Abstract
We tested two sources of lanthanum (La), LaCl3 and La(NO3)3 × 6H2O at a concentration of 40 µM each, in the treatment solution of cut flowers of 15 tulip (Tulipa gesneriana L.) cultivars. Ascorbic acid (AsA; 0.2 g/L) was used as a reference solution, while distilled water was evaluated as an absolute control. With both La sources, bud length and diameter, and stem length were increased; as a result, stem curvature was also significantly increased with La treatments. The cultivars Laura Fygi and Rosario registered the highest relative stem elongation. Lalibela and Acropolis displayed the greatest stem curvature on the last day in vase. At 3, 5, 7, 9 and 11 days after cutting, the highest solution uptake was recorded in flower stems treated with LaCl3, surpassing the control by 5, 11, 15, 18 and 24%, respectively. The relative stem elongations observed were 21.3, 27.4, 35.2 and 35.5% in the control, AsA, LaCl3 and La(NO3)3, respectively. The mean solution uptake per gram of stem fresh biomass weight was 1.44, 1.44, 1.71 and 1.54 mL in the control, AsA, LaCl3 and La(NO3)3, respectively. LaCl3 significantly increased the bud length and solution uptake of flower stems, while La(NO3)3 × 6H2O increased stem fresh weight.
Similar content being viewed by others
Introduction
A preservative solution is used to control ethylene synthesis and microbial proliferation, maintaining an adequate water and respiratory balance as well as the color of petals, while boosting the antioxidant system and inducing opening of the floral buds1. Importantly, management of cut flowers in preservative solutions helps keep the stems for longer periods of time, and thus prolongs the vase life of flowers2. These solutions mainly include sugars, acidifiers and germicides, while other compounds may also be added to induce antioxidant responses3,4. In order to test the individual effect of a novel compound, treatment solutions have a simpler composition than preservative ones. Once the beneficial effect of such a compound is experimentally demonstrated, it can be incorporated into a prepared preservative or holding/vase solution.
In terms of postharvest handling and preservation of cut flowers, L-ascorbic acid (AsA) has been proved n ot only to act as an important antioxidant, but has also been linked to developmental senescence and programmed cell death through a complex signal transduction network5. Consequently, AsA has been used successfully in postharvest management of different cut flowers5,6. In cut lisianthus (Eustoma grandiflorum [Raf.] Shinn) flowers, stems exposed to 200 mg/L AsA exhibited the maximum solution uptake and the highest dry matter weight6, while with 300 mg/L AsA flowers displayed the longest vase life and highest petal water content7.
Beneficial elements can also retard senescence and preserve cut flowers8. Among them, lanthanum (La) can be incorporated into the vase solution during postharvest handling of cut flowers, though its biological functions and effects on tulip (Tulipa gesneriana L.) have been little studied9. Lanthanum belongs to the Lanthanides (Ln3+), which are affiliated to the Rare Earth Elements (REE). The 17 chemical elements that make up the REE group have similar chemical and physical properties10. The symbol Ln3+ is often used as a generic representation of Lanthanides since they typically have trivalent oxidation states. Generally, the parent materials have REE compositions ranging from 0.1 to 100 mg/kg11. Considering the chemical properties of La and the physiological effects it causes in higher plants, La3+ may display similar functions to those of Ca2+ and K+12. For instance, in bell pepper (Capsicum annuum L.), the application of La increased the number of flowers13, which may be correlated with increased endogenous concentrations of cytokinins (important components of floral stimuli in plants)14. In Easter lily (Lolium longiflorum Thunb.), the use of LaCl3 delayed senescence of cut flowers by stimulating their antioxidant defense system and water retaining capacity15. Lanthanum may also influence the gravitropic response of cut tulip stems, while stem bending is dependent on the cultivar and is positively correlated with the rate of postharvest stem elongation. Since La reduces stem elongation, LaCl3 prevents stem bending16. In cut tulip flowers cv. Ile de France, stem diameter and length were increased in comparison to the control, by supplying them with 10 µM La in the nutrient solution17. Although no significant differences in stem diameters among treatments were found, plants treated with nutrient solutions containing 10 µM La showed diameters 2% bigger than the control plants. Indeed, La may stimulate plant growth within a biphasic dose–response hormetic curve18, which is characterized by a low dose stimulation or beneficial effect and a high dose inhibitory or toxic effect19. After analyzing 703 studies demonstrating La-induced hormesis, it was found that the maximum biological response to low La concentrations is frequently below 150% of control response, with a geometric mean of 142% at 56 μM, while the geometric mean concentration of the no-observed-adverse-effect-level (NOAEL) was 249 μM20. Consequently, one can expect no toxic effects in plants if La is applied between the range of approximately 50 and 250 µM, which will depend on the plant genotype, pH of the growth media, La doses below the NOAEL, and time of exposure to La13,20. A summary of the aforementioned findings is presented in Fig. 1.
Schematic model of a representative idealized biphasic dose–response hormetic curve for the effect of lanthanum (La) on plants. MAX: Maximum biological response to low La concentrations, which is commonly below 150% of control response. NOAEL: No-observed-adverse-effect-level. The MAX:NOAEL distance is often below fivefold, with a geometric mean of 4.5-fold. The model is based on experimental data previously analyzed18,20,54.
In preliminary experiments, we tested the effect of a number of different La concentrations on tulip plant growth, development, and nutrient concentration in different plant tissues17,21. Furthermore, we analyzed the literature on La dosage triggering beneficial effects in other plant species. For instance, in tomato (Solanum lycopersicum L.), the application of 40.78 μM La in the nutrient solution increased yield and reduced Cd accumulation in fruits22. In rose (Rosa hybrida L.), the application of 50 μM La increased fresh weight and water balance, maintained flower diameter and the stability of membranes in petals, decreased the solute exosmosis and the respiration rate, and prolonged vase life for 2–3 d more than the control23. In Easter lily, the application of 30–90 µM LaCl3 delayed flower senescence by stimulating the antioxidant defense system and water retaining capacity15. Nevertheless, there is scant information available on the use of La3+ in postharvest aimed at increasing the duration of the tulip flower stems.
