Abstract
The objective of this study was to report that lysosome extracted from egg white could be used as a drug through oral administration for treating diseases by using pH sensitive alginate oligosaccharides. Lysosome-alginate oligosaccharides composite were formulated for oral administration of lysosomes. The dissolution test confirmed the availability of the oral dosage form. When lysosome were used as an independent drug, the activity of protein was lost due to influence of low pH. Its antibacterial activity was also remarkably reduced. However, when lysosome-alginate oligosaccharides composite form was used, antimicrobial activity of lysozyme was maintained. At low pH, a gel-like matrix was formed by alginate oligosaccharides to protect the lysosome. When the pH was increased, alginate oligosaccharides were dissolved and the lysosome was released. SDS–polyacrylamide gel electrophoresis analysis of released lysosomes revealed that alginate oligosaccharide could effectively protect the lysosome from degradation or hydrolysis under acidic conditions for at least 2 h. The results of this study are important for application of lysosomes as therapeutic agents, and also it was confirmed that alginate oligosaccharides have potential as direct delivery system for the oral application of protein derived therapies.
Similar content being viewed by others
Introduction
Lysosomes are cell organelles containing various hydrolytic enzymes capable of digesting intracellular and extracellular materials. Lysosomes are abundant in egg whites. Egg white contains many biologically active proteins (54% albumine, 13% ovotransferin, 11% ovomucoid, and 3.4% lysozyme)1 that are antimicrobial, antiviral, antiphlogistic, and antalgic2. In particular, lysozymes can function as hydrolytic enzymes that can degrade bacteria cell walls3,4. In drug delivery studies, oral route of administration has high acceptability and patient compliance with the advantage of self-administration5. Although steady development of drug delivery technology is underway, much research on the oral administration of protein drugs is still needed. After oral administration, protein drugs face several problems, including rapid systemic degeneration/degradation and malabsorption in the small intestine6. In order to orally administer a protein drug, it is necessary to provide a drug that improves the absorption of the drug by increasing the reversible permeability of the mucosal epithelium and maintains efficacy without protein denaturation7. Therefore, delivery systems are needed to increase the bioavailability of these drugs, and gastrointestinal transit time is one of the challenges to overcome, especially in the development of sustained-release formulations8. This has led to considerable research focusing on overcoming drug's retention time problem at low pH for effective oral delivery of the drug5.
Alginates (alginic acid, sodium alginate, and potassium alginate) have been extensively explored as mucoadhesive biomaterials owing to their very good cytocompatibility, biocompatibility, biodegradation9, sol–gel transition properties, and chemical versatility that make it possible to further modify them to tailor their properties10. Alginate gels tend to be eroded under more neutral and basic pH values than under acid conditions11. This property has motivated its use in chemical stabilization of drugs and biologicals of oral administration that are unstable in gastric fluids.
In general, AOs has a short chain length, so it has improved water solubility when compared to high molecular weight alginates of the same monomer12. Since alginate oligosaccharides (AOs) are considered as non-toxic, non-immunogenic and biodegradable polymers, they are attractive candidates in biomedical applications13. AOs are linear polymers of polysaccharides with alternating gel formation properties consisting of β-(1-4)-D-mannosyluronic acid and α-(1-4)-L-glucosyluronic acid. AOs exhibit important biological activities not only in mammals, but also in plants. AOs have additional properties such as antimicrobial effects14,15, anti-inflammatory effects16, defense-enhancing effects against infection by certain pathogens17, and anti-glycating effects18. AOs can promote growth and proliferation of human cells, including keratinocytes19,20,21 and endothelial cells22. In addition, they can potentially protect nerve cells from oxidative stress23. Finally, AOs have interesting properties for fighting microbial pathogens. AOs can potentiate the action of selected antibiotics by disturbing multidrug-resistant bacteria24. Similarly, they can inhibit fungal cell growth and enhance activities of antifungal agents against Candida and Aspergillus species25. Here, the purpose of this study was to confirm that the lysosome isolated from egg white can be used as an eco-friendly agent for the treatment of metabolic diseases related to microorganisms living in the intestine through oral drug delivery using pH-sensitive AO.
