Abstract
The most frequent mechanism of resistance after 1st/2nd-generation (G) epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) is secondary point mutation Thr790Met (T790M) in EGFR. Afatinib followed by osimertinib (Afa group) may provide better outcomes for T790M-positive non-small cell lung cancer (NSCLC) than 1st-G EGFR-TKI followed by osimertinib (1st-G group). We studied 111 consecutive NSCLC patients with T790M mutation treated with osimertinib after progression following 1st/2nd-G EGFR-TKI between March 28, 2016 and March 31, 2018. We analyzed the ratio of T790M to EGFR-activating mutation (T790M ratio) in post EGFR-TKI resistance re-biopsy tissue using droplet digital polymerase chain reaction. And investigated whether afatinib purified the T790M mutation more than 1st-G EGFR-TKI. Among 60 patients with preserved re-biopsy tissue, we analyzed 38 having adequate DNA content. The response rate in Afa group was 81.8% (n = 11) and 1st-G group was 85.2% (n = 27). The mean T790M ratio in total population was 0.3643. The ratio in those with response to osimertinib was significantly higher than in the non-responders (0.395, 0.202; p = 0.0231) and was similar in Afa and 1st-G group (0.371, 0.362; p = 0.9693). T790M ratio significantly correlated with osimertinib response and was similar between the 1st/2nd-G EGFR-TKIs in 1st/2nd-G EGFR-TKI-refractory tumors.
Similar content being viewed by others
Introduction
Based on randomized trials showing superior progression-free survival (PFS), response rate (RR), and more favorable safety profiles when compared with standard first-line platinum-based doublet chemotherapy in non-small cell lung cancer (NSCLC) patients with activating epidermal growth factor receptor (EGFR) mutation1,2,3,4,5,6, EGFR-tyrosine kinase inhibitors (TKIs), including gefitinib, erlotinib, and afatinib, have been established as the standard first-line treatment. However, cancer cells inevitably develop acquired resistance (AR) to EGFR-TKIs. Although EGFR-TKI treatment shows a durable response against NSCLC harboring EGFR mutations, most patients experience cancer relapse within 1–1.5 years following treatment with first-line 1st- and 2nd-generation (G) EGFR-TKIs. The most common resistance mechanism involves a secondary point mutation Thr790Met (T790M) in EGFR. It impairs the binding of TKIs to EGFR and is detected in approximately 50–60% of patients with 1st and 2nd-G EGFR-TKI-refractory tumors7,8,9. Osimertinib was developed for its activity against T790M by covalently binding to T790M-muted EGFR10, and it has been shown to be effective against AR of T790M-positive NSCLC after 1st- or 2nd-G EGFR-TKI treatment11. The RR in the trial was about 70% and use of osimertinib in 1st- and 2nd-G EGFR-TKI-refractory tumors was established as a standard treatment worldwide.
Moreover, osimertinib is associated with a longer PFS and overall survival (OS) than 1st-G EGFR-TKIs against advanced NSCLC harboring EGFR mutation (exon-19 deletion and L858R) as a first-line treatment12. However, in the Asian subset (especially in the Japanese subset) analysis of OS in the FLAURA study, osimertinib was not superior to 1st-G EGFR-TKIs13. There may be no molecular targets for therapy due to the heterogeneity of resistance mechanisms, which are not well understood14,15. As a result, during clinical care for most patients following cancer progression after osimertinib treatment, chemotherapy is the only remaining option for second-line treatment.
In contrast, afatinib demonstrated superior RR, PFS, and a trend of longer OS compared to gefitinib in the LUX-Lung 716. A study showed that afatinib treatment followed by osimertinib indicated an extremely long treatment time to failure (TTF) and OS, even after removal of selection bias17. Furthermore, we reported that treatment with afatinib followed by osimertinib (Afa group) may provide better outcomes for T790M-positive NSCLC than that with 1st-G EGFR-TKIs (1st-G group)18. These results may be explained by the possibility that afatinib exhibits increased efficacy by targeting co-occurring EGFR mutations that enrich T790M cells.
Therefore, to investigate whether afatinib purifies T790M mutation more effectively than the 1st-G EGFR-TKIs, we analyzed the difference in the ratio of T790M mutation to EGFR-activating mutation ratio (T790M ratio) between the Afa and 1st-G group by using droplet digital polymerase chain reaction (ddPCR).
