Abstract
Obesity is strongly correlated with obstructive sleep apnea (OSA), and bariatric surgery can effectively treat obesity and alleviate OSA. However, the contributing factors are still unclear. We aimed to explore the relationship between betatrophin and OSA in patients undergoing Roux-en-Y gastric bypass (RYGB) surgery. Our study consisted of thirty-seven individuals with OSA and type 2 diabetes (16 males, 21 females) undergoing RYGB surgery. The polysomnography test, anthropometric results, serum betatrophin, and abdominal magnetic resonance images were evaluated both before and 1 year after RYGB surgery. Factors that may correlate with the alleviation of OSA were investigated. In our study, RYGB surgery significantly decreased apnea hypopnea index (AHI) and serum betatrophin concentration (p < 0.001). The abdominal visceral fat area, subcutaneous fat area and HOMA-IR were also significantly decreased (p < 0.001). The preoperative AHI, postoperative AHI and the change in AHI were significantly correlated with the preoperative betatrophin, postoperative betatrophin and the change in betatrophin, respectively (p < 0.05). These correlations were still significant after adjustment for other risk factors. The change in betatrophin was also independently associated with the change in minimum oxygen saturation (p < 0.001). Our data might indicate that serum betatrophin was significantly independently correlated with the improvement of OSA after bariatric surgery.
Similar content being viewed by others
Introduction
Obstructive sleep apnea (OSA) is a common disease characterized by recurrent episodes of partial or complete airway obstruction resulting in hypoxemia, hypercapnia, and respiratory arousal1. The prevalence rate of OSA is ~ 4% among various middle-aged male populations, and represents a health risk through direct and indirect mechanisms2. Moreover, it is associated with a fivefold increased risk of metabolic syndrome, such as abnormal lipid metabolism and insulin resistance, in comparison to healthy individuals3. Obesity is considered the strongest risk factor for OSA, because obesity-related systemic inflammatory mediators, localization of excess adipose tissue, reduced chest wall compliance, disturbances in the relationship between respiratory drive and load compensation, reductions in functional residual capacity and hormonal changes may have adverse effect in pharyngeal neural and mechanical control, and weight loss could significantly alleviate the severity of OSA for these possible mechanisms4. Bariatric surgery, one of the most common and effective treatments for obesity, has also been confirmed to be effective to improve OSA5.
However, the underlying factor by which weight loss contributes to alleviation of OSA is unclear. Previous studies have indicated that greater abdominal fat accumulation was associated with more serious OSA6, and changes in visceral adipose tissue volume showed a strong relationship with improvement of OSA7. However, reduced regional fat distribution can only partially explain the alleviation of OSA8. Weight loss may also influence OSA according to its metabolic consequences9,10. Recent evidence indicated that there are changes in inflammatory biomarkers and adipokines after bariatric surgery11,12, but the associations between these changes and alleviation of OSA have not been studied in detail. Betatrophin, known as angiopoietin-like protein 8 or lipasin, is a hormone found in the liver and adipose tissue, and is a potent regulator of lipid metabolism13. Our previous study indicated a significant decrease in serum betatrophin after Roux-en-Y gastric bypass (RYGB) surgery14. Recently, two studies reported a higher serum betatrophin concentration in the OSA group than in the control group15,16. However, the association between serum betatrophin and OSA have not been well evaluated, especially in people with weight loss. This study was performed to evaluate the changes in serum betatrophin in obese patients with OSA after RYGB surgery, and to examine any association between serum betatrophin and OSA.
Methods
Participants and measurements
Thirty-seven consecutive Chinese obese patients with OSA and type 2 diabetes (T2D), who had received RYGB surgery at our hospital, were recruited to this longitudinal retrospective study. Diagnosis of T2D was according to the American Diabetes Association (ADA) diagnostic standard of 2007. RYGB is performed to treat obesity and T2D and is appropriate for Chinese T2D patients with a BMI of 25–35 kg/m217. OSA was defined according to the American Academic Sleep Medicine (AASM) criteria18. All participants were aged 20–70 years. Patients with psychiatric disturbances and those undergoing continuous positive airway pressure treatment, systemic steroid treatment or hormone-replacement therapy were excluded. All participants provided written informed consent before inclusion in the study. This study was approved by the Ethics Committee of the Shanghai Jiao Tong University Affiliated Sixth People's Hospital and complied with the Declaration of Helsinki.
