Abstract
Traditional fermented Rosa (TFR) is a typical food and medical product among the Dali Bai people, and its popularity is growing. A few studies have looked into TFR's medicinal advantages, linked germplasm resources, traditional processing procedures, and functional food qualities. Our goal was to look into Rosa's traditional processing, examine the dominant strains in TFR, and prove how these strains affected antioxidant and tyrosinase inhibitory activities. We used a snowball selection strategy to pick 371 informants for a semi-structured interview, supplemented with direct observations and sample collection. A microbial strain was isolated and identified from a TFR sample collected in the field. We synthesized TFR in the lab using the traditional way. Both of 2, 2-diphenyl-1 picrylhydrazyl (DPPH) free radical scavenging and tyrosinase inhibitory properties of the fermented solution of Rosa 'Dianhong' have been tested in this study. Altogether 15 species belonging to the genus Rosa, which are utilized in herbal medicine and fermented foods. Rosa 'Dianhong' was the Bai community's principal species with considerable cultural value and consumption. Raw Rosa petals included 15 major flavonoids and phenols, which were identified as TFR's active components. TFR-1 was discovered to be the dominating microbial strain in TFR, increasing total phenolic and flavonoid content in the fermented solution of Rosa 'Dianhong' by 0.45 mg GAE/ml and 0.60 mg RE/ml, respectively, after 30 days. TFR-1 also exhibited promising activity in terms of DPPH free radical scavenging and tyrosinase inhibition. TFR showed potent antioxidant and free-radical scavenger properties and is beneficial in skincare and nutrition, according to the findings. TFR's medicinal and edible properties suggest that it could be used as a cosmetic or nutraceutical product.
Similar content being viewed by others
Introduction
Fermentation has a long history in human food production and is a valuable process in food industries, making food products available throughout the year1,2. Fermentation of edible items improves product quality and creates diverse flavor components, which boosts consumer acceptability3,4,5. Furthermore, by adding salt and producing acid and ethanol, the fermentation process can improve the nutritional and functional qualities of foods while also extend their shelf life2. Microorganisms and their enzymes cause fermentation, which is a biochemical alteration of the fundamental food matrix. Traditional knowledge of the fermentation of edibles has shown pharmacological activity that can be used to cure a variety of diseases. Fermentation, for example, allows for the manufacture of broccoli products that are safe, stable, and high in sulforaphane. These fermented foods are fantastic dietary supplements6. It also contains anti-cancer7,8, anti-diabetes9, and anti-obesity10 properties, as well as helping to alleviate behavioral issues linked with autism spectrum disorder11. Fermentation also enhances the antioxidant activity and tyrosinase inhibitory activity of phenols12 and flavonoid antioxidants13.
The adaptive nature of the fermentation process within a given region arises from centuries of human relationships with the microbial community in the environment. Microorganisms, inorganic components, and their interactions all play a role in fermentation processes and product creation14. In traditional fermentation processes, such as those of Brassica oleracea L., Oryza sativa L., and Glycine max (Linn.) Merr., microbial sources are either internal to plants or derived from external surfaces and the surrounding environment. Fungi and bacteria, particularly yeast and lactic acid bacteria populations among the microorganisms that increase the quality of traditional fermentation products3,4. Traditional fermentation is a promising method for isolating certain microorganisms’ impact on biosynthesis or breakdown of bioactive substances.
Traditional fermented Rosa (TFR) is a biocultural heritage among Dali Bai communities in northwest Yunnan, China, which has evolved over a long time as a result of interactions between local cultures and their environment15. TFR is widely acknowledged in Dlai Bai communities as a medical food that nourishes the body and keeps the skin smooth. However, until today, knowledge of traditional TFR processing has been preserved within the community. TFR’s plant resources, fermentation method, and bioactivity have never been comprehensively described. When the globe is confronting a pandemic like Covid-19, research into traditional knowledge around such a vital food source and its bioactivity is critical. Functional foods with health advantages are in high demand. To that end, the goal of this study was to determine the main microbial strains and relative bioactivities of TFR, as well as clarify the plant resources and conventional processing methods employed in TFR. Our research not only contributes to biocultural preservation, but it also gives critical information for the future development of TFR-based pharmaceuticals and prospective nutraceuticals.
Results
Diversity of the genus of Rosa in Dali Bai communities
Our informants told the interviewers about 15 Rosa species used in Dali for traditional applications. Both wild and cultivated species are in use as food and medicine. Table 1 shows the usage information for these 15 species. The informants reported that Rosa species have anti-aging properties; cure rheumatism and dehydration; activate blood circulation; and possess detoxification, insecticidal, and diuretic properties. These plants are used as medicine, food, and fragrance, with different methods and frequency of use.