Based on these reports and our experience, we hypothesized that low doses of lanthanum may delay senescence and improve postharvest quality in cut tulip flowers. Concomitantly, we decided to perform further analyses by comparing the effect of applying 0 (control) and 40 μM La on flower quality indicators and senescence inhibition of cut tulip flowers. In our experiment, we evaluated the responses of 15 tulip cultivars to two sources of lanthanum [LaCl3 and La(NO3)3 × 6H2O] in order to gain better insight into genotype responses to this beneficial element supplied in the treatment solution.
Results
Regarding ascorbic acid (AsA), it has been widely used as a key component of preservative solutions for postharvest management of cut flowers. Pulse and continuous treatments with AsA (50–1000 mg/L) have been proved to delay senescence, enhance quality, and prolong the vase life of different cut flowers5,6,24,25,26,27,28,29. We further analyzed the literature on AsA dosage triggering preservative effects on cut flowers (Table 1) and selected 0.2 g/L (i.e. 200 mg/L) to test its effect on tulips.
Bud length and diameter
Due to the cultivar factor, bud length and diameter increased from 3 to 11 days after cutting (dac; Fig. 2A–J), with the highest elongation rates occurring from 3 to 5 dac (Fig. 2A,B,F,G). The cultivars Acropolis and Jan van Nes had the greatest bud length (Fig. 2E). The bud diameter in all cultivars increased over time (Fig. 2F–J), displaying its highest values at 11 dac (Fig. 2J). The highest bud diameter values at 11 dac were recorded in Acropolis, followed by Jan van Nes and Golden Parade (Fig. 2J).
Tulip bud length and diameter in postharvest as a function of the cultivars tested. (A) and (F): 3 dac; (B) and (G): 5 dac; (C) and (H): 7 dac; (D) and (I): 9 dac; (E) and (J): 11 dac. Different letters in each subfigure and assessment date indicate statistical differences according to the LSD test with P ≤ 0.05. (Bud length: 3 dac P = < 0.0001; 5 dac P = < 0.0001; 7 dac P = < 0.0001; 9 dac P = < 0.0001; 11 dac P = < 0.0001. Bud diameter: 3 dac P = < 0.0001 = ; 5 dac P = < 0.0001; 7 dac P = < 0.0001; 9 dac P = < 0.0001; 11 dac P = < 0.0001). Ac: Acropolis, Ba: Barcelona, GP: Golden Parade, JN: Jan van Nes, La: Lalibela, LF: Laura Fygi, LM: Lefeber´s Memory, PI: Pink Impression, RI: Red Impression, RS: Red Shine, Ro: Rosario, SL: Snow Lady, SS: Synaeda Show, VB: Violet Beauty, WF: World´s Favorite. dac: days after cutting. Data are means ± SD of six biological replicates.
Figure 3A shows the bud length as affected by the treatment solution. From 5 dac significant differences among treatments were observed. With LaCl3 in the solution, flower stems showed the greatest bud length in measurements performed 5, 7, 9 and 11 dac. The second highest mean was observed in flower stems treated with La(NO3)3 at the same time points analyzed. Indeed, at 11 dac the use of LaCl3 in the treatment solution produced better results than the other treatments, having a bud length of 7.4 cm, while with ascorbic acid and La(NO3)3 it was 7.0 and 7.2 cm, respectively. Stems treated with LaCl3 and La(NO3)3 recorded the greatest bud diameter at 9 to 11 dac (6.07 and 6.68 cm, respectively). From 7 dac the control treatment showed the smallest bud diameter. Thus, at 11 dac the means of LaCl3-treated flowers exceeded those observed in flowers treated with ascorbic acid and the control by 7.4 and 13.2% (Fig. 3B).
Tulip bud length (A) and diameter (B) in postharvest as a function of the treatment solutions used. Different letters in each subfigure and assessment date indicate statistical differences according to the LSD test with P ≤ 0.05. (Bud length: 3 dac P = 0.0016; 5 dac P = < 0.0001; 7 dac P = < 0.0001; 9 dac P = < 0.0001; 11 dac P = < 0.0001. Bud diameter: 3 dac P = < 0.0001; 5 dac P = < 0.0001; 7 dac P = < 0.0001; 9 dac P = < 0.0001; 11 dac P = < 0.0001). Control: distilled water; AsA: L-ascorbic acid, 0.2 g/L; LaCl3: lanthanum(III) chloride, 40 µM; La(NO3)3 × 6H2O: lanthanum(III) nitrate hexahydrate, 40 µM. Data are means ± SD of six biological replicates.
Stem length and curvature
The Acropolis cultivar showed the greatest stem length in vase. The shortest stem length was found in Rosario. The highest relative stem elongation was observed when the initial stem length was the shortest 3 dac, in the cultivars Laura Fygi and Rosario (Table 2).
Stem length increased during vase life, regardless of the treatment solution tested. At 5, 7, 9 and 11 dac the greatest stem length was observed with La(NO3)3, surpassing the control by 5.4, 8.1, 9.2 and 10.3% and the solution with ascorbic acid by 2.0, 3.5, 4.5 and 5.7%, respectively (Fig. 4A). The relative stem elongations observed were 21.3, 27.4, 35.2 and 35.5% in the control, AsA, LaCl3 and La(NO3)3 × 6H2O, respectively. Stem curvature in postharvest showed significant changes as a result of the treatment solutions used. On the three evaluation dates (i.e. 5 and 10 dac, and on the last day in the flower vase) the greatest stem curvature was observed in the treatment solution with LaCl3, followed by the treatment solution with La(NO3)3 × 6H2O. By contrast, the smallest curvature angle was recorded in the control (Fig. 4B).
Tulip stem length (A) and curvature (B) in postharvest as a function of the treatment solutions used. Different letters in each subfigure and assessment date indicate statistical differences according to the LSD test with P ≤ 0.05. (Stem length: 3 dac P = 0.2847; 5 dac P = < 0.0001; 7 dac P = < 0.0001; 9 dac P = < 0.0001; 11 dac P = < 0.0001. Stem curvature: 5 dac P = < 0.0001; 10 dac P = < 0.0001; last day in vase P = < 0.0001). Control: distilled water; AsA: L-ascorbic acid, 0.2 g/L; LaCl3: lanthanum(III) chloride, 40 µM; La(NO3)3 × 6H2O: lanthanum(III) nitrate hexahydrate, 40 µM. Data are means ± SD of six biological replicates.