Materials and methods
We conducted a multicenter-retrospective study across three medical centers (Osaka International Cancer Institute, Osaka Habikino Medical Center, and National Hospital Organization Kinki-Chuo Chest Medical Center) in Japan. The study design and methodology were approved by the Institutional Review Board of each participating institution (Institutional Review Board of Osaka International Cancer Institute, Institutional Review Board of Osaka Habikino Medical Center, and Institutional Review Board of National Hospital Organization Kinki-Chuo Chest Medical Center), and it was conducted in accordance with the Declaration of Helsinki and the World Health Organization’s Guidelines for Good Clinical Practice. We obtained the informed consent from participants and used an opt-out method so that patients and their families could refuse to participate in the study.
Patient selection and ddPCR measurement
Between March 28, 2016 (the date osimertinib was approved in Japan) and March 31, 2018, study participants were consecutively enrolled according to the following criteria: patients with T790M mutation who were treated with osimertinib after AR to EGFR-TKIs at any time for advanced NSCLC, had good Eastern Cooperative Oncology Group (ECOG) performance status (PS): 0–2, and had major EGFR mutation (Exon-19: deletion19 or Exon-21: L858R) before initial EGFR-TKI treatment to reduce bias towards patient conditions. Furthermore, we excluded cases where we detected T790M mutation in the pre-treatment tissue by EGFR test in general practice.
We used RIKEN GENESIS CO., LTD. (Tokyo, Japan) to measure exon-19 deletion, L858R, and T790M by droplet digital polymerase chain reaction (ddPCR). The ddPCR analysis was performed using QX200 AutoDG Droplet Digital PCR System (BioRad). In this study, we excluded samples that had less than 1000 total copies/20 uL well or less than 10 copies with EGFR-activating mutation/20 uL well. T790M ratio was calculated by the following method. First, the fractional abundance (mutant allele/mutant allele + wild type allele) of activating mutation and T790M were measured with droplet count by ddPCR. Next, T790M ratio defined the ratio of T790M to activating mutation.
Statistical analysis
We evaluated the systemic response to osimeritinib using the Response Evaluation Criteria in Solid Tumors (RECIST) ver1.119. We used Fisher’s exact tests for categorical comparisons of data and compared differences in continuous data using the Wilcoxon test. Kaplan–Meier curves were used to evaluate PFS, which were compared using the log-rank test. Median values and 95.0% confidence intervals (CIs) were also reported. All statistical analyses were conducted using R software, version 2.8.1 (http://R-project.org) (The R Foundation for Statistical Computing, Vienna, Austria). p < 0.05 was considered a statistically significant difference.
Results
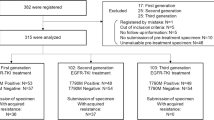
Of the 111 enrolled patients, the re-biopsy tissue obtained from each patient before osimertinib treatment was preserved for 60 patients. Among them, 38 samples yielded enough DNA for ddPCR analysis, and the T790M ratio was assessed (Fig. 1). The patient characteristics are shown in Table 1. The median age was 68 years. Of the 38 patients, 21.1% were men, 23.7% had a history of smoking, 44.7% had L858R mutation, and 55.3% had exon 19 deletion. Eleven (28.9%) were in the Afa group and 27 (71.1%) were in the 1st-G group. The RR in all patients was 84.2%, 81.8% in the Afa group, and 85.2% in the 1st-G group (Table 2).
The mean T790M ratio in the total population was 0.3643 (range: 0.0457–0.8774). The T790M ratio in patients who obtained complete response (CR) or partial response (PR) to osimertinib in the osimertininb group (mean: 0.395) was significantly higher than in patients with stable disease (SD) or progressive disease (PD) (mean: 0.202); p = 0.0231 (Fig. 2a). The T790M ratio in the Afa group (mean: 0.3711, range: 0.1497–0.7465) was the same with the 1st-G group (mean: 03616, range: 0.0457–0.8774); p = 0.9693 (Fig. 2b).