Before and 1 year after RYGB surgery, all subjects were asked to undergo an overnight polysomnography (PSG) test in the sleep center at our hospital. During the sleep center visit, all participants were asked to complete the Epworth Sleepiness Scale (ESS) before the overnight PSG test. Fasting blood samples were taken the next morning in the fasting state and immediately after the PSG test. Body habitus, including weight, height, neck circumference (NC), waist circumference (WC), and hip circumference (HC), was measured using standard anthropometric methods. Blood samples were collected to measure fasting plasms glucose (FPG), fasting insulin, lipids, and betatrophin levels. Both the surgical procedure and the measurements of blood samples were as described previously14. Serum betatrophin was determined using commercially available enzyme-linked immunosorbent assays (ELISAs; SK00528-02, Aviscera Bioscience Inc., Santa Clara, CA, USA) according to the manufacturer’s instructions19. The homeostasis model assessment of insulin resistance (HOMA-IR) was used as a parameter to evaluate the degree of insulin resistance. Subcutaneous fat area (SFA), visceral fat area (VFA), and total fat area (TFA) were assessed in each participant at baseline and 1 year after RYGB using a magnetic resonance imaging (MRI) system (Achieva 3.0-T; Philips Medical Systems, Eindhoven, The Netherlands) with standard array coils, with the subject in the supine position.
Polysomnography test
A laboratory-based PSG (Alice 4; Respironics Inc., Pittsburgh, PA) was used to diagnose OSA. PSG records were evaluated manually according to standard criteria by a single skilled technician20. The apnea hypopnea index (AHI) was defined as the number of apnea and hypopnea events per hour during sleep. The parameters of mean oxygen saturation (SaO2), minimum SaO2 and the percentage of time spent at SaO2 < 90% (CT90%) were also included in the data analysis. Patients with AHI < 5 events/h before RYGB surgery were excluded from the follow-up study.
Statistical analysis
Continuous variables are presented as means ± standard deviation, except for skewed variables, which are presented as the mean (95% confidence interval, CI). Categorical variables are expressed as percentages. Differences between baseline and postoperative characteristics of the participants were examined using the paired Student’s t test, Wilcoxon’s signed-rank test, Kruskal–Wallis test, or χ2 test, as appropriate. Correlations of the various variables and PSG parameters were analyzed using Spearman’s correlation test or Pearson correlation test. Parameters that may influence the effect of RYGB surgery on OSA were evaluated by partial correlation analysis. We considered p < 0.05 to indicate statistical significance for a two-sided test. All statistical analyses were performed using SPSS software (version 19.0 for Windows; SPSS Inc., Chicago, IL).
Results
Clinical characteristics of the subjects at baseline and 1 year after RYGB surgery
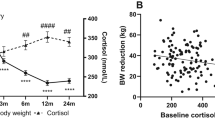
The demographics and clinical characteristics of the subjects are shown in Table 1. Of the total population of 37 patients, 16 were male and 21 were female. The mean age of the study population was 49.3 ± 10.6 years. Compared with baseline values, BMI, NC, WC, HC, FPG, fasting insulin, HOMA-IR, and the ESS score were all significantly decreased 1 year later (all p < 0.001). The mean BMI dropped from 31.0 ± 3.4 to 24.0 ± 2.2 kg/m2. The mean total weight loss was 17.9 ± 6.8 kg. Significant changes were also observed in lipid profiles, such as total cholesterol, triglyceride, low-density lipoprotein, and high-density lipoprotein (all p < 0.001). As shown in Fig. 1, serum betatrophin level decreased significantly from 109.55 ng/mL (95% CI 83.26–135.84) to 76.71 ng/mL (95% CI 51.09–102.33) (p < 0.001). The parameters of abdominal fat accumulation were also significantly decreased at follow-up; the baseline values of TFA, SFA, and VFA were 408.6 cm2 (95% CI 366.9–450.3), 269.7 cm2 (95% CI 234.4–305.0), and 138.9 ± 45.3 cm2, while those at 1 year later had decreased to 207.4 cm2 (95% CI 178.3–236.5), 161.4 cm2 (95% CI 137.7–185.2), and 45.9 ± 26.6 cm2, respectively (all p < 0.001). PSG indicators showed that the AHI changed significantly from 21.5 events/h (95% CI 16.5–26.4) to 6.4 events/h (95% CI 4.2–8.7) (p < 0.001). The minimum SaO2 was 87% (95% CI 86–89%) after surgery compared to 80% (95% CI 76–83%) at baseline (p < 0.001).