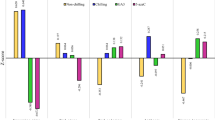
A wide variation in stem curvature among cultivars in vase was observed, with statistically significant differences occurring at 5 and 10 dac, and on the last day in vase. During the last measurement, the cultivars Lalibela and Acropolis displayed the greatest curvature (Fig. 5).
Tulip stem curvature in postharvest as a function of the cultivars tested. Different letters in each subfigure and assessment date indicate statistical differences according to the LSD test with P ≤ 0.05. (5 dac P = < 0.0001; 10 dac P = < 0.0001; last day in vase P = < 0.0001). Ac: Acropolis, Ba: Barcelona, GP: Golden Parade, JN: Jan van Nes, La: Lalibela, LF: Laura Fygi, LM: Lefeber’s Memory, PI: Pink Impression, RI: Red Impression, RS: Red Shine, Ro: Rosario, SL: Snow Lady, SS: Synaeda Show, VB: Violet Beauty, WF: World´s Favorite. dac: days after cutting. Data are means ± SD of six biological replicates.
The application of ascorbic acid, LaCl3 and La(NO3)3 increased solution uptake in the tulip flower stems. Particularly with LaCl3 the highest values were observed at 3, 5, 7, 9 and 11 dac, surpassing the control by 5, 11, 15, 18 and 24%, respectively, which showed the lowest values. The mean solution uptake per gram of stem fresh biomass weight (FBW) was 1.44, 1.44, 1.71 and 1.54 mL in the control, AsA, LaCl3 and La(NO3)3 × 6H2O, respectively (Fig. 6A).
Accumulated solution uptake (A) and fresh weight of tulip stems (B) in postharvest as a function of the treatment solutions used. Different letters in each subfigure and assessment date indicate statistical differences according to the LSD test with P ≤ 0.05. (Accumulated solution uptake: 3 dac P = 0.0687; 5 dac P = < 0.0001; 7 dac P = < 0.0001; 9 dac P = < 0.0001; 11 dac P = < 0.0001. Fresh weight of tulip stems: 3 dac P = 0.0027; 5 dac P = 0.0275; 7 dac P = 0.0002; 9 dac P = < 0.0001; 11 dac P = < 0.0001). Control: distilled water; AsA: L-ascorbic acid, 0.2 g/L; LaCl3: lanthanum(III) chloride, 40 µM; La(NO3)3 × 6H2O: lanthanum(III) nitrate hexahydrate, 40 µM. Data are means ± SD of six biological replicates.
At 5 and 7 dac, the greatest stem weight was found by adding La3+, either in the form of chloride or nitrate. The highest fresh weight (43.7 g) was found 9 dac in stems treated with La(NO3)3, followed by those treated with LaCl3 (42.1 g) and ascorbic acid (41.5 g); the control had the lowest weight (Fig. 6B).
From 3 dac, stem fresh weight increased, reaching its peak values in most cultivars between 7 and 9 dac. With the results obtained, we could identify cultivars that maintain their constant weight during postharvest, including World´s Favorite, Violet Beauty, Laura Fygi and Rosario, in which the difference between the weight at 3 dac and the maximum weight is less than 6 g (Fig. 7).
Fresh weight of tulip stems in postharvest as a function of the cultivars tested. Different letters in each assessment date indicate statistical differences according to the LSD test with P ≤ 0.05. (3 dac P = < 0.0001; 5 dac P = < 0.0001; 7 dac P = < 0.0001; 9 dac P = < 0.0001; 11 dac P = < 0.0001). Ac: Acropolis, Ba: Barcelona, GP: Golden Parade, JN: Jan van Nes, La: Lalibela, LF: Laura Fygi, LM: Lefeber’s Memory, PI: Pink Impression, RI: Red Impression, RS: Red Shine, Ro: Rosario, SL: Snow Lady, SS: Synaeda Show, VB: Violet Beauty, WF: World’s Favorite. dac: days after cutting. Data are means ± SD of six biological replicates.
Discussion
Lanthanum’s major uses include petroleum cracking and chemical industry catalysts. Furthermore, La can be used in glasses, studio lights and projectors, lighters and torches, electron cathodes, and scintillators, among others. Lanthanum carbonate is used as a phosphate binder in cases of renal failure or hypophosphatemia in humans. Additionally, La may have positive effects on plant physiology and may improve some crop yield indicators30. It has been less frequently studied in ornamental plants or cut flowers than it has in cereal grains and industrial crops8.
In cut tulip flowers, bud length of different commercial cultivars may range from 4.8 to 9.2 cm31. Under our experimental conditions, these values varied from 3.2 to 3.9 cm 3 dac, from 4.1 to 5.5 cm 5 dac, from 4.8 to 7.1 cm 7 dac, and from 5.3 to 7.8 cm 9 dac, respectively. In general, values observed at the end of vase life (11 dac) in our study are in full agreement with those reported elsewhere31. On average, the bud diameter in the cultivars evaluated 3, 5, 7, 9 and 11 dac was 1.9, 3.6, 4.8, 5.8 and 6.3 cm, respectively. In a previous study, we reported that the longest vase life (13 days) occurred in Laura Fygi treated with 40 µM La (either as LaCl3 or as La[NO3]3), which was associated with greater water uptake and concentrations of sugars, proteins and chlorophylls9. In most plant species, flowering is a highly intricate process that depends on both endogenous and exogenous factors. Regarding endogenous factors, flowering may be determined by local elongation growth and local ion accumulation, which in turn depend on the genetic background of the genotype studied. External or exogenous factors include temperature, quality and quantity of light, and duration of both light and darkness32. Senescence is also a highly regulated active process, which involves metabolic changes that are part of a genetically-based program leading to death of the cells involved33. Flower senescence refers to a series of deterioration phases involving petals, corolla and tepals, and translocation of nutrients out of the flower to sustain growth and development of seeds34,35. Under our experimental conditions, La improved flower quality parameters and hence delayed senescence.
