NH), 9.88 (s, 1H, NH), 7.80 (d, J = 7.4 Hz, 2H), 7.49 (d, J = 8.3 Hz, 2H), 7.38 (d, J = 8.1 Hz, 2H), 7.30 (d, J = 7.7 Hz, 2H), 7.26 (d, J = 8.3 Hz, 1H), 6.92 (t, J = 7.2 Hz, 2H), 6.85 (s, 2H), 6.32 (d, J = 8.1 Hz, 1H), 6.31 (s, 1H), 5.97 (s, 1H); 13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 138.9, 138.1, 137.8, 135.5, 135.5, 132.9, 130.7, 130.5, 130.1, 129.6, 129.6, 129.5, 129.5, 127.4, 127.2, 124.7, 124.7, 127.4, 123.0, 123.0, 121.0, 121.0, 116.4, 116.4, 113.2, 113.2, 112.1, 112.1, 54.6: HREI-MS: m/z Calcd for C31H19Br2Cl2N5O2S2 [M + 6]+ 790.8644, [M + 5]+ 789.8701, [M + 4]+ 788.8661, [M + 3]+ 787.8722, [M + 2]+ 786.8698, [M + 1]+ 785.8742 [M + ]+ 784.8718.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-2,4-dinitrobenzenesulfonamide (8)
Orange solid, Yield: 83.0%; Rƒ. 0.50 (ethylecetate/hexane 3:7); m.p.: 315–314 °C; IR (KBr): 3242 cm−1 (2°amine N-H Str), 1610 cm−1 (Ar C=C), 1565 cm−1 (N-O str), 1358 cm−1 (N-S=O), 630 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ 11.91 (s, 1H, NH), 11.62 (s, 1H, NH), 8.51 (s, 1H, NH), 7.85 (d, J = 6.8 Hz, 2H), 7.50 (d, J = 6.8 Hz, 2H), 7.38 (d, J = 8.3 Hz, 2H), 7.30 (d, J = 7.4 Hz, 2H), 6.92 (t, J = 7.5 Hz, 2H), 6.87 (s, 1H), 6.84 (d, J = 7.3 Hz, 1H), 6.72 (t, J = 7.5 Hz, 1H), 5.94 (s, 1H); 13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 152.0, 148.1, 140.5, 138.1, 135.5, 135.5, 130.5, 130.3, 129.5, 129.5, 129.1, 127.4, 127.4, 124.7, 124.7, 123.0, 123.0, 121.0, 121.0, 116.4, 116.4, 114.5, 113.2, 113.2, 112.1, 112.1, 54.6: HREI-MS: m/z Calcd for C31H19Br2N7O6S2 [M + 4]+ 810.9155, [M + 3]+ 809.9205, [M + 2]+ 808.9176, [M + 1]+ 806.9195 [M + ]+ 806.9198.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-3-chloro-4-nitrobenzenesulfonamide (9)
Red solid. Yield: 79.0%; Rƒ. 0.58 (ethylecetate/hexane 3:7); m.p.: 318–319 °C; IR (KBr): 3210 cm−1 (2°amine N-H Str), 1617 cm−1 (Ar C=C), 1568 cm−1 (N-O str), 1365 cm−1 (N-S=O), 792 cm−1 (C-Cl str), 657 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ11.46(s, 1H, NH), 9.47 (s, 1H, NH), 9.22 (s, 1H, NH), 7.83 (d, J = 8.4 Hz, 2H), 7.47 (d, J = 8.4 Hz, 2H), 7.36 (d, J = 8.1 Hz, 2H), 7.32 (d, J = 7.6 Hz, 2H), 7.21 (s, 1H), 6.96–6.92 (m, 3H), 6.85 (s, 2H), 6.76 (d, J = 8.3 Hz, 1H), 5.96 (s, 1H);13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 150.8, 147.2, 138.1, 135.5, 135.5, 130.5, 129.7, 129.6, 129.6, 129.5, 129.5, 127.5, 127.4, 127.4, 126.3, 125.6, 124.7, 124.7, 123.0, 123.0, 121.0, 121.0, 116.4, 116.4, 113.2, 113.2, 112.1, 112.1, 54.6: HREI-MS: m/z Calcd for C31H19Br2ClN6O4S2 [M + 4]+ 799.8902, [M + 3]+ 798.8969, [M + 2]+ 797.8932, [M + 1]+ 796.8986 [M]+ 795.8954.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-3,4-dichlorobenzenesulfonamide (10)