Comparisons of parameters of betatrophin (a), AHI (b), mean SaO2 (c), minimum SaO2 (d), CT90% (e), VFA (f), SFA (g) and TFA (h) between baseline and one year after RYGB. **p < 0.001. Data were shown as mean (95% confidence interval). RYGB Roux-en-Y gastric bypass, AHI apnea hypopnea index, SaO2 oxygen saturation, CT90% percentage of time spent at SaO2 < 90%, VFA visceral fat area, SFA subcutaneous fat area, TFA total fat area.
Factors associated with the alleviation of OSA
Spearman correlation analysis indicated that preoperative AHI was significantly correlated with preoperative betatrophin (r = 0.603, p < 0.001, Fig. 2a), ESS score (r = 0.636, p < 0.001), BMI (r = 0.431, p = 0.008) and NC (r = 0.382, p = 0.02) at baseline. When adjusted for the other factors, preoperative AHI was significantly independently correlated with preoperative betatrophin (r = 0.439, p = 0.009). Preoperative minimum SaO2 was significantly correlated with preoperative betatrophin (r = − 0.458, p = 0.004,), ESS score (r = − 0.476, p = 0.003), VFA (r = − 0.434, p = 0.007), BMI (r = − 0.397, p = 0.015), NC (r = − 0.439, p = 0.007), WC (r = − 0.360, p = 0.029) and HC (r = − 0.365, p = 0.026) at baseline. However, when adjusted for the other factors, preoperative minimum SaO2 was not significantly correlated with preoperative betatrophin (p > 0.05).
One year after bariatric surgery, 23 patients with OSA were cured, while the other 14 patients had residual OSA. Parameters that were significantly different between these two groups were shown in Table 2. Postoperative AHI was significantly correlated with postoperative betatrophin (r = 0.356, p = 0.031, Fig. 2b), ESS score (r = 0.533, p = 0.001), and waist–hip ratio (r = 0.346, p = 0.036). When adjusted for the other factors, postoperative AHI was significantly independently correlated with postoperative betatrophin (r = 0.431, p = 0.01).
Spearman correlation analysis also indicated the change in AHI was significantly associated with the change in betatrophin (r = 0.421, p = 0.009, Table 3) and ESS score (r = 0.540, p < 0.001). When adjusted for the change in ESS score, the change in AHI was significantly independently correlated with the change in betatrophin (r = 0.515, p = 0.001). The change in the minimum SaO2 was also significantly associated with changes in betatrophin (r = − 0.468, p = 0.004), the ESS score (r = − 0.388, p = 0.018) and HC (r = − 0.356, p = 0.031). When adjusted for the other factors, the change in betatrophin was independently associated with the change in AHI (r = 0.515, p = 0.001) and the minimum SaO2 (r = − 0.579, p < 0.001).
Discussion
Bariatric surgery can effectively treat obesity and improve OSA21. In this study, betatrophin, physical parameters (BMI, NC, WC, etc.), indices of lipid metabolism (triglyceride, cholesterol, etc.), OSA indictors (AHI, the minimum SaO2, etc.), and body fat distribution parameters (SFA, VFA, and TFA) showed significant changes after surgery. The change of betatrophin was found to be independently correlated with the alleviation of OSA. Therefore, adipokines, such as betatrophin, may be associated with the improvement of OSA after RYGB surgery.