Stem length was different among the cultivars evaluated. In a study involving the tulip cultivars Apeldoorn, Paul Richter and Rose Copland, stem elongation was associated with a higher concentration and activity of gibberellins, while differences in stem length were observed among cultivars36. In addition, a higher concentration of gibberellins increased transport of auxins and enhanced their biosynthesis37.
Regarding the treatment solutions employed, from 5 dac the LaCl3 treatment stood out by producing the highest bud length and bud diameter values. The AsA treatment generally resulted in higher bud length and diameter values than the control treatment. It has been well documented that in cut flowers, AsA promotes stomatal closure, decreasing respiration and water loss by transpiration38.
We observed a positive relationship between bud length and stem length measured during vase life, as a function of the treatment solution. In particular, stem length was always greater when La was added, regardless of the source used. In Ile de France tulip, the use of 10 µM La in the nutrient solution during the production cycle increased bud length and diameter compared to the control17. The effects of La on cell division and elongation processes have not yet been fully elucidated and they have been generally studied during the production cycle, by supplying La to the soil, in nutrient solutions or as foliar sprays, with differential results among species. In durum wheat (Triticum durum L.), there was a reduction in root length and a decrease in the mitotic index, from 21.53% in the control to 9.91, 1.90 and 0.69% in plants treated with 0.01, 1.0 and 10.0 mM La3+, respectively12. In tulip, the application of 2.5 mM La3+ inhibited stem elongation, while the addition of 25 mM La3+ resulted in smaller flower diameters compared to the control16.
In daylily (Hemerocallis lilioasphodelus L.), narcissus (Narcissus spp. L.) and snapdragon (Antirrhinum majus L.), the application of La3+ has been shown to favor different yield attributes and floral stem growth39,40. In soybean (Glycine max L.), La induced cell division in roots and consequently increased the mitotic index, but the occurrence of abnormalities such as c-metaphases, which occur simultaneously in the root, adversely affect their growth41.
Stem curvature (negative gravitropism) is another valuable attribute to be evaluated in cut flowers, being a problem that affects the quality of the stems on the market and thus lowers their sales potential. In order to reduce negative gravitropism in tulip floral stems it is necessary to select cultivars that show minimum stem growth in postharvest, as stem elongation is a genetically controlled trait passed down by the parents to their offspring42. In our study, differences among the cultivars evaluated are evident. Importantly, Labilela and Acropolis showed the most pronounced curvatures at the end of vase life (> 50°). Crossing of genotypes that show minimum stem elongation in postharvest can generate new cultivars with less stem growth, and therefore less curvature.
It has been demonstrated that cytosolic Ca2+ acts as a second messenger involved in the gravitropic curvature of floral stems, since it causes a redistribution of auxins along the stems and increases the production of ethylene, thereby generating the curvature43. La3+ inhibits several Ca-dependent processes, blocks Ca channels and stimulates Ca2+-ATPase activity, preventing an increase in cytosolic Ca2+ concentration44, thus reducing stem curvature. However, herein we observed increased stem curvature caused by the La treatment, which is positively associated with stem elongation in the vase. This means that with greater stem elongation the curvature thereof increases in the vase. In snapdragon stems, LaCl3 inhibited stem curvature in a horizontal and vertical position, since the La3+ decreased stem elongation and inhibited various processes dependent on gravitropism43. Furthermore, high doses of LaCl3 (20 to 30 mM) decreased the stem elongation rate40, which indicates that LaCl3 supply inhibits stem curvature as a result of its antagonism against Ca39.
In tulip cultivars Peer Gynt, Maureen and Kingsblood, the application of 25 mM LaCl3 resulted in stem curvature and elongation similar to that observed in snapdragon stems. It is well documented that stem responses to gravistimulation are differential among cultivars, probably caused by a genetic variation in stem elongation, which is positively correlated with stem curvature in response to LaCl316.
During the final stage of senescence, one of the most evident symptoms is the loss of fresh weight due to dehydration, mainly of the petals, which causes wilting45. In cut flowers, vase life is determined by curvature of the flower stem, dehydration and loss of fresh weight46. In the cultivars evaluated, we observed a positive relationship between the accumulated water and the weight of the stems in the vase; this trend was also observed in response to the treatment solutions evaluated. Indeed, we have previously demonstrated that La treatments (either as LaCl3 or as La[NO3]3) extend the vase life of tulip flowers from 9 days (in the control) to 12 days9. In the cultivars Burgundy, Gander, Don Quichott, Upstar and King Blood, vase life had a maximum of 9.0 days, with a minimum of 5.8 days47, while the cultivar Triumph registered 11.8 vase days when treated with a solution containing 75 mL/L humic acid + 10 g/L NPK, while the control (distilled water) resulted in 6.3 vase days48. This behavior was associated with the genetic background of each cultivar evaluated, the culture management and the environment.
In regard to the treatment solutions tested, the longer vase life observed with La3+ was due to the greater stem length, stem fresh weight and solution uptake. As mentioned before, La3+ may exert an antagonistic action with ethylene and increase cellular antioxidant activity, reducing the formation of reactive oxygen species (ROS), which retards senescence49.
It is well known that La promotes antioxidant activity in plants. For instance, the application of 40 µM LaCl3 in rice stimulated the redox system, but concentrations higher than 40 µM drastically depress such activity50. In cut Easter lily flowers, La significantly increased the activities of antioxidant enzymes, while decreasing the concentrations of reactive oxygen species, as compared to the control11. Similar responses have been observed in tomato51,52 and rice49,53. In our study, we tested the effect of LaCl3 and La(NO3)3 × 6H2O at a concentration of 40 µM each on floral quality indicators of 15 commercial tulip cultivars. We measured some physiological and biochemical responses of tulips, though the effect of La on the antioxidant capacity of cut flowers was beyond the scope of our study. In future approaches, we are planning to perform further biochemical and molecular analyses aimed at deciphering the effect of different sources of La on the antioxidant capacity of different plant genotypes.