Brown solid. Yield: 80.0%; Rƒ. 0.70 (ethylecetate/hexane 3:7); m.p.: 302–303 °C; IR (KBr): 3250 cm−1 (2°amine N-H Str), 1616 cm−1 (Ar C=C), 1355 cm−1 (N-S=O), 798 cm−1 (C-Cl str), 634 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ 11.62(s, 1H, NH), 9.81 (s, 1H, NH), 9.70 (s, 1H, NH), 7.83 (d, J = 8.4 Hz, 2H),7.81 (d, J = 8.1 Hz, 2H), 7.48 (d, J = 8.1 Hz, 2H), 7.32–7.27 (m, 4H), 6.93 (t, J = 7.5 Hz, 2H), 6.87 (s, 2H), 6.57 (s, 2H), 6.24 (s, 1H), 5.94 (s, 1H); 13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 139.2, 138.1, 136.6, 135.5, 135.5, 133.7, 130.5, 130.5, 129.6, 129.6, 129.5, 129.5, 128.0, 127.4, 127.4, 126.8, 124.7, 124.7, 123.0, 123.0, 121.0, 121.0, 116.4, 116.4, 113.2, 113.2, 112.1, 112.1, 54.6: HREI-MS: m/z Calcd for C31H19Br2Cl2N5O2S2 [M + 6]+ 790.8642, [M + 5]+ 789.8695, [M + 4]+ 788.8654, [M + 3]+ 787.8719, [M + 2]+ 786.8694, [M + 1]+ 785.8744 [M + ]+ 784.8715.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)naphthalene-2-sulfonamide (11)
Orange solid. Yield: 80.0%; Rƒ. 0.61 (ethylecetate/hexane 2:8); m.p.: 313–314 °C; IR (KBr): 3240 cm−1 (2°amine N-H Str), 1640 cm−1 (Ar C=C), 1369 cm−1 (N-S=O), 670 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ11.88(s, 1H, NH), 11.28 (s, 1H, NH), 10.48 (s, 1H, NH), 7.80 (t, J = 7.3 Hz, 3H), 7.52–7.45 (m, 6 H), 7.38 (d, J = 8.4 Hz, 2H), 7.31 (d, J = 7.5 Hz, 2H), 7.11 (t, J = 7.6 Hz, 2H), 6.93 (t, J = 7.2 Hz, 2H), 6.85 (s, 2H), 5.96 (s, 1H); 13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 138.1, 137.0, 136.7, 135.5, 135.5, 134.1, 130.5, 129.6, 129.6, 129.5, 129.5, 129.4, 128.1, 128.1, 127.4, 127.4, 126.2, 126.2, 126.0, 124.7, 124.7, 123.4, 123.0, 123.0, 121.0, 121.0, 116.4, 116.4, 113.2, 113.2, 112.1, 112.1, 54.6: HREI-MS: m/z Calcd for C35H23Br2N5O2S2 [M + 4]+ 770.9606, [M + 3]+ 769.9662, [M + 2]+ 767.9683, [M + 1]+ 785.8744 [M + ]+ 766.952.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-2-chloro-5-methylbenzenesulfonamide (12)
Yellow solid. Yield: 83.0%; Rƒ. 0.68 (ethylecetate/hexane 3:7); m.p.: 301–302 °C; IR (KBr): 3236 cm−1 (2°amine N-H Str), 1632 cm−1 (Ar C=C), 1349 cm−1 (N-S=O), 770 cm−1 (C-Cl str), 639 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ11.72(s, 1H, NH), 11.89 (s, 1H, NH), 9.61 (s, 1H, NH), 7.80 (d, J = 8.3 Hz, 2H), 7.51 (d, J = 8.3 Hz, 2H), 7.39 (d, J = 8.4 Hz, 2H), 7.33 (d, J = 7.6 Hz, 2H), 7.18–7.11 (m, 3H), 6.91 (t, J = 7.2 Hz, 2H), 6.85(s, 2H), 5.96 (s, 1H), 1.92 (s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 139.6, 138.1, 136.8, 135.5, 135.5, 133.6, 130.5, 129.6, 129.6, 129.5, 129.5, 129.0, 128.5, 128.1, 127.4, 127.4, 124.7, 124.7, 123.0, 123.0, 121.0, 121.0, 116.4, 116.4, 113.2, 113.2, 112.1, 112.1, 54.6, 21.3: HREI-MS: m/z Calcd for C32H22Br2ClN5O2S2 [M + 4]+ 768.9219, [M + 3]+ 767.9272, [M + 2]+ 766.9240, [M + 1]+ 765.9297 [M + ]+ 764.918.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-5 methyl-2-nitrobenzenesulfonamide (13)