The Dali Bai communities primarily use 10 of the 15 species for medicine, 7 for food, and 2 for fragrance. Rosa damascena is one of the main species used for essential oil extraction. Of the seven edible species, Rosa ‘Dianhong’ scored the highest informant consensus factor (ICF)16 and use frequency (f)17 values, indicating its cultural value and extent of consumption (Table 1). Rosa ‘Dianhong’ is used in petal tea, wine, sugar, and TFR. TFR is the most popular among these uses.
The TFR in Dali Bai communities
TFR in Dali was prepared by natural fermentation. Petals picked from locally available edible Rosa plants were the main ingredient in TFR (Fig. 1). The traditional planting of edible Rosa resources including Rosa ‘Dianhong’, Rosa ‘Mohong’, Rosa laevigata, Rosa rugosa, Rosa roxburghii, Rosa gallica, and Rosa centifolia. Rosa ‘Dianhong’ was mainly used in TFR preparations, while Rosa ‘Mohong’ was used as an ingredient of TFR only in Heqing county. According to our informants, the thinner petals of Rosa ‘Dianhong’ have better flavor after fermentation compared with those of Rosa ‘Mohong’.
All informants mentioned that TFR benefits the skin. The majority of informants (83.3%) told us that TFR can nourish the stomach, and a few informants mentioned that TFR is hepatoprotective and refreshing. Although none of the informants could explain the pharmacological reasons, these traditional uses hint at potential therapeutic value.
Microbial strain identification
Microbial strains were isolated from TFR prepared traditionally by the Bai people in Dali. The ITS fragment from the dominant strain, TFR-1 (Table 2), shared 99.84% similarity with that of Saccharomyces rouxii. Therefore, the strain TFR-1 was named Saccharomyces rouxii TFR-1. The strain is now preserved in the China General Microbial Culture Collection Center (CGMCC) with the sample number CGMCC19335.
Pharmacological properties of FSR
Antioxidant activity
The free radical scavenging activity of FSR after different periods of fermentation were examined. As shown in Fig. 2. At low concentration (i.e., 0.025 mg/ml, 0.05 mg/ml, and 0.1 mg/ml), samples FSR-3, FSR-7, FSR-14, FSR-21, and FSR-30 showed better DPPH free scavenging activities than FSR-0. At a concentration of 0.05 mg/ml, this difference was significant (P < 0.05). FSR-21 had the highest DPPH scavenging activity, reaching 44.60%. At concentrations greater than 0.2 mg/ml, there was no significant difference in the DPPH scavenging activity of FSR-3, FSR-7, FSR-14, FSR-21, and FSR-30 compared with that of FSR-0. However, with increasing concentration, the trend for greater scavenging activity with longer fermentation began to flatten. DPPH radical scavenging rates of these samples were higher than 85% after 30 days. Table 3 shows the IC50 values for FSR and the positive control.
Effects of fermentation time and concentration of FSR on DPPH radical scavenging activity. Each value represents the mean ± SD (n = 3). Different numbers of asterisks represent different levels of significance from one-way ANOVA: a, compared with FSR-0; b, compared with the previous sample; *P < 0.05; **P < 0.01; ***P < 0.001.
Tyrosinase inhibition activity
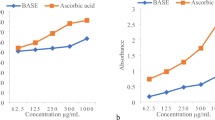
At a concentration of 0.125 mg/ml, tyrosinase inhibition activity of FSR was insignificant at all time points compared with FSR-0, except in the FSR-30 sample (5.48%; P < 0.05) (Fig. 3). However, as the concentration of FSR increased, tyrosinase inhibition activity also increased. At a concentration of 0.5 mg/ml, tyrosinase inhibition by FSR-7, FSR-14, FSR-21, and FSR-30 was significantly different from that by FSR-0 (P < 0.05). The confidence level for the FSR-30 sample was higher (P < 0.001), and FSR-30 was also significantly different from the FSR-21 sample (P < 0.05). Although only the inhibitory activity of FSR-30 showed a statistical difference compared with FSR-0 at a concentration of 2 mg/ml, the mean value of tyrosinase inhibition increased with increasing fermentation time. At a concentration of 10 mg/ml, tyrosinase inhibition by FSR-3, FSR-7, FSR-14, FSR-21, and FSR-30 was significantly higher than that of FSR-0 (P < 0.001). The highest inhibition rate was 86.58% and was for FSR-30. Among all samples, longer FSR fermentation duration resulted in greater inhibition of tyrosinase activity. Table 4 shows the IC50 values for FSR and the positive control.
Effects of fermentation time and concentration of fermentation solution of Rosa ‘Dianhong’ (FSR) on tyrosinase inhibition. Each value represents mean ± SD (n = 3). Different numbers of asterisks represent different levels of significance from one-way ANOVA: a, compared with FSR-0; b, compared with the previous sample; *P < 0.05; **P < 0.01; ***P < 0.001.