Rare Earth Elements such as La can accumulate in the environment with potential effects in living organisms and ecosystems. Hence, special attention should be paid to control for desired effects, such as stimulation of productivity, while maintaining quality at acceptable standards54. Though La has not yet demonstrated a defined biological role in humans, it can indeed trigger proliferation, osteogenic differentiation, and mineralization of MC3T3-E1 cells55, while its role as an effective phosphate binder that can control serum phosphate in patients with hyperphosphatemia is well recognized56. Nevertheless, surpassing the toxicological threshold for adverse effects may lead to disruption of diverse food chains, with potential implications for the homeostasis of living systems57. Consequently, according to current scientific surveys, environmental standards should maintain La concentrations below 250 µM. Currently, the development of novel analytical technologies may expand the application of La and other REE in parallel58.
Discoveries on the effects of La on cut tulip flower senescence and quality parameters are of paramount importance to determine the role of this beneficial element on postharvest management of ornamental species. Herein we have demonstrated that La delays senescence and improves postharvest quality in cut tulip flowers. Particularly, La increased bud length and diameter, and stem length, and consequently stem curvature was also increased. Flower stems treated with LaCl3 showed the highest solution uptake. Furthermore, relative stem elongation was the highest in flower stems exposed to either LaCl3 or La(NO3)3, while La(NO3)3 increased stem fresh weight. Regarding the cultivars, Laura Fygi and Rosario registered the highest relative stem elongation, while Lalibela and Acropolis displayed the greatest stem curvature on the last day in vase.
Since lanthanum-containing compounds are commercialized by different companies worldwide, the use of this beneficial element in holding solutions for cut flower postharvest management is feasible. Importantly, the cost of each gram of LaCl3 ranges between 0.002 and 0.7 US dollar (USD) cents, while the price of each gram of La(NO3)3 × 6H2O is around 0.2 and 0.4 USD cents. Therefore, the cost of 100 mL of the solution containing 40 µM La (needed for each tulip floral stem) is approximately 0.15–0.11 USD cents, depending on the use of LaCl3 or La(NO3)3 × 6H2O, respectively. If agrochemical companies are able to develop commercial solutions containing La at a lower price, then the use of this element for cut flowers can be expanded.
Materials and methods
Treatment and experimental design
To test the effect of lanthanum on cut tulip flowers, we used the stems of 15 commercial tulip cultivars. Tulip bulbs of 12 + grade were bought from the Mexican company Akiko, which is the exclusive distributor of the Dutch company Jan de Wit en Zonen B. V. (https://www.jandewitenzonen.com/en/home/) in Mexico. It is important to note that the number 12 refers to the circumference length in cm, while the + symbol is used in commercialization to indicate bulbs which are 12 cm or more in size. Bulbs were sown individually in 2.25 L pots, which contained a 30:70 (v:v) mixture of peatmoss and a volcanic gravel (particle size 3 mm), respectively. Plants were irrigated with half-strength Steiner nutrient solution59, which was prepared using analytical grade reagents (J. T. Baker; Phillipsburg, NJ, USA), and the pH was adjusted to 5.5 every other day. This initial phase of the experiment was carried out under greenhouse conditions with a minimum temperature of 1.5 °C and a maximum of 22 °C, light intensity of 165.4 µmol/m/s (produced with a 50% shade mesh), and average relative humidity of 84%. A total of 150 mL of the Steiner nutrient solution were applied to each individual pot every other day. Plants reached the mature stage at different time points, which depended on each cultivar evaluated. In general, flower stems were cut when flower buds reached < 50 of their color, which corresponded to stage 5 of the scale developed elsewhere60. Immediately after cut, flower stems were placed in individual vases containing the solutions to be tested, and then evaluated in the laboratory.
In the treatment solution of cut tulip flowers, two sources of lanthanum at 40 µM La each were tested: lanthanum chloride (LaCl3) and lanthanum nitrate hexahydrate [La(NO3)3 × 6H2O], while L-ascorbic acid (AsA) at 0.2 g/L was used as a reference solution. The absolute control was distilled water. In all cases, distilled water was used to prepare the treatment solutions tested. L-ascorbic acid (AsA), LaCl3 and La(NO3)3 × 6H2O were provided by Sigma Aldrich (Darmstadt, Germany). One assay per cultivar was established in order to test our treatments, so that 15 independent trials were carried out in the laboratory at room temperature in a 15 × 4 factorial experiment with completely randomized distribution. The study factors were the commercial tulip cultivar (Ac: Acropolis, Ba: Barcelona, GP: Golden Parade, JN: Jan van Nes, La: Lalibela, LF: Laura Fygi, LM: Lefeber’s Memory, PI: Pink Impression, RI: Red Impression, RS: Red Shine, Ro: Rosario, SL: Snow Lady, SS: Synaeda Show, VB: Violet Beauty, and WF: World’s Favorite) and the treatment solution (distilled water as absolute control; AsA, 0.2 g/L as reference solution; LaCl3, 40 µM; and La(NO3)3 × 6H2O, 40 µM). Our experimental unit was a 500 mL glass jar containing one of the four treatment solutions and two flower stems of each cultivar. Consequently, we evaluated 180 experimental units in total. The environmental conditions in the laboratory during the conducting of the experiment were as follows: mean day/night temperature of 20 °C/17 °C, respectively; mean relative humidity of 40%; and 12 h light (12 µmol/m/s light intensity) photoperiod. The stems of all cultivars received the same agronomic and nutritional management during the experimental period. Stems were cut at the beginning of flowering and exposed to the treatment solutions as previously described61. All treatment solutions tested [control, L-ascorbic acid, LaCl3 or La(NO3)3 × 6H2O] were applied as steady solutions throughout the duration of the experiment.
Variables evaluated
Once flower stems were cut (day 0), they were immediately placed in glass jars containing the different treatment solutions to be tested. Then, at 3, 5, 7, 9 and 11 days after cutting (dac), we measured bud length and diameter, stem length and curvature, solution uptake and fresh weight of flower stems. Bud length (cm) was measured with a millimeter ruler, from the top of the bud to its receptacle, while flower stem length (cm) was recorded from the junction of the stem and bulb (bottom) to the start of the receptacle (top). Relative Stem Elongation (RSE) was calculated considering the methodology described elsewhere62, according to the following equation: RSE = [Stem length in each treatment or variety 3 dac/Stem length in each treatment or variety 11 dac] × 100, with treatment referring to either AsA, LaCl3 or La(NO3)3 × 6H2O applied in the solution. Bud diameter (cm) was determined using a digital vernier caliper, according to the methodology described elsewhere63,64,65.