Results
Formation of lysosome-AOs composite
Three types of alginate oligosaccharides used in this study were depolymerized with alginate by treating sodium alginate at 37 °C for 6 h, 12 h, and 24 h with alginate decomposing enzyme, respectively, to prepare three types of alginate oligosaccharides. The prepared alginate oligosaccharide was analyzed by MALDI-TOF–MS spectroscopy, and each alginate oligosaccharide had an intensity of 379.25 m/z peak corresponding to [M + Na] as 41.96%, 72.03%, and 88.86%. Results of observing the appearance of wild type lysosome, lysosome-2% alginate composite, or lysosome-AOs composite under low pH environment are shown in Fig. 1. Wild type lysosomes without alginate or AOs were used as negative controls. Wild type lysosomes were almost the same before and after the dissolution test. Denaturation of protein was observed in simulated gastric fluid due to its low pH. Lysosome-2% alginate composite was used as a positive control. In acid phase (simulated gastric juice, pH 1.2), alginate gel containing lysosome was formed because pH-sensitive alginate could form spherical lysosome-2% alginate. In the buffer phase (simulated intestinal juice, pH 6.8), the alginate gel dissolved to release lysosome. Next, lysosome-AOs (lysosome-Type 1 AOs, lysosome-Type 2 AOs, lysosome-Type 3 AOs) composites formed spherical gel containing lysosome at low pH. In the buffer phase, the gel formed by AOs was dissolved and lysosome was released. Among these three types of AOs, Type 1 AOs behaved like alginate the most.
Antimicrobial activity of the released lysosome
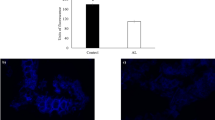
To use lysosomes as a drug, lysosome-2% alginate composite and lysosome-AOs composite were manufactured and applied as oral delivery systems. The antimicrobial activity of wild type lysosome against cell mortality of E. coli was 95% or more (Fig. 2). Figure 3 shows results of dissolution test, confirming the antimicrobial activity of lysosomes released into the intestine. Wild type lysosomes used for the dissolution test showed approximately 80% decreased antimicrobial activity. However, the antimicrobial activity of the lysosomes used in the dissolution test was maintained after mixing 2% alginate with three types of AOs. The antimicrobial activity of lysosome mixed with 2% alginate was the highest, followed by lysosome mixed with Type 1 AOs.
Antimicrobial activity of lysosomes immobilized on pH-sensitive biopolymers passing simulated gastrointestinal environment against Escherichia coli. In the graph, lysosomes without any treatment, lysosomes immobilized on 2% alginate, and antimicrobial activities against three types of AO-immobilized lysosomes are shown in order.
Protein of the released lysosome
Figure 4 shows SDS-PAGE results. Lane M shows protein marker (DokDo-MARK). Lane 1 shows lysosomal protein extracted from wild type lysosome (without alginate or AOs) as a control. Lanes 2 to 4 schematically describe lysosomal protein patterns extracted from wild type lysosome, lysosome-2% alginate composite, and lysosome-AOs composites formed in simulated gastric fluid (pH 1.2). As a result, the pattern was similar to that shown in lane 1. Lanes 1 and 2 and lanes 3 and 4 showed almost the same pattern. After reaction in simulated gastric fluid, wild type lysosome, lysosome-2% alginate composites, and lysosome-AOs composites were collected and reacted in simulated intestinal fluid. Lanes 5, 6, and 7 show lysosomal protein patterns extracted from wild type lysosomes, lysosome-2% alginate composites, and lysosome-AOs composites dissolved in simulated intestinal fluid (pH 6.8), respectively. As a result, protein pattern extracted from the wild type lysosome (without alginate/AOs) appeared in simulated gastric fluid. This protein pattern did not appear in simulated intestinal fluid. On the other hand, lanes 6 and 7 were lysosomal proteins extracted from lysosome-2% alginate composites and lysosome-AOs composites dissolved and released in simulated intestinal fluid, respectively. Although expression levels and patterns were slightly changed, these results were similar to those under acidic conditions. In particular, lysozyme is another significant protein discovered in egg white. The molecular weight of lysozyme is 14,400 Da. Lysozyme consists of a single polypeptide chain with 129 amino acids25. These results confirmed that the pattern of protein extracted from released lysosome appeared below 15 kDa molecular weight (Fig. 4, arrow). In other words, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis showed that mixed alginate and alginate oligosaccharides could effectively protect lysosomes from degradation or hydrolysis under acidic conditions for at least 2 h.