Nutrients in FSR and phytochemical profile
The total phenolic contents of FSR-3, FSR-7, FSR-14, FSR-21, and FSR-30 were significantly higher than that of FSR-0 (P < 0.001) (Table 5). Longer duration of fermentation increased the total phenolic contents of FSR. However, none of the differences between adjacent fermentation intervals, FSR-3, FSR-7, FSR-14, FSR-21, and FSR-30, were significant.
FSR-7, FSR-14, FSR-21, and FSR-30 showed a significant difference in total flavonoid content (P < 0.01) compared with FSR-0 (Table 5). However, different from the total phenolic contents, across the whole fermentation process, the values for total flavonoid contents of FSR-7, FSR-21, and FSR-30 were significantly different from those at the previous time point (P < 0.05). This indicated that fermentation duration plays an important role in increasing total flavonoid content in FSR.
Correlation analysis
Our results revealed that an increase in total phenolic and flavonoid contents produced by increased fermentation time enhances the DPPH free radical scavenging rate and tyrosinase inhibition activity of FSR (Fig. 4). Pearson analysis demonstrated a moderate correlation between total phenolic content and DPPH free radical scavenging activity, but the difference was not statistically significant (P > 0.5) (Fig. 4A). Total phenolic contents were highly correlated with tyrosinase inhibition activity (P < 0.5) (Fig. 4B). These results indicate an increase in phenolic content after fermentation as an important factor triggering tyrosinase inhibition activity of FSR.
Pearson analysis showed that total flavonoid contents after fermentation were highly correlated with DPPH free radical scavenging rate and tyrosinase inhibitory activity (P < 0.5) (Fig. 4C,D). Moreover, the total flavonoid contents changed significantly with increases in fermentation time (Table 5).
Main effective compounds in the genus Rosa used by Bai people in Dali
Petals from 15 plant species of the genus Rosa used in traditional applications in Dali Bai communities were collected; most were included in the previous phytochemical and pharmacological studies. The contents of the petals varied, but most of them contained flavonoids and phenols, which are known to be bioactive in vitro. Antioxidation is an important bioactivity of TFR in vitro. For example, according to Vinokur et al.18, the radical scavenging activity in rose petals is mostly due to the high content of phenolic compounds, especially free gallic acid (1; Fig. 5). protocatechuic acid (2), syringic acid (3), anthocyanin (4), 4-hydroxybenzoic acid (5), chlorogenic acid (6), and catechin hydrate (7) are also predominant phenols in rose petals19. These phenols have more hydroxyls, including o-dihydroxy, which demonstrate strong free radical scavenging and antioxidant abilities20. Flavonoids possess a galloyl ester in the C ring, which are important structures for chelation of metal ions, formation of complexes with metal ions, and inhibition of metal-initiated lipid oxidation. Therefore, flavonoids are able to effectively scavenge hydroxyl and peroxyl radicals21,22,23. The high concentration of flavonoids in Rosa resulted from the presence of a large amount of naringenin (8), with quercitrin (9), hesperidin (10), quercextin (11), luteolin (12), apigenin (13), and kaempferol (14) also comprising the main effective flavonoid compounds in rose petals; these flavonoid compounds contribute towards the antioxidant capacities of rose19. According to Jin24, the main flavonoids in rose petals include rubin (15), quercetin (11), kaempferol (14), and their derivatives; these compounds contributed more than 60% to the flavonoids in TFR rose petals, and our correlation analysis showed that they were significantly related to antioxidant activity. Flavonoids also slow tyrosinase activities by interacting with copper ions essential to the active site of tyrosinase. Tyrosinase, a key enzyme in skin pigmentation, catalyzes hydroxylation of monophenols to o-diphenols and oxidation of o-diphenols to o-quinones, which generates melanin25. Excessive tyrosinase can cause freckles, melasma, skin cancer, and age spots26,27. Therefore, flavonoids are considered to be a natural tyrosinase inhibitor.