Stem curvature was measured using a 180° protractor at 5 dac, 10 dac and on the last day of vase life and expressed with respect to the angle at time of cutting (0°) as described elsewhere59. Solution uptake (mL) was determined by the difference between solution supplied and solution taken up in covered vases, during the different time points measured, using 250 mL graduated cylinders. Fresh weight of flower stems (g) was measured using a digital weighing balance.
Statistical analysis
Data obtained were subjected to an analysis of variance and the Least Significant Difference (LSD) test with a 95% confidence level, using the Statistical Analysis System software66.
References
Sun, J., Jameson, P. E. & Clemens, J. Water relations and stamen abscission in cut flowers of selected Myrtaceae. Acta Hortic. 543, 185–189. https://doi.org/10.17660/ActaHortic.2001.543.22 (2001).
Ketsa, S. & Narkbua, N. Effect of amino oxyacetic acid and sucrose on vase life of cut roses. Acta Hortic. 543, 227–234. https://doi.org/10.17660/ActaHortic.2001.543.27 (2001).
Asrar, A. W. A. Effects of some preservative solutions on vase life and keeping quality of snapdragon (Antirrhinum majus L.) cut flowers. J. Saudi Soc. Agric. Sci. 11(1), 29–35. https://doi.org/10.1016/j.jssas.2011.06.002 (2012).
Ahmad, I., John, M. & Dole, J. M. Homemade floral preservatives affect postharvest performance of selected specialty cut flowers. HortTechnology 24(3), 3384–3393. https://doi.org/10.21273/HORTTECH.24.3.384 (2014).
Barth, C., De Tulio, M. & Conklin, P. L. The role of ascorbic acid in the control of flowering time and the onset of senescence. J. Exp. Bot. 57, 1657–1665. https://doi.org/10.1093/jxb/erj198 (2006).
Azizi, S., Onsinejad, R. & Kaviani, B. Effect of ascorbic acid on post-harvest vase life of cut lisianthus (Eustoma grandiflorum L.) flowers. ARPN J. Agric. Biol. Sci. 10, 417–420 (2015).
Sheikh, F., Neamati, S. H., Vahdati, N. & Dolatkhahi, A. Study on effects of ascorbic acid and citric acid on vase life of cut lisianthus (Eustoma grandiflorum) ‘Mariachi Blue’. J. Ornam. Plants 4(4), 57–64 (2014).
Gómez-Merino, F. C. & Trejo-Téllez, L. I. The role of beneficial elements in triggering adaptive responses to environmental stressors and improving plant performance. In Biotic and Abiotic Stress Tolerance in Plants (ed. Vats, S.) 137–172 (Springer, Berlin, 2018). https://doi.org/10.1007/978-981-10-9029-5_6.
Kotelnikova, A., Fastovets, I., Rogova, O. & Volkov, D. S. La, Ce and Nd in the soil-plant system in a vegetation experiment with barley (Hordeum vulgare L.). Ecotoxicol. Environ. Saf. 206, 111193. https://doi.org/10.1016/j.ecoenv.2020.111193 (2020).
IUPAC. Nomenclature of Inorganic Chemistry, Recommendations (RCS Publishing, Norfolk, 2005).
Aide, M. T. & Aide, C. Rare earth elements: their importance in understanding soil genesis. ISRN Soil Sci. 2012, Article ID 783876, 1‒11. https://doi.org/10.5402/2012/783876 (2012).
d’Aquino, L., de Pinto, M. C., Nardi, L., Morgana, M. & Tommasi, F. Effect of some light rare earth elements on seed germination, seedling growth and antioxidant metabolism in Triticum durum. Chemosphere 75, 900–905. https://doi.org/10.1016/j.chemosphere.2009.01.026 (2009).
García-Jiménez, A., Gómez-Merino, F. C., Tejeda-Sartorius, O. & Trejo-Téllez, L. I. Lanthanum affects bell pepper seedling quality depending on the genotype and time of exposure by differentially modifying plant height, stem diameter and concentrations of chlorophylls, sugars, amino acids, and proteins. Front. Plant Sci. 8, 308. https://doi.org/10.3389/fpls.2017.00308 (2017).
He, Y. W. & Loh, C. S. Induction of early bolting in Arabidopsis thaliana by triacontanol, cerium and lanthanum is correlated with increased endogenous concentration of isopentenyl adenosine (iPAdos). J. Exp. Bot. 53, 505–512. https://doi.org/10.1093/jexbot/53.368.505 (2002).
Shan, C. & Zhao, X. Lanthanum delays the senescence of Lilium longiflorum cut flowers by improving antioxidant defense system and water retaining capacity. Sci. Hortic. 197, 516–520. https://doi.org/10.1016/j.scienta.2015.10.012 (2015).
Kim, H. J., Holcomb, E. J. & Brown, K. M. Lanthanum effects on gravitropic responses of cut tulip flowers. Acta Hortic. 669, 417–423. https://doi.org/10.17660/ActaHortic.2005.669.55 (2005).
Ramírez-Martínez, M. et al. Effect of lanthane on quality of tulip flower “Ile de France”. Acta Hortic. 847, 295–300. https://doi.org/10.17660/ActaHortic.2009.847.39 (2009).
Agathokleous, E., Kitao, M. & Calabrese, E. J. The rare earth element (REE) lanthanum (La) induces hormesis in plants. Environ. Pollut. 238, 1044–1047. https://doi.org/10.1016/j.envpol.2018.02.068 (2018).
Mattson, M. P. Hormesis defined. Ageing Res. Rev. 7(1), 1–7. https://doi.org/10.1016/j.arr.2007.08.007 (2008).
Agathokleous, E., Kitao, M. & Calabrese, E. J. Hormetic dose responses induced by lanthanum in plants. Environ. Pollut. 244, 332–341. https://doi.org/10.1016/j.envpol.2018.10.007 (2019).
Ramírez-Martínez, M. et al. Bioaccumulation of potassium, calcium and lanthanum in tulip treated with lanthanum. Terra Latin. 30, 229–238 (2012).