Expression patterns of lysosomal protein in egg white lysosome. Arrow indicates lysozyme. Lane 1 indicates total protein of wild type. Lanes 2, 3, and 4 indicate total proteins isolated from wild type, lysosome-2% alginate composites, and lysosome-AOs composites from Simulated Gastric Fluid. Lanes 5, 6, and 7 indicate total proteins isolated from wild type, lysosome-2% alginate composites, and lysosome-AOs composites from Simulated Intestinal Fluid.
Morphology of complex
Figure 5 shows morphologies of lysosomes observed according to environmental conditions during oral administration. In Fig. 5a, wild type lysosome (unused for experiments) in DW (distilled water) was observed. Figure 5b confirmed the morphology formed by oral administration of lysosomes. The picture showed lysosomes in pH 1.2 solution (instead of gastric fluid, and lysosomes in pH 6.8 solution (instead of intestinal fluid). As shown in Fig. 5b, the control group (without alginate or AOs) did not have spherical lysosomes at pH 1.2 or pH 6.8. On the other hand, in the case of treatment of 2% alginate with Type 1 AOs, Type 2 AOs, or Type 3 AOs, it was found that spheres seen as lysosomes in pH 1.2 were more clustered by surrounding substrates. Further, at pH 6.8, a spherical lysosome was found independently.
Discussion
Alginate hydrogel exists in the units of D-mannuronic acid and L-guluronic acid, and is a pH-sensitive biopolymer. Alginate has a sol–gel transition characteristic, and Alginate gels tend to be eroded under more neutral and basic pH values than under acid conditions27. In other words, alginate hydrogel is an ideal matrix for use in oral sustained release drugs28. To overcome the drawback of using the properties of alginate hydrogels but not being degraded in vivo, this study proposes alginate oligosaccharides as a drug matrix in oral sustained release formulations9. In this study, we devised a method to utilize low molecular weight AOs to use lysosomes as oral drugs. Lysosome-AOs (lysosome-Type 1 AOs, lysosome-Type 2 AOs, lysosome-Type 3 AOs) composites formed spherical gel containing lysosome at low pH. In the buffer phase, the gel formed by AOs was dissolved and lysosome was released. Of these three types of AO, type 1 AO behaves like the alginate, making gel formation most effective. Alginate has the property of changing to alginate sol form under acidic conditions. Similarly, it was confirmed that AOs changed to sol form under acidic conditions, so that AOs act as a substrate surrounding lysosomes.
Dissolution testing is the most common method for evaluating oral modified release delivery systems for colon-specific drug delivery. In-vitro evaluation of oral modified drug delivery systems is generally performed by using standard process procedures or by dissolution testing as appropriate29. The dissolution test confirmed that the lysosome activity was maintained up to the intestine when the lysosomes were used for oral administration in the form of AOs complex.