Discussion
Traditional edible flowers have been utilized as a therapeutic food in China for thousands of years28 because of their emollient, antibacterial, and anti-inflammatory properties. Many Dali Bai groups use specific plants because they are considered healthy, according to our ethnobotanical survey. Specific species, biochemical ingredients, or pharmacological qualities are the subject of most studies on the beneficial properties of food plants. Many researchers, for example, are looking for potential nutritional supplements against cancer in food plants. Antioxidants play a role in cancer prevention, and many types of research have been conducted on these substances. Apium nodiflorum, Humulus lupulus, Silene vulgaris29, Nasturtium officinale30, and Leopoldia comosa bulbs31 all have strong antioxidant capabilities. Our survey showed that Rosa 'Dianhong' petals are most commonly employed in traditional meals as a classic sauce, and their phenolic and flavonoid components have been linked to health benefits. Fermentation changes the chemical composition of food, potentially increasing its biological activity32. Fermentation is traditionally employed for food preservation3,33, but it is also an important biotransformation process for producing new products or crude materials6,34. Fermentation appears to increase the total phenolic and flavonoid content of FSR, as suggested by our findings. According to Pearson analysis, there is a strong link between total phenolic and flavonoid content in FSR and tyrosinase inhibition, consistent with prior research35. TFR can play a crucial role in conferring antioxidant and tyrosinase inhibitory action by boosting phenols and flavonoids. PDA isolated TFR-1, which was later recognized as belonging to Saccharomyces rouxii. It is a yeast commonly employed in soy sauce fermentation and safe to consume as it has been consumed for a long time in human history. This strain can withstand high osmotic pressure because it produces sugars, alcohols, acids, and other chemicals involved in sugar and energy metabolism to protect cells under hypertonic culture conditions36. Traditional Rosa fermentation uses a 50 percent sugar concentration to retain sweetness and taste under sugar stress to generate a high osmotic pressure environment that inhibits putrefactive, pathogenic, and other bacteria, most beneficial bacteria do not adapt to high osmotic pressure conditions, their usage is limited37. Only one strain, TFR-1, could tolerate the high osmotic pressure and play an important role in TFR processing in Dali Bai populations. Because of its excellent osmotic pressure resistance, non-toxicity to humans, and other possible functions in healthcare, the strain in this study is worth further investigation.
Conclusion
In the Dali Bai villages in Northwest Yunnan, China, TFR is a traditional medical food. As evidenced by our ethnobotanical survey, local people sustain the traditional TFR process, which has been passed down from generation to generation through these activities. Our findings show that TFR prepared traditionally contains strain TFR-1 (Saccharomyces rouxii) as the most important microbial content that facilitates fermentation and impacts TFR quality. It increased the antioxidant activity and tyrosinase inhibitory activity of FSR. These critical functional activity alterations provide applicable research for TFR products, mainly cosmetic or nutraceutical products. Traditional medical food culture promotes environmental protection, protects fast vanishing traditional knowledge, and has numerous applications in other domains of human activity. To that end, this work underlines the importance of continuing research on traditional fermented food products and related traditional knowledge as a method of discovering new strains and expanding the commercial potential. Our findings also highlight the importance of traditional items in creating modern healthcare, food, and cosmetic businesses.
Materials and methods
Study area
This study was conducted in Dali Bai Autonomous Prefecture in northwest Yunnan Province, China. The average elevation of this area is 2090 m. The area receives an average of 776 mm annual precipitation, and the average annual temperature is 16.5 °C38. It is the homeland of the Bai people, and therefore Bai communities are most densely settled. Diverse geographical conditions mean Dali possesses rich flower resources, forming the necessary material for the inheritance of an edible flower culture. “Selling flowers by weighing” is an edible flower custom among 26 ethnic groups in Yunnan, where 140 species of edible flowers have been reported39. Dali Bai people’s “hundred flowers banquet” has a long history and is famous in Yunnan. Rosa species including Rosa ‘Dianhong’, Rosa gallica L., Rosa banksiae R. Br., and Rosa multiflora Thunb. var. Carnea Thory are among the common edible flowers used by the Bai people. These flowers are fried, steamed, boiled, pickled, and fermented prior to consumption. Fermentation ensures the availability of edible flower resources all year.
Field work and ethnobotanical investigation
After consultation with local government officials and preliminary field visits, 15 traditional communities were selected for the investigation. The investigation was conducted between April 2017 and October 2018 in five counties: Dali, Weishan, Eryuan, Jianchuan, and Heqing (Fig. 6). Field work strictly obeyed the International Society of Ethnobiology Code of Ethics (2006 convention with 2008 additions)40. First, the purpose of the study was explained to key informants of Bai communities. Local community committees were then visited to obtain field study permission and request assistance. The assistance included introduction to community members and heirs of the intangible cultural heritage of TFR and organization of representative workshops. All field studies were carried out with informed consent. An ethnobotanical survey was conducted among 371 informants (167 men and 204 women) (Table 6) using the snowball sampling method to select potential informants41. Herbalists, farmers, merchants, and indigenous people were among the informants. They were engaged in the collection, production, sales, and use of the genus Rosa. Women are frequent users of the genus Rosa and accounted for 55% of informants. Men who are herbalists, farmers, and vendors are associated with the value chain of Rosa. All had long-term experience with the applications of the genus Rosa.
Location map of study sites. (Figure is created by ArcGIS 10.8, https://developers.arcgis.com, the satellite imagery was generated by Google Earth 7.3.4.8248, https://google-earth.en.softonic.com).