Orange solid. Yield: 78.0%; Rƒ. 0.59 (ethylecetate/hexane 3:7); m.p.: 312–313 °C; IR (KBr): 3237 cm−1 (2°amine N-H Str), 1623 cm−1 (Ar C=C), 1563 cm−1 (N-O str), 1345 cm−1 (N-S=O), 687 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ12.02(s, 1H, NH), 11.12 (s, 1H, NH), 10.98 (s, 1H, NH), 7.82 (d, J = 7.8 Hz, 2H), 7.74 (t, J = 7.6 Hz, 1H), 7.50 (d, J = 8.3 Hz, 2H), 7.38 (d, J = 8.6 Hz, 2H), 7.32 (d, J = 7.7 Hz, 2H), 7.11 (t, J = 7.4 Hz, 2H), 6.94 (t, J = 7.4 Hz, 2H), 6.87 (s, 2H), 5.94 (s, 1H), 1.91 (s, 3H, CH3);13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 144.8, 144.2, 138.1, 135.5, 135.5, 134.3, 133.1, 130.5, 129.6, 129.6, 129.5, 129.5, 127.6, 127.4, 127.4, 124.7, 124.7, 124.1, 123.0, 123.0, 121.0, 121.0, 116.4, 116.4, 113.2, 113.2, 112.1, 112.1, 54.6, 21.3: HREI-MS: m/z Calcd for C32H22Br2N6O4S2 [M + 4]+ 768.9219, [M + 3]+ 779.9461, [M + 2]+ 778.9514, [M + 1]+ 776.9527 [M + ]+ 775.9502.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-2-bromo-5-methoxybenzenesulfonamide (14)
Yellow solid. Yield: 87.0%; Rƒ. 0.71 (ethylecetate/hexane 3:7); m.p.:309–310 °C; IR (KBr): 3190 cm−1 (2°amine N-H Str), 1609 cm−1 (Ar C=C), 1341 cm−1 (N-S=O), 716 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ 11.95 (s, 1H, NH), 10.68 (s, 1H, NH), 8.61(s, 1H, NH), 7.82 (d, J = 7.3 Hz, 2H), 7.50 (d, J = 8.2 Hz, 2H), 7.38 (d, J = 8.6 Hz, 2H), 7.33 (d, J = 7.7 Hz, 2H), 7.14–7.09 (m, 3H), 6.94–6.90 (m, 2H), 6.86 (s, 2H), 6.85 (d, J = 7.4 Hz, 1H), 5.96 (s, 1H), 3.74 (s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 158.3, 143.6, 138.1, 135.5, 135.5, 132.9, 130.5, 129.6, 129.6, 129.5, 129.5, 127.4, 127.4, 124.7, 124.7, 123.0, 123.0, 121.5, 121.0, 121.0, 116.4, 116.4, 113.4, 113.2, 113.2, 112.4, 112.1, 112.1, 55.8, 54.6: HREI-MS: m/z Calcd for C32H22Br3N5O3S2 [M + 6]+ 830.8641, [M + 5]+ 829.8695, [M + 4]+ 828.8662, [M + 3]+ 827.8709, [M + 2]+ 826.8679, [M + 1]+ 825.8732 [M + ]+ 824.8714.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-5-methoxy-2-nitrobenzenesulfonamide (15)
Brown solid. Yield: 81.0%; Rƒ. 0.58 (ethylecetate/hexane 3:7); m.p.: 308–309 °C; IR (KBr): 3234 cm−1 (2°amine N-H Str), 1625 cm−1 (Ar C=C), 1554 cm−1 (N-O str), 1359 cm−1 (N-S=O), 1129 cm−1 (C-O str), 682 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ11.62(s, 1H, NH), 11.47 (s, 1H, NH), 8.38 (s, 1H, NH), 7.80 (d, J = 8.4 Hz, 2H), 7.64 (d, J = 7.8 Hz, 2H), 7.49 (d, J = 8.1 Hz, 2H), 7.38 (d, J = 8.3 Hz, 2H), 7.31 (d, J = 7.7 Hz, 2H), 7.04 (d, J = 8.6 Hz, 2H), 6.90 (t, J = 7.2 Hz, 2H), 6.86 (s, 2H), 5.96 (s, 1H), 3.82 (s, 3H, OCH3);13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 165.4, 139.5, 138.1, 135.5, 135.5, 135.4, 130.5, 129.6, 129.6, 129.5, 129.5, 127.4, 127.4, 125.2, 124.7, 124.7, 123.0, 123.0, 121.0, 121.0, 118.4, 116.4, 116.4, 113.2, 113.2, 112.1, 112.1, 112.1, 55.8, 54.6: HREI-MS: m/z Calcd for C32H22Br2N6O5S2 [M + 4]+ 795.9402, [M + 3]+ 794.9464, [M + 2]+ 793.9423, [M + 1]+ 792.9482 [M + ]+ 791.932.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-2-methylbenzenesulfonamide (16)