Lysosome activity mediates several processes for cell feeding and antimicrobial defense, including lysosomal fusion with endosomes and phagosomes2,30. Thus, lysosomal enzymes are capable of cell digestion31. Antimicrobial effects of lysosomes isolated from egg white on different strains including Gram-positive and Gram-negative bacteria have been reported32. Wild type lysosomes used for the dissolution test showed remarkably decreased antimicrobial activity than before it was used in the experiment. However, the antimicrobial activity of the lysosomes used in the dissolution test was maintained after mixing 2% alginate or three types of AOs. The antimicrobial activity of lysosome mixed with 2% alginate was the highest, followed by lysosome mixed with Type 1 AOs. Therefore, it can be seen that it is effective to use type 1 AOs together to maintain lysosomal activity up to the intestine.
Lysozyme is another significant protein discovered in egg white. The molecular weight of lysozyme is 14,400 Da. Lysozyme consists of a single polypeptide chain with 129 amino acids26. In this study, it was shown that lysosomes mixed with alginate or alginate oligosaccharide were protected so that antibacterial activity could be maintained in acidic conditions (artificial gastric juice) for at least 2 h.
Conclusions
The purpose of this research was to describe that lysosome isolated from egg white could be used as an environmentally friendly antimicrobial agent for treating diseases through oral drug delivery. This study applied sol–gel transition characteristics of biopolymer by pH change. AOs, when processed together with lysosome to use lysosome as a drug, allows drug release in the intestine with a high pH and limits drug exposure in the upper part of the gastrointestinal tract at low pH. Although results described in this study are from preliminary experiments for applying lysosomes as therapeutic agents, AOs have possibility as delivery systems for direct application of drugs, proteins, and lysosomes.
Materials and methods
Isolation of lysosome from egg white
Lysosome isolation was performed by homogenizing egg white followed by centrifugation twice under different conditions. First, egg white was homogenized for 30 min, divided into centrifuge bottles, and centrifuged at 1000 × g for 10 min at 4 °C. The supernatant was collected, homogenized for 2 h, and centrifuged at 20,000 × g for 30 min at 4 °C. After the second centrifugation, the supernatant was discarded and only the remaining pellet was collected. The collected pellet was tested for antimicrobial activity and used as lysosomes in all subsequent experiments. The collected pellet was stored at 4 °C in the dark.
AOs production by enzymatic degradation of sodium alginate
To prepare Type 1 AOs, Type 2 AOs, and Type 3 AOs, alginate lyase (Sigma-Aldrich, A1603) was used to catalyze the cleavage of alginate. To make Type 1 AOs, 2% (w/v) of sodium alginate (Sigma-Aldrich, A0682) was prepared by dissolving sodium alginate in 100 ml of distilled water (DW) followed by incubation at 37 °C for 6 h in the presence of 20 U/mg alginate lyase in a rotary shaker at 150 rpm. After the incubation, alginate lyase in the sample was inactivated at 100 °C for 10 min and removed after centrifugation at 12,000×g for 10 min at 4 °C33. Type 2 and Type 3 AOs were prepared with the same method except that alginate lyase treatment time was changed from 6 to 12 h for Type 2 AOs and 24 h for Type 3 AOs. Fractions of AOs were collected, freeze-dried, and store at − 4 °C.
Formation of lysosome-AOs composite using three types of AOs
Lysosomes and 2% alginate, or lysosomes and AOs were mixed at a ratio of 1:1 and prepared separately. Wild type lysosome was mixed with DW (ratio 1:1). A spherical composite was formed by dropping wild type lysosome, lysosome-2% alginate, or lysosome-AOs into a 1 ml disposable plastic syringe (Ormond Beach, FL, USA) in a simulated gastric fluid (pH 1.2). Particles formed in the acid solution were separated by centrifugation at 3000 rpm for 5 min.