Semi-structured interviews were conducted with the consent of local people. The interviews focused on the following questions:
-
(1)
Does the community use the Rosa species? If yes, how many species are in traditional use?
-
(2)
Where do you get these plants?
-
(3)
How do you use these plants?
-
(4)
Does the community prepare TFR? If yes, which species of Rosa are in use?
-
(5)
Which ingredients are necessary for making TFR, and how do you prepare TFR?
-
(6)
How long does it require to make a TFR?
-
(7)
How do you consume TFR?
-
(8)
What are the benefits of TFR to health, and what effects have been noticed?
Voucher specimens of 20 Rosa species naturally occurring in the study area were collected. A taxonomist and ethnobotanist at Kunming Institute of Botany identified the voucher specimens. All specimens collected during the field survey were deposited in the Herbarium of Kunming Institute of Botany.
Isolation, purification, and identification of strains
TFR prepared traditionally by the Bai people in Dali was used for strain isolation. TFR solution was prepared in sterile water at different concentrations (10–1–10–5). The dilution coating method was applied42. The diluent was inoculated onto a PDA (potato dextrose agar) medium for culture of fungi43 and onto NA (nutrient agar) medium for culture of bacteria44,45. After growth, fungal and bacterial colonies were purified using the plate streak method45. Purified materials, which were similar to yeast, were stored at 4 °C for preservation. PDA and NA medium were both from Qingdao Rishui Biotechnology Co., Ltd. The strain was named TFR-1 and deposited at the Kunming Institute of Botany, Chinese Academy of Sciences.
To ascertain the identity of strain TFR-1, its total genomic DNA was extracted using a DNA extraction kit from Beijing Tsingke Xinye Biotechnology Co., Ltd. (Tsingke) using the CTAB/SDS method46. The ITS1 region from the DNA sample was amplified by PCR using an Applied Biosystems 2720 thermal cycler, while fungal sequences were amplified using universal primers (Tsingke). Amplified PCR fragments were sequenced by Tsingke and used as a query sequence in a BLASTN search against the NCBI public database, following the method described by Blanc et al.47.
Preparation of Rosa fermentation and sampling
TFR was prepared in the laboratory following the traditional method. Petals of Rosa ‘Dianhong’ that is traditionally used in TFR preparation in Dali were purchased, and 0.5 kg of petals was cut into small pieces to maximize extraction. Brown sugar (0.5 kg) was added and the mixture was kneaded manually until fully wet. The mixture was then placed into a 1-l fermentation bottle and 500 ml sterile water was added. Three bottles of this solution were prepared as replicates. The bottles were exposed to ultraviolet light for 45 min for sterilization on a super purgative working table. After 24 h, 10 ml Rosa ‘Dianhong’ solution from each bottle was removed and stored as sample FSR-0. Then, TFR-1 solution (15 ml) with a concentration of 4.15 × 106 CFU/ml was added to the fermentation bottles, which were sealed with caps and stored at 28 °C in a thermostatic incubator. Fermentation solution of Rosa ‘Dianhong’ (FSR) from each bottle (10 ml) was sampled on days 3, 7, 14, 21, and 30 and labeled FSR-3, FSR-7, FSR-14, FSR-21, and FSR-30, respectively. Each of the samples was centrifuged at 4000 rpm for 15 min, and the supernatant liquid was stored at − 20 °C to cease further fermentation.
Determination of total phenolic contents
Total phenolic contents of FSR were determined spectrophotometrically using Folin–Ciocalteu’s method as described by Kang et al.48 with slight modification. Diluted FSR (0.4%, v/v) was used in the analysis. A 10-μl aliquot of FSR was mixed with 0.25 ml 1 N Folin–Ciocalteu’s reagent in a 5-ml test tube. The mixture was covered and kept still for 2 min in the dark before 0.5 ml 12% (w/v) aqueous solution of Na2CO3 and 1.24 ml distilled water were added. The mixture was incubated for 1 h at room temperature and then ultraviolet absorbance was measured at 765 nm using a UV-5500PC (METASH) against a control. The control sample contained the same amount of chemicals with the FSR replaced by distilled water. Gallic acid (GA) was used as a standard for preparing a calibration curve. Total phenolic contents were expressed as mg GA equivalents per FSR.
Determination of total flavonoid contents
Total flavonoid contents of FSR were measured using the aluminum chloride colorimetric assay49 with slight modification. Diluted FSR (concentration at 1.6%, v/v) was used in the analysis. A 40-μl aliquot of FSR was added into a 5-ml test tube containing 1.31 ml of distilled water and 75 μl of 5% (w/v) NaNO2 was then added. After 5 min, 75 μl 10% (w/v) AlCl3 was added and allowed to react for 6 min before 1 ml of 4% (w/v) NaOH dissolved in distilled water was added. The solution was mixed and kept for 12 min at room temperature before ultraviolet absorbance was measured against the control at 510 nm using a UV-5500PC (METASH). Control samples contained the same amount of chemicals except the FSR was replaced with distilled water. Rutin was used as a standard for constructing a calibration curve. Total flavonoid contents were expressed as mg rutin equivalents per of FSR.