Light brown. Yield: 82.0%; Rƒ. 0.75 (ethylecetate/hexane 3:7); m.p.: 291–292 °C; IR (KBr): 3232 cm−1 (2°amine N-H Str), 1620 cm−1 (Ar C=C), 1354 cm−1 (N-S=O), 722 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ11.72(s, 1H, NH), 10.96 (s, 1H, NH), 8.72 (s, 1H, NH), 7.81 (t, J = 7.4 Hz, 2H), 7.49 (d, J = 7.7 Hz, 2H), 7.38 (d, J = 8.3 Hz, 2H), 7.31 (d, J = 7.6 Hz, 2H), 7.30–7.21 (m, 3H), 6.94 (t, J = 7.3 Hz, 2H), 6.86 (s, 2H), 5.96 (s, 1H), 2.40 (s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 138.9, 138.1, 136.6, 131.8, 131.5, 130.5, 129.7, 129.6, 129.6, 129.5, 129.5, 127.4, 127.4, 124.7, 124.7, 123.0, 123.0, 121.0, 121.0, 120.8, 116.4, 116.4, 113.2, 113.2, 112.1, 112.1, 54.6, 22.0: HREI-MS: m/z Calcd for C32H23Br2N5O2S2 [M + 4]+ 734.9606, [M + 3]+ 733.9662, [M + 2]+ 732.9624, [M + 1]+ 731.9682 [M + ]+ 730.9652.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-3-methylbenzenesulfonamide (17)
Yellow. Yield: 82.0%; Rƒ. 0.74 (ethylecetate/hexane 3:7); m.p.: 289–290 °C; IR (KBr): 3236 cm−1 (2°amine N-H Str), 1629 cm−1 (Ar C=C), 1361 cm−1 (N-S=O), 730 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ 12.52 (s, 1H, NH), 11.74 (s, 1H, NH), 8.42 (s, 1H, NH),7.80 (d, J = 7.7 Hz, 2H), 7.52 (s, 1H), 7.49 (d, J = 8.4 Hz, 2H), 7.39 (d, J = 8.3 Hz, 2H), 7.31 (d, J = 7.6 Hz, 2H), 7.22 (d, J = 8.3 Hz, 2H), 6.91 (t, J = 7.2 Hz, 2H), 6.86 (s, 2H), 5.95 (s, 1H), 2.40 (s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 139.6, 138.1, 138.7, 135.5, 135.5, 132.2, 130.5, 129.6, 129.6, 129.5, 129.5, 128.9, 127.4, 127.4, 126.7, 124.7, 124.7, 124.3, 123.0, 123.0, 121.0, 121.0, 116.4, 116.4, 113.2, 113.2, 112.1, 112.1, 54.6, 21.3: HREI-MS: m/z Calcd for C32H23Br2N5O2S2 [M + 4]+ 734.9609, [M + 3]+ 733.9659, [M + 2]+ 732.9622, [M + 1]+ 731.9679 [M + ]+ 730.9647.
N-(5-(4-(bis(5-bromo-1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)-4-methylbenzenesulfonamide (18)
Brown. Yield: 75.0%; Rƒ. 0.72 (ethylecetate/hexane 3:7); m.p.: 285–286 °C; IR (KBr): 3220 cm−1 (2°amine N-H Str), 1631 cm−1 (Ar C=C), 1359 cm−1 (N-S=O), 726 cm−1 (C-Br str); 1H NMR (500 MHz, DMSO-d6): δ 12.08 (s, 1H, NH), 11.74 (s, 1H, NH), 8.37 (s, 1H, NH),7.83 (d, J = 8.3 Hz, 2H), 7.60 (d, J = 7.3 Hz, 2H), 7.48 (d, J = 7.6 Hz, 2H), 7.39 (d, J = 7.8 Hz, 2H), 7.30 (d, J = 7.4 Hz, 2H), 7.24 (d, J = 7.3 Hz, 2H), 6.93 (t, J = 7.3 Hz, 2H), 6.85 (s, 2H), 5.94 (s, 1H), 2.37 (s, 3H, CH3);13C-NMR (125 MHz, DMSO-d6): δ174.1, 173.0, 138.1, 137.6, 136.7, 135.5, 135.5, 130.5, 129.6, 129.6, 129.5, 129.5, 129.3, 129.3, 128.3, 128.3, 127.4, 127.4, 124.7, 124.7, 123.0, 123.0, 121.0, 121.0, 116.4, 116.4, 113.2, 113.2, 112.1, 112.1, 54.6, 21.3: HREI-MS: m/z Calcd for C32H23Br2N5O2S2 [M + 4]+ 734.9602, [M + 3]+ 733.9656, [M + 2]+ 732.9618, [M + 1]+ 731.9675 [M + ]+ 730.9649.