Obesity has been widely reported and accepted as the most frequent risk factor for OSA22,23. Weight reduction has been demonstrated as an important treatment for OSA, because even a small decrease in BMI can significantly improve the AHI24. However, the extent of weight loss did not correlate with OSA improvement25, which has also been found in our previous study26. Some authors have attributed this to the interaction of anatomic factors27 and weight-independent metabolic effects28, such as the cytokines, gut hormones and adipokines. However, the current evidence could only partially explain the alleviation of OSA, which means further research are required to reveal the underlying mechanisms of OSA resolution after weight loss.
Betatrophin is a hormone highly enriched in the liver and adipose tissues, and has been shown to be relevant to obesity and glucose/lipid homeostasis29,30. Both in vivo and in vitro models, inhibition of betatrophin leads to the phenotype change of adipocytes characterized by increased mitochondria contents, beige adipocytes and mitochondria biogenesis-specific markers31. Betatrophin has also been reported to regulate the lipoprotein lipase activity in the heart and skeletal muscles32, and regulate the energy homeostasis. Suppressed betatrophin could lead to lipoprotein lipase activation in muscles and triglyceride mobilization to muscles for oxidation and energy supply with greater expression of genes related to beta-oxidation33,34, which may enhance upper airway muscle function and result in the alleviation of OSA. The lipoprotein lipase has also been demonstrated to be downregulated by hypoxia32. Considering the association between OSA and obesity and dyslipidemia, the potential interaction between OSA and betatrophin is worth exploring. However, there have been few studies regarding the association between betatrophin and OSA. It was not until recently that two studies reported the serum betatrophin concentration was higher in the OSA group than in the control group15,16, and this was consistent with our study. Moreover, our study showed a reduction of serum betatrophin after the alleviation of OSA, and the change of betatrophin was significantly correlated with the change of AHI and the change of minimum SaO2. Increasing evidence has indicated that serum betatrophin concentration is influenced by serum lipid profile, obesity and T2D. However, when adjusted for the influence factors in our study, the change of betatrophin was still significantly correlated with the change of AHI and the minimum SaO2. The results of these previous studies taken together with our findings suggest that betatrophin may be associated with the alleviation of OSA. Although the complex association between betatrophin and OSA could not reveal causal relations, our results might provide supplementary clinical evidence for a new perspective for the study of OSA.
This study also had some limitations. First, it was carried out in subjects with OSA and T2D rather than patients with simple obesity, so the generalizability of the results to other populations is unclear. Second, adipose tissue-related factors and gastrointestinal hormones were not exhaustively measured in this study, and the research evidence between betatrophin and OSA could not reveal underlying mechanism. Moreover, the small sample size, lack of a nonsurgical weight loss group and limited follow-up duration may also limit the generalizability of the results. Therefore, further studies including larger samples are required to further examine the underlying role of betatrophin, as well as other metabolic factors, on the therapeutic effects of RYGB surgery on OSA.
Conclusions
This is the first study showing significant reduction in the serum betatrophin level after RYGB surgery in obese Chinese patients with T2D. Serum betatrophin may be associated with the improvement of OSA after bariatric surgery. Further prospective studies are required to determine the mechanisms underlying these observations.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Duchna, H. W. Sleep-related breathing disorders—A second edition of the International Classification of Sleep Disorders (ICSD-2) of the American Academy of Sleep Medicine (AASM). Pneumologie 60, 568–575. https://doi.org/10.1055/s-2006-944248 (2006).
Jordan, A. S., McSharry, D. G. & Malhotra, A. Adult obstructive sleep apnoea. Lancet 383, 736–747. https://doi.org/10.1016/s0140-6736(13)60734-5 (2014).
Toyama, Y. et al. Association between sleep apnea, sleep duration, and serum lipid profile in an urban, male, working population in Japan. Chest 143, 720–728. https://doi.org/10.1378/chest.12-0338 (2013).