Free radical scavenging activity
The free radical scavenging activity of FSR was examined in vitro using DPPH (2, 2-diphenyl-1 picrylhydrazyl) radical as described by El Atki et al.49. Different concentrations of FSR or GA were added to ethanolic solution and mixed with DPPH dissolved in ethanolic solution so that the final concentration of DPPH was 0.1 mmol. The absorbance of the mixture was measured using a UV-5500PC (METASH) at 517 nm after 30 min of incubation at room temperature in darkness. A control mixture in which FSR was replaced by the equivalent amount of ethanolic solution was prepared following the same procedures. The percentage of inhibition was calculated using the following equation:
Here, ODcontrol is the absorbance of the negative control and ODsample is the absorbance of the sample. GA served as positive control. IC50 values were calculated as the concentration of causing a 50% inhibition of DPPH radical.
Tyrosinase inhibition activity
Tyrosinase inhibitory activity was examined in vitro as described by Elena et al.50. Different concentrations of FSR or kojic acid (KA) were mixed with tyrosinase dissolved in 0.2 M phosphate buffer (pH 6.8) and kept still for 15 min at 37 °C. Next, 12.5 mM L-Dopa dissolved in 0.2 M phosphate buffer (pH 6.8) was added, so that the final concentration of tyrosinase was 25 U/ml and that of L-Dopa was 1.25 mM in the solution. Absorbance at 475 nm was measured using a UV-5500PC (METASH) after incubation for 5 min at room temperature. FSR was replaced by the equivalent amount of phosphate buffer in the control mixture prepared following the same procedures. The percentage of inhibition was calculated using the following equation:
ODcontrol is the absorbance of the negative control and ODsample is the absorbance of the sample. KA served as a positive control. IC50 values were calculated as the concentration of causing a 50% inhibition of tyrosinase.
Statistical analysis
SPSS 22 (Statistical Product and Service Solutions, https://www.ibm.com/products/spss-statistics) was used for statistical analysis. Probit regression analysis was used to analyze the IC50 of DPPH free radical and tyrosinase inhibition. Pearson correlation analysis was used to determine the correlation of total phenolic and flavonoid contents with DPPH free radical scavenging activity and tyrosinase inhibition activity. Differences between means were determined using the least significant difference test at P < 0.05, and figures were drawn using OriginPro 2017 (https://www.originlab.com).
Ethics approval and consent to participate
The Authors confirm that no animal/human studies have been carried out in the present. This study was part of a wider project entitled “Study on traditional fermented rose in Dali Bai Nationality”. We conducted this research in accordance with International Society of Ethnobiology (2006), ISE Code of Ethics (with 2008 additions), and the protocol was approved by Kunming Institute of Botany, Chinese Academy of Sciences (KIB) ethics committee (Supporting documents S1) and Center of Biodiversity and Indigenous Knowledge(CBIK) ethics committee (Supporting documents S2). Before data collection, we described the goals of this research to local informants and asked them to sign a Free andInformed Consent Term. We were authorized to collect plant specimens by Forestry and grassland Bureau of Dali Bai Autonomous Prefecture.
Consent for publication
This manuscript does not contain any individual person's data, not applicable.
Data availability
All data, materials, and information are collected from the study sites.
Abbreviations
- TFR:
-
Traditional fermented Rosa
- FSR:
-
Fermentation solution of Rosa ‘Dianhong’
- TFC:
-
Total flavonoid contents
- TPC:
-
Total phenolic contents
- PDA:
-
Potato dextrose agar (Medium)
- NA:
-
Nutrient agar (Medium)
- DPPH:
-
2, 2-Diphenyl-1 picrylhydrazyl
- GAE:
-
Gallic acid equivalents
- RE:
-
Rutin equivalents
- KA:
-
Kojic acid
References
Nkhata, S. G., Ayua, E., Kamau, E. H. & Shingiro, J. B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 6, 2446–2458 (2018).
Xiang, H., Sun-Waterhousea, D. X., Waterhouse, G. I. N., Cui, C. & Ruan, Z. Fermentation-enabled wellness foods: a fresh perspective. Food Sci. Hum. Wellness 8, 203–243 (2019).
Xiao, Y. S. et al. Correlation between microbiota and flavours in fermentation of Chinese Sichuan Paocai. Food Res. Int. 114, 123–132 (2018).
Pang, X. N. et al. Effect of the environment microbiota on the flavour of light flavour Baijiu during spontaneous fermentation. Sci. Rep. 8, 3396 (2018).