Xie, W. et al. Effect of exogenous lanthanum on accumulation of cadmium and its chemical form in tomatoes. Wuhan Univ. J. Nat. Sci. 19, 221–228. https://doi.org/10.1007/s11859-014-1005-5 (2014).
Chen, W. H. & Lu, X. F. Effects of lanthanum and samarium on senescence of cut rose (Rosa hybrida Hort.) flowers. Plant Physiol. Commun. 42, 239–241 (2006).
Anjum, M. A., Naveed, F., Shakeel, F. & Amin, S. Effect of some chemicals on keeping quality and vase-life of tuberose (Polianthes tuberosa L.) cut flowers. J. Res. (Sci.) 12, 1–7 (2001).
Mensuali-Sodi, A. & Ferrante, A. Physiological changes during postharvest life of cut sunflowers. Acta Hortic. 669, 219–224. https://doi.org/10.17660/ActaHortic.2005.669.28 (2005).
Jin, J., Shan, N., Ma, N., Bai, J. & Gao, J. Regulation of ascorbate peroxidase at the transcript level is involved in tolerance to postharvest water deficit stress in the cut rose (Rosa hybrida L.) cv. Samantha. Postharv. Biol. Technol. 40, 236–243. https://doi.org/10.1016/j.postharvbio.2006.01.014 (2006).
Ieamtim, P., Buanong, M. & Kanlayanarat, S. Role of ascorbic acid on vase life of red ginger (Alpinia purpurata (Vieill.) K. Schum). Acta Hortic. 804, 287–290. https://doi.org/10.17660/ActaHortic.2008.804.39 (2008).
Bhaskar, V. V., Venkata, R. P. & Reddy, R. S. Effect of different chemicals on the microbial growth during vase life period of cut rose cv. ‘First Red’ Int. J. Curr. Microbiol. App. Sci. 6, 812–820 (2017).
Budiarto, K. Effects of ascorbic acids on post-harvest longevity of chrysanthemum cut flowers. Planta Trop. 7(1), 34–40. https://doi.org/10.18196/pt.2019.091.33-40 (2019).
Hu, Z., Richter, H., Sparovek, G. & Schnug, E. Physiological and biochemical effects of rare earth elements on plants and their agricultural significance: a review. J. Plant Nutr. 27, 183–220. https://doi.org/10.1081/PLN-120027555 (2004).
Bashir, M., Khan, M. A., Qasim, M. & Basra, S. M. A. Evaluation of commercial tulip accessions for flowering potential in climatic conditions of Faisalabad. Int. J. Agric. Biol. 20, 25–32. https://doi.org/10.17957/IJAB/15.0323 (2018).
van Doorn, G. W. & van Mesteren, U. Flower opening and closure. A review. J. Exp. Bot. 54, 1801–1812. https://doi.org/10.1093/jxb/erg213 (2003).
Woltering, E. J. Postharvest biology-Flower senescence. In Encyclopedia of Applied Plant Science (eds Denis, B. T. et al.) 292–299 (Elsevier, Amsterdam, 2017). https://doi.org/10.1016/B978-0-12-394807-6.00010-1.
van Doorn, W. G. & Woltering, E. J. Physiology and molecular biology of petal senescence. J. Exp. Bot. 59, 453–480. https://doi.org/10.1093/jxb/erm356 (2008).
Rogers, H. J. From models to ornamentals: how is flower senescence regulated?. Plant Mol. Biol. 82, 563–574. https://doi.org/10.1007/s11103-012-9968-0 (2013).
Hanks, R. G. & Menhenett, R. Responses of potted tulips to novel growth-retarding chemicals and interactions with time of forcing. Sci. Hortic. 21, 73–83. https://doi.org/10.1016/0304-4238(83)90188-7 (1983).
Sanlewski, M. & Kawa, L. Hormonal control of growth and development of tulips. Acta Hortic. 325, 43–54. https://doi.org/10.17660/ActaHortic.1992.325.3 (1992).
Leamtim, P., Buanong, M. & Kanlayanarat, S. Role of ascorbic acid on vase life of red ginger (Alpinia purpurata (Vieill.) K. Schum). Acta Hortic. 804, 287–290. https://doi.org/10.17660/ActaHortic.2008.804.39 (2008).
Brown, P. H., Rathjen, A. H., Graham, R. D. & Tribe, D. E. Rare earth elements in biological systems. In Handbook on the Physics and Chemistry of Rare Earths (eds Gschneidner, K. A. & Eyring, L.) 423–452 (Elsevier, Amsterdam, 1990).
Friedman, H. et al. Inhibition of the gravitropic response of snapdragon spikes by the calcium-channel blocker lanthanum chloride. Plant Physiol. 118, 483–492. https://doi.org/10.1104/pp.118.2.483 (1998).
De Oliveira, C. et al. Bioaccumulation and effects of lanthanum on growth and mitotic index in soybean plants. Ecotoxicol. Environ. Saf. 122, 136–144. https://doi.org/10.1016/j.ecoenv.2015.07.020 (2015).
van der Meulen-Muisers, J. J. M., van Oeveven, J. C. & van Tuyl, J. M. Breeding as a tool for improving postharvest quality characters of lily and tulip flowers. Acta Hortic. 430, 569–575. https://doi.org/10.17660/ActaHortic.1997.430.91 (1997).
Philosoph-Hadas, S., Meir, S., Rosenberger, I. & Halevy, A. H. Regulation of the gravitropic response and ethylene biosynthesis in gravistimulated Snapdragon spikes by calcium chelators and ethylene inhibitors. Plant Physiol. 116, 301–310. https://doi.org/10.1104/pp.110.1.301 (1996).
Belyauskaya, N. A. Calcium and graviperception in plants inhibitor analysis. Int. Rev. Cytol. 168, 123–185. https://doi.org/10.1016/S0074-7696(08)60884-0 (1996).
Navabpour, S. et al. Expression of senescence-enhanced genes in response to oxidative stress. J. Exp. Bot. 54, 2285–2292. https://doi.org/10.1093/jxb/erg267 (2003).
Francescangeli, P. & Frangi, R. F. Adaptación del tulipán a zonas de inviernos templados de Argentina. Inf. Técnica Econ. Agrar. 102, 278–287 (2006).