Tuomilehto, H., Seppa, J. & Uusitupa, M. Obesity and obstructive sleep apnea—Clinical significance of weight loss. Sleep Med. Rev. 17, 321–329. https://doi.org/10.1016/j.smrv.2012.08.002 (2013).
Quintas-Neves, M., Preto, J. & Drummond, M. Assessment of bariatric surgery efficacy on Obstructive Sleep Apnea (OSA). Rev. Port. Pneumol. 2006(22), 331–336. https://doi.org/10.1016/j.rppnen.2016.05.006 (2016).
Harada, Y. et al. Differences in associations between visceral fat accumulation and obstructive sleep apnea by sex. Ann. Am. Thorac. Soc. 11, 383–391. https://doi.org/10.1513/AnnalsATS.201306-182OC (2014).
Sutherland, K. et al. Effect of weight loss on upper airway size and facial fat in men with obstructive sleep apnoea. Thorax 66, 797–803. https://doi.org/10.1136/thx.2010.151613 (2011).
Greenburg, D. L., Lettieri, C. J. & Eliasson, A. H. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am. J. Med. 122, 535–542. https://doi.org/10.1016/j.amjmed.2008.10.037 (2009).
Pannain, S. & Mokhlesi, B. Bariatric surgery and its impact on sleep architecture, sleep-disordered breathing, and metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 24, 745–761. https://doi.org/10.1016/j.beem.2010.07.007 (2010).
Tirado, R. et al. Impact of bariatric surgery on heme oxygenase-1, inflammation, and insulin resistance in morbid obesity with obstructive sleep apnea. Obes. Surg. 27, 2338–2346. https://doi.org/10.1007/s11695-017-2635-4 (2017).
Iantorno, M. et al. Obesity, inflammation and endothelial dysfunction. J. Biol. Regul. Homeost. Agents 28, 169–176 (2014).
Herder, C. et al. Adiponectin and bariatric surgery: Associations with diabetes and cardiovascular disease in the Swedish Obese Subjects Study. Diabetes Care 37, 1401–1409. https://doi.org/10.2337/dc13-1362 (2014).
Ren, G., Kim, J. Y. & Smas, C. M. Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 303, E334-351. https://doi.org/10.1152/ajpendo.00084.2012 (2012).
Guo, K. et al. Decreased serum betatrophin levels correlate with improved fasting plasma glucose and insulin secretion capacity after Roux-en-Y gastric bypass in obese Chinese patients with type 2 diabetes: a 1-year follow-up. Surg. Obes. Relat. Dis. 12, 1343–1348. https://doi.org/10.1016/j.soard.2016.01.024 (2016).
Sertogullarindan, B. et al. Betatrophin association with serum triglyceride levels in obstructive sleep apnea patients. Ann. Thorac. Med. 14, 63–68. https://doi.org/10.4103/atm.ATM_52_18 (2019).
Al-Terki, A. et al. Increased level of angiopoietin like proteins 4 and 8 in people with sleep apnea. Front. Endocrinol. 9, 651. https://doi.org/10.3389/fendo.2018.00651 (2018).
Huang, C. K. et al. Laparoscopic Roux-en-Y gastric bypass for the treatment of type II diabetes mellitus in Chinese patients with body mass index of 25–35. Obes. Surg. 21, 1344–1349. https://doi.org/10.1007/s11695-011-0408-z (2011).
Iber, C., Ancoli-Israel, S., Chesson, A. L. & Quan, S. F. For the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (American Academy of Sleep Medicine, Westchester, 2007).
Barja-Fernández, S. et al. Circulating betatrophin levels are increased in anorexia and decreased in morbidly obese women. J. Clin. Endocrinol. Meta. 100, E1188–E1196. https://doi.org/10.1210/jc.2015-1595 (2015).
Zou, J. et al. An effective model for screening obstructive sleep apnea: A large-scale diagnostic study. PLoS ONE 8, e80704. https://doi.org/10.1371/journal.pone.0080704 (2013).