Box plot of the comparison with the T790M ratio. (a) SD/PD versus CR/PR, (b) 1st-G EGFR-TKIs versus Afatinib. T790M ratio T790M mutation to EGFR-activating mutation ratio, CR complete response, PR partial response, SD stable diseases, PD progression diseases, 1st-G EGFR-TKIs first generation epidermal growth factor receptor-tyrosine kinase inhibitors.
The median PFS in the CR or PR subgroup of the Osimertinib group (446 days [95% CIs 347–710]) was significantly longer than that in the SD or PD subgroup (77 days [95%CIs 52-not available (NA)]); p < 0.0001 (Fig. 3a). The median PFS in the Afa group (258 [95%CIs 239-NA] days) was also similar to the 1st-G group (414 days [95%CIs 241–710]); p = 0.6 (Fig. 3b).
Kaplan-Meyer curves for progression free survival of osimertinib by (a) SD/PD versus CR/PR, (b) 1st-G EGFR-TKIs versus Afatinib, (c) T790M ratio ≥ 0.35 versus < 0.35. CR complete response, PR partial response, SD stable diseases, PD progression diseases, 1st-G EGFR-TKIs first generation epidermal growth factor receptor-tyrosine kinase inhibitors, T790M ratio T790M mutation to EGFR-activating mutation ratio.
Furthermore, we divided two groups based on a threshold T790M ratio of 0.35 from the mean T790M ratio in total population. The response rate in patients with T790M ratio ≥ 0.35 (100% [18/18]) was higher than that in patients with T790M ratio < 0.35 (70% [14/20]); p = 0.03691. The median PFS in patients with T790M ratio ≥ 0.35 was longer when compared with patients with T790M ratio < 0.35 (387 days [95%CIs 214-NA] versus 356 days [95%CIs 241-NA]; p = 0.2), however this was not statistically significant (Fig. 3c). Pearson correlation analysis was performed to examine the relationship between T790M ratio and PFS. The analysis showed a correlation coefficient of r = 0.06123 (p = 0.715) (Fig. 4).
Discussion
This is the first analysis to reveal a potential implication that afatinib purifies the T790M mutation more than the 1st-G EGFR-TKI. This study suggested that the T790M ratio in 1st/2nd-G EGFR-TKI-refractory tumors significantly correlated with response to osimertinib. The T790M ratio in CR or PR subgroup of the osimertinib group (mean: 0.395) was significantly higher than in the SD or PD subgroup (mean: 0.202); p = 0.0231. However, we did not observe a difference in the T790M ratio between the 1st- and 2nd-G EGFR-TKIs. The T790M ratio in the Afa group (mean: 0.3711) was similar to the 1st-G group (mean: 03616); p = 0.9693.
Osimertinib was developed as a drug against T790M that works by covalently binding to T790M-muted EGFR10. Therefore, although osimertinib is also effective against sensitive EGFR mutations, the most effective targets are T790M-positive EGFR mutations. In contrast, afatinib irreversibly binds to EGFR, ERBB2, and ERBB4 and blocks transphosphorylation of ERBB3 to inhibit all ERBB family signaling20. Two hypotheses were proposed: the first is that the broader inhibition of the EGFR family may improve the efficacy of EGFR-TKIs for NSCLC with EGFR mutation, and the second is that the broader inhibition of the EGFR family may attenuate T790M in cancer cells during relapse after EGFR-TKI treatment. Osimertinib could also be more effective against the AR of T790M after EGFR-TKI. The results of LUX-Lung7 and ARCHER1050 supported this theory in the first-line treatment for NSCLC with EGFR mutation16,21. Furthermore, afatinib provided the efficacy for uncommon EGFR mutations in preclinical and clinical studies22,23. In the analysis of GIO-TAG trials, the duration of response to osimertinib in patients receiving afatinib and subsequent osimertinib was highly encouraging17,24,25.
In our study, the concentration of T790M correlated with the response to osimertinib, and our results suggested that the degree of attenuation of T790M is important in eliciting the effects of osimertinib. Some prior studies have also shown similar results: the ratio of T790M to EGFR-activating mutation in cell samples or plasma samples may predict the response to osimertinib or rociletinib26,27,28.