Urease inhibition assay
Urease is an enzyme (jack bean urease) that catalyzes the hydrolysis of urea into carbon dioxide and ammonia. The production of ammonia was measured by the indophenol method and used to determine the urease inhibitory activity57,58. The percentage remaining activity was calculated from the formula % Remaining Activity = [(ODtest)/(ODcontrol) × 100]. Thiourea was used as a standard inhibitor. To calculate IC50 values, different concentrations of synthesized compounds and standards were assayed at the same reaction conditions.
Docking studies
The binding modes between selected bis-indole bearing thiadiazol derivatives and the active residues of urease have been investigated using Autodock package59. The staring geometries of urease and the original docked acetohydroxamic acid were download from the RCSB data bank web site (PDB code 1FWE)60. Water molecules were removed; polar hydrogen atoms and Kollman charge were added to the extracted receptor using the automated tool in AutoDock Tools 4.2. The active site is identified based on co-crystallized receptor-ligand complex structure of urease. The re-docking of the original ligand acetohydroxamic acid into the active site is well reproduced with a RMSD value less than 0.717 Å. Molecular geometries of selected diindolylmethane bearing thiadiazol derivatives were minimized at Merck molecular force field 94 (MMFF94) level44. The optimized structures were saved as PDB files. Nonpolar hydrogens were merged and rotatable bonds were defined for each docked ligand. Docking studies were performed by Lamarckian genetic algorithm, with 500 as total number of run for binding sites for original ligand the synthesized derivatives. In each respective run, a population of 150 individuals with 27000 generations and 250000 energy evaluations were employed. Operator weights for crossover, mutation, and elitism were set to 0.8, 0.02, and 1, respectively. The docking calculations have been carried out using an Intel Core i5–3770 CPU 3.40 GHz workstation.
References
Li, M., Ding, W., Baruah, B., Crans, D. C. & Wang, R. Inhibition of protein tyrosine phosphatase 1B and alkaline phosphatase by bis (maltolato) oxovanadium (IV). J. Inorg. Biochem. 102, 1846–1853 (2008).
Collins, C. M. & D’Orazio, S. E. Bacterial ureases: structure, regulation of expression and role in pathogenesis. Mol. Micro. 9, 907–913 (1993).
Krajewska, B. Ureases I. Functional, catalytic and kinetic properties: A review. J. Mol. Cat. B: Enzym. 59, 9–21 (2009).
Amtul, Z. et al. Cysteine based novel noncompetitive inhibitors of urease (s) Distinctive inhibition susceptibility of microbial and plant ureases. Bioorg. Med. Chem. 14, 6737–6744 (2006).
Seneviratne, G., Van Holm, L. H. J. & Ekanayake, E. M. H. G. S. Agronomic benefits of rhizobial inoculant use over nitrogen fertilizer application in tropical soybean. Field. Crops. Research. 68, 199–203 (2000).
Samtoy, B. & DeBeukelaer, M. M. Ammonia encephalopathy secondary to urinary tract infection with Proteus mirabilis. Pediatrics. 65, 294–297 (1980).
Mobley, H. L., Island, M. D. & Hausinger, R. P. Molecular biology of microbial ureases. Microbiol. Rev. 59, 451–480 (1995).
Lodhi, M. A., Abbasi, M. A., Choudhary, M. I. & Ahmad, V. U. Kinetics studies on triacontanyl palmitate: a urease inhibitor. Nat. Prod. Res. 21, 721–725 (2007).
Lodhi, M. A. et al. A new Bacillus pasteurii urease inhibitor from Euphorbia decipiens. J. Enz. Inhib. Med. Chem. 21, 531–535 (2006).
Barraja, P. et al. Pyrrolo [3, 4-h] quinolinones a new class of photochemotherapeutic agents. Bioorg. Med. Chem. 19, 2326–2341 (2011).