Dixon, J. B. et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: A randomized controlled trial. JAMA 308, 1142–1149. https://doi.org/10.1001/2012.jama.11580 (2012).
Patil, S. P., Schneider, H., Schwartz, A. R. & Smith, P. L. Adult obstructive sleep apnea: Pathophysiology and diagnosis. Chest 132, 325–337. https://doi.org/10.1378/chest.07-0040 (2007).
Deflandre, E., Gerdom, A., Lamarque, C. & Bertrand, B. Understanding pathophysiological concepts leading to obstructive apnea. Obes. Surg. 28, 2560–2571. https://doi.org/10.1007/s11695-018-3325-6 (2018).
Peppard, P. E., Young, T., Palta, M., Dempsey, J. & Skatrud, J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 284, 3015–3021. https://doi.org/10.1001/jama.284.23.3015 (2000).
Xanthopoulos, M. S., Berkowitz, R. I. & Tapia, I. E. Effects of obesity therapies on sleep disorders. Metabolism 84, 109–117. https://doi.org/10.1016/j.metabol.2018.01.022 (2018).
Zou, J. et al. Effect of laparoscopic Roux-en-Y gastric bypass surgery on obstructive sleep apnea in a Chinese population with obesity and T2DM. Obes. Surg. 25, 1446–1453. https://doi.org/10.1007/s11695-014-1510-9 (2015).
Malhotra, A. et al. Upper-airway collapsibility: Measurements and sleep effects. Chest 120, 156–161. https://doi.org/10.1378/chest.120.1.156 (2001).
Ashrafian, H. et al. Metabolic surgery and obstructive sleep apnoea: The protective effects of bariatric procedures. Thorax 67, 442–449. https://doi.org/10.1136/thx.2010.151225 (2012).
Abu-Farha, M. et al. Circulating ANGPTL8/betatrophin is increased in obesity and reduced after exercise training. PLoS ONE 11, e0147367. https://doi.org/10.1371/journal.pone.0147367 (2016).
Tseng, Y. H., Yeh, Y. H., Chen, W. J. & Lin, K. H. Emerging regulation and function of betatrophin. Int. J. Mol. Sci. 15, 23640–23657. https://doi.org/10.3390/ijms151223640 (2014).
Liao, Z. Z. et al. Betatrophin knockdown induces beiging and mitochondria biogenesis of white adipocytes. J. Endocrinol. 245, 93–100. https://doi.org/10.1530/JOE-19-0447 (2020).
Fu, Z., Abou-Samra, A. B. & Zhang, R. A lipasin/Angptl8 monoclonal antibody lowers mouse serum triglycerides involving increased postprandial activity of the cardiac lipoprotein lipase. Sci. Rep. 5, 18502. https://doi.org/10.1038/srep18502 (2015).
Luo, M. & Peng, D. ANGPTL8: An important regulator in metabolic disorders. Front. Endocrinol. (Lausanne) 9, 169. https://doi.org/10.3389/fendo.2018.00169 (2018).
Izumi, R. et al. CRISPR/Cas9-mediated Angptl8 knockout suppresses plasma triglyceride concentrations and adiposity in rats. J. Lipid Res. 59, 1575–1585. https://doi.org/10.1194/jlr.M082099 (2018).
Author information
Authors and Affiliations
Contributions
Literature search: Z.S., K.G., W.H., J.G. Data collection: Z.S., K.G., W.H., H.X., Y.L. Study design: J.Z., H.Y., H.Y. and S.Y. Analysis of data: J.Z. Manuscript preparation: Z.S., K.G. Manuscript revision: J.Z., H.Y. and H.Y. Review of manuscript: All authors
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, Z., Guo, K., Huang, W. et al. Decreased serum betatrophin may correlate with the improvement of obstructive sleep apnea after Roux-en-Y Gastric Bypass surgery. Sci Rep 11, 1808 (2021). https://doi.org/10.1038/s41598-021-81379-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-81379-1