However, we did not observe differences in the clonality of T790M after AR between afatinib and 1st-G EGFR-TKI. One reason was that the response of osimertinib after AR was the same in Afa group and 1st-G group when we collected the available tissue, although our previous study revealed that the objective RR and disease control rate were significantly higher in the Afa group than in the 1st-G group18. We intended to collect all the samples in our previous study; however, this was not possible. Another reason is that later lines of therapy may have advanced clonal evolution and may not reflect the effect of osimertinib after 1st-G EGFR-TKI and afatinib treatment.
Next, Pearson correlation analysis showed no significant relationship between T790M ratio and PFS of osimertinib after AR of EGFR-TKIs (r = 0.06123, p = 0.715). We believed that the PFS of osimertinib after AR in NSCLC with T790M mutation was influenced by co-mutation.
Based on clinal evolution, which was reported for the first time as early as 197629, lung adenocarcinoma seems to follow a multistep progression from atypical adenomatous hyperplasia to adenocarcinoma in situ, and finally invasive adenocarcinoma30. EGFR driver alterations are acquired in the early step of cancer progression and can be identified in most neoplastic cancer cells31. Furthermore, by layering exacerbations of disease after treatment, especially EGFR-TKI use, cell progression will show different genomic patterns of selection through different lines of therapy32. In this setting, EGFR-sensitive mutant tumor cells may coexist with sub-clonal tumor cells harboring other gene mutations, and co-mutations such as TP53 affect the efficacy of osimertinib33,34. Furthermore, histologic transformation and other off-target molecular alterations are frequent early emerging resistance mechanisms to osimertinib and are associated with poor clinical outcomes35.
We must consider some limitations of the present study. First, this was a retrospective study, and the participants were selected based on obtainment of adequate samples. Second, the line of treatment for EGFR-TKIs was not determined. Since the lines of EGFR-TKIs varied from case to case, the effectiveness of osimertinib may also vary accordingly. Although osimertinib response was not different between the two groups, we focused on the attenuation of tumor cells by EGFR-TKIs immediately before the administration of osimertinib to clarify the correlation between T790M ratio and osimertinib response. Third, in this study, we examined only exon-19 deletion, L858R and T790M by ddPCR and did not perform next generation sequencing. Therefore, we cannot know about the presence of other EGFR mutations, amplifications, alternative bypass pathways or co-mutations that may provide a mechanism of resistance to osimerutinib.
Conclusion
In our analysis, the T790M ratio in 1st/2nd-G EGFR-TKI-refractory tumors significantly correlated with osimertinib response. Furthermore, the T790M ratio between the 1st- and 2nd-G EGFR-TKIs was almost similar in patients with EGFR-TKI-refractory NSCLC who showed response to osimertinib. Further analysis in a larger number of prospective studies is warranted to confirm our results.
References
Maemondo, M. et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 362, 2380–2388 (2010).
Mitsudomi, T. et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 11, 121–128 (2010).
Rosell, R. et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 13, 239–246 (2012).
Sequist, L. V. et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 31, 3327–3334 (2013).
Wu, Y. L. et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 15, 213–222 (2014).
Zhou, C. et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 12, 735–742 (2011).
Yun, C. H. et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl. Acad. Sci. U. S. A. 105, 2070–2075 (2008).
Arcila, M. E. et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin. Cancer Res. 17, 1169–1180 (2011).
Oxnard, G. R. et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin. Cancer Res. 17, 1616–1622 (2011).
Cross, D. A. et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 4, 1046–1061 (2014).
Mok, T. S. et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med. 376, 629–640 (2017).
Soria, J. C. et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 378, 113–125 (2018).
Ohe, Y. et al. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn. J. Clin. Oncol. 49, 29–36 (2019).
Oxnard, G. R. et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M-positive lung cancer and acquired resistance to osimertinib. J. A. M. A. Oncol. 4, 1527–1534 (2018).
Leonetti, A. et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 121, 725–737 (2019).
Park, K. et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 17, 577–589 (2016).
Hochmair, M. J. et al. Sequential treatment with afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: an observational study. Future Oncol. 14, 2861–2874 (2018).
Tamiya, M. et al. Which is better EGFR-TKI followed by osimertinib: afatinib or gefitinib/erlotinib?. Anticancer Res. 39, 3923–3929 (2019).
Schwartz, L. H. et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur. J. Cancer 62, 132–137 (2016).