Agarwal, A., Srivastava, K., Puri, S. K. & Chauhan, P. M. Synthesis of substituted indole derivatives as a new class of antimalarial agents. Bioorg. Med. Chem. Lett. 15, 3133–3136 (2005).
Meanwell, N. A. et al. Inhibitors of HIV-1 attachment. Part 3: a preliminary survey of the effect of structural variation of the benzamide moiety on antiviral activity. Bioorg. Med. Chem. Lett. 19, 5136–5139 (2009).
Lakshmi, N. V., Thirumurugan, P., Noorulla, K. M. & Perumal, P. T. InCl3 mediated one-pot multicomponent synthesis, anti-microbial, antioxidant and anticancer evaluation of 3-pyranyl indole derivatives. Bioorg. Med. Chem. Lett. 20, 5054–5061 (2010).
Reddy, B. S. et al. Iodine-catalyzed conjugate addition of indoles onto en-1,4-dione: A novel synthesis of 3-(1-(1H-indol-3-yl)-2-oxo-2-phenylethyl)indolin-2-ones as antibacterial and antifungal agents. Bioorg. Med. Chem. Lett. 21, 6510–6514 (2011).
Taha, M. et al. Synthesis of indole-2-hydrazones in search of potential leishmanicidal agents. Med. Chem. Res. 23, 5282–5293 (2014).
Taha, M. et al. Synthesis of novel derivatives of oxindole, their urease inhibition and molecular docking studies. Bioorg. Med. Chem. lett. 25, 3285–3289 (2015).
Gu, X. H., Wan, X. Z. & Jiang, B. Syntheses and biological activities of bis (3-indolyl) thiazoles, analogues of marine bis (indole) alkaloid nortopsentins. Bioorg. Med. Chem. Lett. 9, 569–572 (1999).
Jin, G. et al. Chemical genetics-based discovery of indole derivatives as HCV NS5B polymerase inhibitors. Eur. J. Med. Chem. 75, 413–425 (2014).
Bahekar, R. H. et al. Design, synthesis, and biological evaluation of substituted-N-(thieno[2,3-b]pyridin-3-yl)-guanidines, N-(1H-pyrrolo[2,3-b]pyridin-3-yl)-guanidines, and N-(1H-indol-3-yl)-guanidines. Bioorg. Med. Chem. 15, 3248–3265 (2007).
Mohareb, R. M., Ahmed, H. H., Elmegeed, G. A., Abd-Elhalim, M. M. & Shafic, R. W. Development of new indole-derived neuroprotective agents. Bioorg. Med. Chem. 19, 2966–2974 (2011).
Hall, A. et al. Discovery of a novel indole series of EP1 receptor antagonists by scaffold hopping. Bioorg. Med. Chem. Lett. 18, 2684–2690 (2008).
Singh, P., Mittal, A., Bhardwaj, A., Kaur, S. & Kumar, S. 1-Toluene-sulfonyl-3-[(3′-hydroxy-5′-substituted)-γ-butyrolactone]-indoles: Synthesis, COX-2 inhibition and anti-cancer activities. Bioorg. Med. Chem. Lett. 18, 85–89 (2008).
Khan, K. M. et al. Oxindole derivatives: Synthesis and antiglycation activity. Med. Chem. 9, 681–688 (2013).
Madadi, N. R., Penthala, N. R., Janganati, V. & Crooks, P. A. Synthesis and anti-proliferative activity of aromatic substituted 5-((1-benzyl-1H-indol-3-yl)methylene)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione analogs against human tumor cell lines. Bioorg. Med. Chem. Lett. 24, 601–603 (2014).
Adam, J. M. et al. Design, synthesis, and structure–activity relationships of indole-3-carboxamides as novel water soluble cannabinoid CB1 receptor agonists. Med. Chem. Commun. 1, 54–60 (2010).
Madadi, N. R. et al. Evaluation of (Z)-2-((1-benzyl-1H-indol-3-yl) methylene)-quinuclidin-3-one analogues as novel, high affinity ligands for CB1 and CB2 cannabinoid receptors. Bioorg. Med. Chem. Lett. 23, 2019–2021 (2013).
Dembitsky, V. M., Gloriozova, T. A. & Poroikov, V. V. Novel antitumor agents: marine sponge alkaloids, their synthetic analogs and derivatives. Mini-Rev. Med. Chem. 5, 319–336 (2005).
Bao, B. et al. Cytotoxic Bisindole Alkaloids from a Marine Sponge Spongosorites sp. J. Nat. Prod. 68, 711–715 (2005).