Huang, X. N., Yu, S., Han, B. Z. & Chen, J. Y. Bacterial community succession and metabolite changes during sufu fermentation. LWT Food Sci. Technol. 97, 537–545 (2018).
Cai, Y. X., Wang, J. H. & Catherine, M. A. Fermentation for enhancing the bioconversion of glucoraphanin into sulforaphane and improve the functional attributes of broccoli puree. J. Funct. Foods 61, 103461 (2019).
Matusheski, N. V. & Jeffery, E. H. Comparison of the bioactivity of two glucoraphanin hydrolysis products found in broccoli, sulforaphane and sulforaphane nitrile. J. Agric. Food Chem. 49(12), 5743–5749 (2001).
Soundararajan, P. & Kim, J. Anti-carcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancers. Molecules 23(11), 2983 (2018).
Axelsson, A. S. et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci. Translat. Med. 9(394), eaah4477 (2017).
Martins, T. et al. Poten-tial effects of sulforaphane to fight obesity. J. Sci. Food Agric. 98(8), 2837–2844 (2018).
Singh, K. et al. Sulforaphane treatment of autism spectrum disorder (ASD). Proc. Natl. Acad. Sci. U.S.A. 111(43), 15550–15555 (2014).
Cíntia, L. H. et al. Parameters of the fermentation of soybean flour by Monascus purpureus or Aspergillus oryzae on the production of bioactive compounds and antioxidant activity. Food Chem. 271, 274–283 (2019).
Grażyna, B., Elżbieta, K., Joanna, G., Ilona, G. C. & Radosław, M. Lactic acid fermentation of legume seed sprouts as a method of increasing the content of isoflavones and reducing microbial contamination. Food Chem. 285, 478–484 (2019).
Scott, R. & Sullivan, W. C. Ecology of fermented foods. Hum. Ecol. Rev. 15(1), 25–31 (2008).
Nabhan, G. P. Ethnobiology for a diverse world: microbial ethnobiology and the loss of distinctive food cultures. J. Ethnobiol. Ethnomed. 30(2), 181–183 (2010).
Gazzaneo, L., de Lucena, R. & de Albuquerque, U. Knowledge and use of medicinal plants by local specialists in a region of Atlantic Forest in the state of Pernambuco (Northern Brazil). J. Ethnobiol. Ethnomed. 1(1), 9–9 (2005).
Lozada, M. Nontimber forest product use in two human populations from northwest patagonia: a quantitative approach. Hum. Ecol. 29(4), 367–380 (2001).
Vinokur, Y. et al. Rose petal tea as an antioxidant-rich beverage: cultivar effects. J. Food Sci. 71(1), 542–547 (2006).
Zheng, J. Y., Meenua, M. & Xu, B. J. A systematic investigation on free phenolic acids and flflavonoids profifiles of commonly consumed edible flowers in China. J. Pharm. Biomed. Anal. 172, 268–277 (2019).
Yokozawa, T. et al. Study on the inhibitory effect of tannins and flflavonoids against the 1,1-diphenyl-2- picrylhydrazyl radical. Biochem. Pharmacol. 56, 213–222 (1998).
Chu, S. C. & Chen, C. Effects of origins and fermentation time on the antioxidant activities of kombucha. Food Chem. 98(3), 502–507 (2006).
Khokhar, S. & Owusu Apenten, R. K. Iron binding characteristics of phenolic compounds: some tentative structure–activity relations. Food Chem. 81(1), 133–140 (2003).
Lee, J., Koo, N. & Min, D. B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 3(1), 21–33 (2004).
Jin, J. The Comparative Analysis of Antioxidant Activity and Flavonoid Compositions from Petals of Four Rosa Species, 31–65 (Guizhou Normal University, 2018).
Gou, L. et al. The efect of oxaloacetic acid on tyrosinase activity and structure: integration of inhibition kinetics with docking simulation. Int. J. Biol. Macromol. 101, 59–66 (2017).
Nguyen, H. et al. Tyrosinase inhibitory activity of flavonoids from Artocarpus heterophyllous. Chem. Cent. J. 10, 1–6 (2016).
Zhang, L., Zhao, X., Tao, G., Chen, J. & Zheng, Z. Investigating the inhibitory activity and mechanism dierences between norartocarpetin and luteolin for tyrosinase, a combinatory kinetic study and computational simulation analysis. Food Chem. 223, 40–48 (2017).
Yang, L. X., Gao, Y. & Fan, Y. X. Ethnobotanical study and textual research on skin care efficacy of Rosa. J. Chin. Med. Mater. 41(2), 498–502 (2018).
Morales, P. et al. Tocopherol composition and antioxidant activity of Spanish wild vegetables. Genet. Resour. Crop Evol. 59(5), 851–863 (2012).