Solca, F. et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J. Pharmacol. Exp. Ther. 343, 342–350 (2012).
Wu, Y. L. et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 18, 1454–1466 (2017).
Yang, J. C. et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 16, 830–838 (2015).
Kobayashi, Y. et al. EGFR exon 18 mutations in lung cancer: molecular predictors of augmented sensitivity to afatinib or neratinib as compared with first- or third-generation TKIs. Clin. Cancer Res. 21, 5305–5313 (2015).
Hochmair, M. J. et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: final analysis of the GioTag study. Future Oncol. 16, 2799–2808 (2020).
Yamamoto, N. et al. Observational study of sequential afatinib and osimertinib in EGFR mutation-positive NSCLC: patients treated with a 40-mg starting dose of afatinib. Adv. Ther. 37, 759–769 (2020).
Ariyasu, R. et al. High ratio of T790M to EGFR activating mutations correlate with the osimertinib response in non-small-cell lung cancer. Lung Cancer 117, 1–6 (2018).
Piotrowska, Z. et al. Heterogeneity underlies the emergence of EGFRT790 wild-type clones following treatment of T790M-positive cancers with a third-generation EGFR inhibitor. Cancer Discov. 5, 713–722 (2015).
Chabon, J. J. et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat. Commun. 7, 11815 (2016).
Nowell, P. C. The clonal evolution of tumor cell populations. Science 194, 23–28 (1976).
Sun, W. et al. Clonality assessment of multifocal lung adenocarcinoma by pathology evaluation and molecular analysis. Hum. Pathol. 81, 261–271 (2018).
de Bruin, E. C. et al. Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science 346, 251–256 (2014).
Kohsaka, S., Petronczki, M., Solca, F. & Maemondo, M. Tumor clonality and resistance mechanisms in EGFR mutation-positive non-small-cell lung cancer: implications for therapeutic sequencing. Future Oncol. 15, 637–652 (2019).
Li, X. M. et al. Predictive and prognostic potential of TP53 in patients with advanced non-small-cell lung cancer treated with EGFR-TKI: analysis of a phase III randomized clinical trial (CTONG 0901). Clin. Lung Cancer. https://doi.org/10.1016/j.cllc.2020.11.001 (2020).
Wang, F. et al. Prognostic value of TP53 co-mutation status combined with EGFR mutation in patients with lung adenocarcinoma. J. Cancer Res. Clin. Oncol. 146, 2851–2859 (2020).
Schoenfeld, A. J. et al. Tumor analyses reveal squamous transformation and off-target alternation as early resistance mechanisms to first-line osimertinib in EGFR-mutant lung cancer. Clin. Cancer Res. 26, 2654–2663 (2020).
Acknowledgements
The authors thank all the participants in the present study and RIKEN GENESIS CO., LTD. (Tokyo, Japan) for their excellent work in performing ddPCR.
Funding
The study was funded by Boehringer Ingelheim.
Author information
Authors and Affiliations
Contributions
M.T. designed the study. M.T. and A.T. wrote the initial draft of the manuscript. M.T. and H.S. contributed to analysis the results of ddPCR from RIKEN GENESIS CO., LTD. All other authors have contributed in data collection and in review of the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
Motohiro Tamiya has a research grant from Boehringer Ingelheim and receives lecture fee from Boehringer Ingelheim, Chugai Pharmaceutical, and AstraZeneca. Akihiro Tamiya has a research grant from AstraZeneca and receives lecture fee from Boehringer Ingelheim, Chugai Pharmaceutical, and AstraZeneca. Yoshihiko Taniguchi receives lecture fee from AstraZeneca. Kazuo Nishino has a research grant from Boehringer Ingelheim and receives lecture fee from Boehringer Ingelheim, Chugai Pharmaceutical, and AstraZeneca. Fumio Imamura has a research grant from AstraZeneca. Other authors have no conflicts of interest to declare under consideration for publication.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tamiya, M., Tamiya, A., Okamoto, N. et al. The ratio of T790M to EGFR-activating mutation predicts response of osimertinib in 1st or 2nd generation EGFR-TKI-refractory NSCLC. Sci Rep 11, 9629 (2021). https://doi.org/10.1038/s41598-021-89006-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89006-9