Ahmed, J. M. & Khurshid, S. Performance of tulip (Tulipa gesneriana) varieties under Rawalakot conditions. Asian J. Plant Sci. 3, 170–173. https://doi.org/10.3923/ajps.2004.170.173 (2004).
Ali, A. et al. Enhancing the vase life of tulip (Tulipa gesneriana L.) using various pulsing solutions of humic acid and NPK. Int. J. Plant Anim. Environ. Sci. 4(2), 193–200 (2014).
Fashui, H. et al. Effect of La (III) on the growth and aging of root of loquat plantlet in vitro. Biol. Trace Elem. Res. 104, 185–191. https://doi.org/10.1385/BTER:104:2:185 (2005).
Zheng, H., Zhang, C., Zhao, Z., Ma, J. & Huang, X. Effects of lanthanum chloride on activity of redox system in plasma membrane of rice seedling roots. J. Rare Earths 20, 156–157 (2002).
Ippolito, M. P., Fasciano, C., d’Aquino, L. & Tommasi, F. Responses of antioxidant systems after exposition to rare earths and their role in chilling stress in common duckweed (Lemna minor L.): a defensive weapon or a boomerang?. Arch. Environ. Contam. Toxicol. 58, 42–52. https://doi.org/10.1007/s00244-009-9340-9 (2010).
Huang, G. & Shang, C. Lanthanum improves the antioxidant capacity in chloroplast of tomato seedlings through ascorbate-glutathione cycle under salt stress. Sci. Hortic. 232, 264–268. https://doi.org/10.1016/j.scienta.2018.01.025 (2018).
Liu, D., Zheng, S. & Wang, X. Lanthanum regulates the reactive oxygen species in the roots of rice seedlings. Sci. Rep. 6, 31860. https://doi.org/10.1038/srep31860 (2016).
Agathokleous, E., Kitao, M. & Calabrese, E. J. Hormesis: a compelling platform for sophisticated plant science. Trends Plant Sci. 24(4), 318–327. https://doi.org/10.1016/j.tplants.2019.01.004 (2019).
Liu, D. et al. The dual-effects of LaCl3 on the proliferation, osteogenic differentiation, and mineralization of MC3T3-E1 cells. Biol. Trace Elem. Res. 150(1–3), 433–440. https://doi.org/10.1007/s12011-012-9486-6 (2012).
Ohno, M. et al. Lanthanum carbonate for hyperphosphatemia in patients on peritoneal dialysis. Perit. Dial. Int. 33(3), 297–303. https://doi.org/10.3747/pdi.2012.00600 (2013).
Pagano, G., Guida, M., Tommasi, F. & Oral, R. Health effects and toxicity mechanisms of rare earth elements. Knowledge gaps and research prospects. Ecotoxicol. Environ. Saf. 115, 40–48. https://doi.org/10.1016/j.ecoenv.2015.01.030 (2015).
Li, J. et al. Coherent toxicity prediction framework for deciphering the joint effects of rare earth metals (La and Ce) under varied levels of calcium and NTA. Chemosphere 254, 126905. https://doi.org/10.1016/j.chemosphere.2020.126905 (2020).
Steiner, A. A. The universal nutrient solution. In Proceedings of the Sixth International Congress on Soilless Culture held on 29 April–5 May 1984, Lunteren, The Netherlands (1984).
Aros, D., Orellana, K. & Escalona, V. Modified atmosphere packaging as a method to extend postharvest life of tulip flowers. New Zeal. J. Crop Hortic. 45, 202–215. https://doi.org/10.1080/01140671.2017.1296872 (2017).
Gómez-Merino, F. C., Ramírez-Martínez, M., Castillo-González, A. M. & Trejo-Téllez, L. I. Lanthanum prolongs vase life of cut tulip flowers by increasing water consumption and concentrations of sugars, proteins and chlorophylls. Sci. Rep. 10, 4209. https://doi.org/10.1038/s41598-020-61200-1 (2020).
Oki, L., Mattson, N. & Lieth, J. Predicting stem length of cut flower roses at harvest using stem elongation rates in relationship to developmental events. Acta Hortic. 718, 113–120. https://doi.org/10.17660/ACTAHORTIC.2006.718.12 (2006).
Cheng, F., Zhong, Y., Long, F., Yu, X. & Kamenetsky, R. Chinese herbaceous peonies: Cultivar selection for forcing culture and effects of chilling and gibberellin (GA3) on plant development. Isr. J. Plant Sci. 57, 357–367. https://doi.org/10.1560/IJPS.57.4.357 (2009).
Liu, C. et al. Segregation correlation of SSR markers and flower traits in aneuploid tobacco. World J. Eng. Technol. 6, 225–240. https://doi.org/10.4236/wjet.2018.62013 (2018).
Pérez-Harguindeguy, N. et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 61, 167–234. https://doi.org/10.1071/BT12225 (2013).
SAS. SAS Institute Inc. SAS/STAT Users Guide. Version 9.3. Carry, N. C.: SAS Institute Inc. (2011).
Acknowledgements
FCGM acknowledges the infrastructure facilities and support provided by the Institute of Horticulture of Chapingo Autonomous University (Mexico) during his sabbatical leave.
Author information
Authors and Affiliations
Contributions
F.C.G.-M.: Conceptualization, Investigation, Writing – original draft. A.M.C.-G.: Methodology, Supervision, Writing – review & editing. M.R.-M.: Investigation, Methodology, Writing – original draft. L.I.T.-T.: Conceptualization, Funding, Methodology, Supervision, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gómez-Merino, F.C., Castillo-González, A.M., Ramírez-Martínez, M. et al. Lanthanum delays senescence and improves postharvest quality in cut tulip (Tulipa gesneriana L.) flowers. Sci Rep 10, 19437 (2020). https://doi.org/10.1038/s41598-020-76266-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-020-76266-0
This article is cited by
-
GABA-Mediated Defense Mechanisms Against Oxidative Stress: A Case Study in Lilium tigrinum Cut Flowers
Journal of Plant Growth Regulation (2025)
-
Nitric oxide effectively curtails neck bending and mitigates senescence in isolated flowers of Calendula officinalis L.
Physiology and Molecular Biology of Plants (2021)