Aires, A., Carvalho, R., Rosa, E. A. S. & Saavedra, M. J. Phytochemical characterization and antioxidant properties of baby—leaf water cress produced under organic production system. CyTA-J. Food 11(4), 343–351 (2013).
Pieroni, A. et al. In vitro antioxidant activity of non-cultivated vegetables of ethnic Albanians in southern Italy. Phytother. Res. 16, 467–473 (2002).
Di Cagno, R., Coda, R., De Angelis, M. & Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 33(1), 1–10 (2013).
Ye, J. H., Huang, L. Y., Netsanet, S. T. & Mary, A. A. Fermentation-based biotransformation of glucosinolates, phenolics and sugars in retorted broccoli puree by lactic acid bacteria. Food Chem. 286, 616–623 (2019).
Wu, R. et al. Changes in flavour and microbial diversity during natural fermentation of suan-cai, a traditional food made in Northeast China. Int. J. Food Microbiol. 211, 23–31 (2015).
Jacob, V., Hagai, T. & Soliman, K. Structure-activity relationships of flavonoids. Curr. Org. Chem. 15(15), 2641–2657 (2011).
Han, X. J., Xu, Z. J. & Yue, T. L. Metabolic fingerprinting analysis of Zygosaccha-romyces rouxii under sugar stress. Food Sci. 39(12), 167–173 (2018).
Sperber, W. & Doyle, M. P. Compendium of the Microbiological Spoilage of Foods and Beverages 235–236 (Springer, 2009).
Fan, Y. X. et al. Indigenous knowledge of dye-yielding plants among Bai communities in Dali, Northwest Yunnan, China. J. Ethnobiol. Ethnomed. 14, 74 (2018).
Yang, Y. et al. Investigation on Vegetable-Edible flower plant resources and autophagy culture in Yunnan province. J. Plant Genet. Resour. 18(6), 1125–1136 (2017).
International Society of Ethnobiology. ISE Code of Ethics (with 2008 additions). http://ethnobiology.net/code-of-ethics/ (2006).
Heckathorn, D. D. Snowball versus respondent-driven sampling. Sociol. Methodol. 41, 355–366 (2011).
Dong, H. X. & Shi, M. Z. Isolation and determination of soil microorganisms. Soil Fertil. 20, 072 (2018).
Jiang, M. D. & Hu, X. F. Preliminary study on detection of bacteria in maize seeds. Nong Yi Nong Ji 15, 59–60 (2017) (in Chinese).
Fang, Z. D. Plant Research Methods 47 (China Agricultural Press, 1998).
Chen, Z. B., Chen, X. W. & Xia, T. Y. Study on diversity of cultivable endophytic bacteria isolated from petals of edible roses. Southwest China J. Agric. Sci. 06(29), 1408–1413 (2016).
Zhou, J., Bruns, M. A. & Tiedje, J. M. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 62(2), 316–322 (1996).
Blanc, G. & Wolfe, K. H. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell 16, 1667–1678 (2004).
Kang, W. Y., Li, C. F. & Liu, X. Y. Antioxidant phenolic compounds and flavonoids of Mitragyna rotundifolia (Roxb.) Kuntze in vitro. Med. Chem. Res. 19, 1222–1232 (2010).
El Atki, Y. et al. Total phenolic and flavonoid contents and antioxidant activities of extracts from Teucrium polium growing wild in Morocco. Mater. Today Proc. 13, 777–783 (2019).
Elena, N., Gabriel, L. R., Camelia, A. & Gabriela, P. Antioxidant activity, acetylcholinesterase and tyrosinase inhibitory potential of Pulmonaria officinalis and Centarium umbellatum extracts. Saudi J. Biol. Sci. 25, 578–585 (2018).
Acknowledgements
We gratefully thank the local people and government in Dali, Yunnan Province, China, especially Ms. Chen, who assisted us with the valuable information. In particular, I would like to thank Mr. Zhang and Ms. Du for their valuable comments on the revision of the manuscript.
Funding
This study was funded by Natural Science Foundation of China (Nos. 31670340 and 31970357) and Bio-Innovation Center of DR PLANT, Kunming Institute of Botany, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
L.Y. design and elaborated the project, coordinated the entire study and revised the manuscript; B.L. made all the experiments, and data analysis, S.R. and R.Y. had written and revised the final manuscript; Y.Z. and A.L. conducted field investigation and data collation, analyzed the data and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lang, B., Zhao, Y., Yang, R. et al. Antioxidant and tyrosinase inhibitory activities of traditional fermented Rosa from Dali Bai communities, Northwest Yunnan, China. Sci Rep 11, 22700 (2021). https://doi.org/10.1038/s41598-021-02160-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02160-y
This article is cited by
-
Advancements and prospects in China’s edible rose industry: breeding, cultivation, and processing
Environment, Development and Sustainability (2024)