Oh, K. B. et al. Antimicrobial activity and cytotoxicity of bis (indole) alkaloids from the sponge Spongosorites sp. Biol. Pharm. Bull. 29, 570–573 (2006).
Oh, K. B. et al. Bis (indole) alkaloids as sortase A inhibitors from the sponge Spongosorites sp. Bioorg. Med. Chem. Lett. 15, 4927–4931 (2005).
Yang, S. W. & Cordell, G. A. Metabolism Studies of Indole Derivatives Using a Staurosporine Producer, Streptomyces staurosporeus. J. Nat. Prod. 60, 44–48 (1997).
Tsuda, M., Takahashi, Y., Fromont, J., Mikami, Y. & Kobayashi, J. I. Dendridine A, a Bis-indole Alkaloid from a Marine Sponge Dictyodendrilla Species. J. Nat. Prod. 68, 1277–1278 (2005).
Shin, J., Seo, Y., Cho, K. W., Rho, J. R. & Sim, C. J. New Bis(Indole) Alkaloids of the Topsentin Class from the Sponge Spongosorites genitrix. J. Nat. Prod. 62, 647–649 (1999).
Ryan, K. S. & Drennan, C. L. Divergent pathways in the biosynthesis of bisindole natural products. Chem. Biol. 16, 351–364 (2009).
Diana, P. et al. Synthesis and antitumor properties of 2, 5-bis (3′-indolyl) thiophenes: Analogues of marine alkaloid nortopsentin. Bioorg. Med. Chem. Lett. 17, 2342–2346 (2007).
Baltaş, N., Yılmaz, F. & Menteşe, E. Synthesis, antioxidant, xanthine oxidase and urease inhibitory activities of some chlorine containing benzimidazoles. J. Biol. Chem. 44, 293–305 (2016).
Akhtar, T., Hameed, S., Khan, K. M., Khan, A. & Choudhary, M. I. Design, synthesis, and urease inhibition studies of some 1, 3, 4-oxadiazoles and 1, 2, 4-triazoles derived from mandelic acid. J. Enzym. Inhib. Med. Chem. 25, 572–576 (2010).
Beale, J. M. & Block, J., Hill, In Textbook of Organic Chemistry Medicinal and Pharmaceutical Chemistry; Wilson, Giswold’s. (1998).
Bekircan, O., Mentese, E. & Ulker, S. Synthesis and Pharmacological Activities of Some New 2-[1-Heptyl-3-(4-methoxybenzyl)-5-oxo-1, 5-dihydro-4H-1, 2, 4-triazol-4-yl] acetohydrazide Derivatives. Naturforsch. 69, 969–981 (2014).
Menteşe, E. et al. Synthesis and molecular docking study of some 5,6-dichloro-2-cyclopropyl-1H-benzimidazole derivatives bearing triazole, oxadiazole, and imine functionalities as potent inhibitors of urease. Bioorg. Med. Chem. Lett. 27, 3014–3018 (2017).
Bekircan, O., Menteşe, E., Ülker, S. & Kucuk, C. Synthesis of Some New 1,2,4‐Triazole Derivatives Starting from 3‐(4‐Chlorophenyl)‐5‐(4‐methoxybenzyl)‐4H‐1,2,4‐triazol with Anti‐Lipase and Anti‐Urease Activities. Arch. Pharm. Chem. Life Sci. 347, 387–397 (2014).
Amtul, Z., Rasheed, M., Choudhary, M. I., Rosanna, S. & Khan, K. M. Kinetics of novel competitive inhibitors of urease enzymes by a focused library of oxadiazoles/thiadiazoles and triazoles. Biochem. Biophys. Res. Commun. 319, 1053–1057 (2004).
Serwar, M., Akhtar, T., Hameed, S. & Khan, K. M. Synthesis, urease inhibition and antimicrobial activities of some chiral 5-aryl-4-(1-phenylpropyl)-2H-1, 2, 4-triazole-3 (4H)-thiones. Arkivoc 7, 210–221 (2009).
Taha, M. et al. Synthesis, α-glucosidase inhibitory, cytotoxicity and docking studies of 2-aryl-7-methylbenzimidazoles. Bioorg. Chem. 65, 100–109 (2016).
Imran, S. et al. Synthesis, In vitro and Docking Studies of New Flavone Ethers as α‐Glucosidase Inhibitors. Chem. Biol. & Drug Desig. 87, 361–373 (2016).
Taha, M. et al. Synthesis, molecular docking and α-glucosidase inhibition of 5-aryl-2-(6′-nitrobenzofuran-2′-yl)-1,3,4-oxadiazoles. Bioorg. Chem. 66, 117–123 (2016).
Zawawi, N. K. N. A. et al. Synthesis, in vitro evaluation and molecular docking studies of biscoumarin thiourea as a new inhibitor of α-glucosidases. Bioorg. Chem. 63, 36–44 (2015).
Taha, M. et al. Evaluation of 2-indolcarbohydrazones as potent α-glucosidase inhibitors, in silico studies and DFT based stereochemical predictions. Bioorg. Chem. 63, 24–35 (2015).
Taha, M. et al. Novel quinoline derivatives as potent in vitro α-glucosidase inhibitors: in silico studies and SAR predictions. Med. Chem. Commun. 6, 1826–1836 (2015).
Rahim, F. et al. Isatin based Schiff bases as inhibitors of α-glucosidase: Synthesis, characterization, in vitro evaluation and molecular docking studies. Bioorg. Chem. 60, 42–48 (2015).
Taha, M. et al. Synthesis crystal structure of 2-methoxybenzoylhydrazones and evaluation of their α-glucosidase and urease inhibition potential. Med. Chem. Res. 24, 1310–1324 (2015).
Khan, K. M. et al. Synthesis and molecular docking studies of potent α-glucosidase inhibitors based on biscoumarin skeleton. Eur. J. Med. Chem. 81, 245–252 (2014).
Rahim, F. et al. Synthesis, in vitro evaluation and molecular docking studies of thiazole derivatives as new inhibitors of α-glucosidase. Bioorg. Chem. 62, 15–21 (2015).
Rahim, F. et al. Triazinoindole analogs as potent inhibitors of α-glucosidase: synthesis, biological evaluation and molecular docking studies. Bioorg. Chem. 58, 81–87 (2015).
Imran, S. et al. Synthesis of novel flavone hydrazones: in-vitro evaluation of α-glucosidase inhibition, QSAR analysis and docking studies. Euro. J. Med. Chem. 105, 156–170 (2015).
Almandil, N. B. et al. Synthesis of novel quinoline-based thiadiazole, evaluation of their antileishmanial potential and molecular docking studies. Bioorg. Chem. 85, 109–116 (2019).
Javid, M. T. et al. Synthesis, in vitro α-glucosidase inhibitory potential and molecular docking study of thiadiazole analogs. Bioorg. Chem. 78, 201–209 (2018).
Menteşe, E., Akyüz, G., Emirik, M. & Baltaş, N. Synthesis, in vitro urease inhibition and molecular docking studies of some novel quinazolin-4(3H)-one derivatives containing triazole, thiadiazole and thiosemicarbazide functionalities. Bioorg. Chem. 83, 289–296 (2019).
Morris, G. M. et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. computational. chem. 30, 2785–2791 (2009).
Pearson, M. A., Michel, L. O., Hausinger, R. P. & Karplus, P. A. Structures of Cys319 Variants and Acetohydroxamate-Inhibited Klebsiella aerogenes Urease. Biochemistry 36, 8164–8172 (1997).
Acknowledgements
Authors would like to acknowledge financial support for this study by Deanship of Scientific Research, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia, under Project No. 2019-211-IRMC.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.T. and A.A.K..; methodology, M.T.; software, E.H.A.; validation, F.R., A.A.K. and N.A.; formal analysis, N.A..; investigation, S.A.A.S.; resources, M.T.; data curation, M.I. and S.A.A.S.; Writing—Original Draft preparation, M.T. and Z.A.Z.; Writing—Review and Editing, F.R. and Z.A.Z.; visualization, S.A.A.S.; supervision, F.R.; project administration, Z.A.Z. and M.T.; funding acquisition, M.T. and M.I.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taha, M., Rahim, F., Khan, A.A. et al. Synthesis of diindolylmethane (DIM) bearing thiadiazole derivatives as a potent urease inhibitor. Sci Rep 10, 7969 (2020). https://doi.org/10.1038/s41598-020-64729-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64729-3
This article is cited by
-
The anti-inflammatory drugs diclofenac and ketorolac inhibit urease activity and biofilm formation by uropathogenic Proteus mirabilis
Folia Microbiologica (2025)
-
Synthesis, enzyme inhibition and docking studies of isatin derivatives from aliphatic esters
Journal of the Iranian Chemical Society (2025)
-
[1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives as new therapeutic candidates against urease positive microorganisms: design, synthesis, pharmacological evaluations, and in silico studies
Scientific Reports (2023)