Dissolution test of lysosome-AOs composite
Dissolution test was carried out under two conditions, acid stage (simulated gastric fluid; pH 1.2) and buffer stage (simulated intestinal fluid; pH 6.8), to confirm the role of gastric-resistant coating agent or the effect of coating on drug diffusion. A dissolution study of lysosome-AOs composite was performed according to the Korean Food and Drug Administration (KFDA). To make simulated gastric fluid, 0.4 g of NaCl was dissolved in 200 ml of DW and the pH was adjusted to 1.2 using HCl. Then 25 ml of 0.2 M KH2PO4 and 11.8 ml of 0.2 N NaOH were mixed with DW to prepare 100 ml of simulated intestinal fluid. Dissolution test was performed with simulated gastric fluid of pH 1.2 at 50 rpm for 2 h at 37 °C. After 2 h, simulated gastric fluid was discarded and simulated colon fluid of pH 6.8 was added. The dissolution test was continued under the same conditions as acidic conditions for 1 h. After the dissolution test, the release of lysosome was confirmed by antimicrobial test and protein pattern analysis of lysosomal protein.
Confirmation of antimicrobial activity of the released lysosome
To confirm the antimicrobial activity of the lysosome released to the colon, lysosomes isolated from wild type lysosome composite, lysosome-2% alginate composite, and three types of lysosome-AOs composite were used. Escherichia coli BL21 was used for antimicrobial activity tests. For antimicrobial test, after E. coli grew up to OD600 at 0.7–0.8, cultures were diluted to 106 cells/mL with DW. After that, 100 µl of diluted E. coli was mixed with 900 µl lysosome and 100 µl of the mixture was spread onto LB agar plate. The antimicrobial activity of lysosome was measured using colony counts. Results are expressed as the percentage cell mortality (%) according to reference34.
Analysis of proteins extracted from released lysosome
To analyze lysosomal proteins during drug delivery, proteins were extracted from wild type lysosomes, lysosome-2% alginate composite, and lysosome-AOs composite in simulated gastric fluid and intestinal fluid condition followed by SDS-PAGE. Lysosomal proteins were extracted from separated lysosomes. In this experiment, lysis buffer (0.1% NP-40, 0.5 mM DTT, 0.1 mM PMSF) was used. After wild type lysosome and lysis buffer were mixed at a ratio of 1:1, vortexing and cooling were repeated for 20 min and stabilized on ice for 30 min. Finally, we performed centrifugation at 13,000 rpm, 10 min, and 4 °C and collected separated supernatants containing lysosomal proteins extracted from egg white. After filtration of protein through 10 K Amicon Ultra filter, protein concentration was measured with Bradford assay using BSA (bovine serum albumin) as control. Proteins in the gastrointestinal tract were prepared in the same way.
To analyze protein pattern, the amount of lysosomal protein extracted from wild type lysosome, lysosome-2% alginate composite, or lysosome-AOs composite was adjusted 20 µg. Protein was denatured with 5 × SDS-PAGE sample buffer and boiled at 100 °C for 5 min. Then 12.5% separating gel was used to separate proteins by electrophoresis for 3 h. The gel was stained with Coomassie Brilliant Blue overnight and de-stained with de-staining buffer (45% methanol, 10% acetic acid) until Coomassie Brilliant Blue stain was removal.
Observation of the surface by Field emission scanning electron microscope
Surfaces of wild type lysosome, lysosome-2% alginate composite, and lysosome-Types 1, 2, and 3 AOs composite under different pH conditions were observed by Field emission scanning electron microscope (FE-SEM) (Carl Zeiss SUPRA, Germany). Samples were observed after freeze-drying and storage at − 4 °C.
Data analysis
Each data point was taken from three independent samples that were run simultaneously for error analysis. Means were reported to correlate with the standard deviation of some experimental conditions. The data was analyzed using SigmaPlot (Systat Software, Inc., USA). A p-value < 0.05 was considered significant.
Data availability
All the data generated and analyzed during this study are included in the published article.
Abbreviations
- AOs:
-
Alginate Oligosaccharides
- SDS-PAGE:
-
Sodium dodecyl sulfate—polyacrylamide gel electrophoresis
- DW:
-
Distilled water
References
Trombetta, E., Ebersold, M., Garrett, W., Pypaert, M. & Mellman, I. Activation of lysosomal function during dendritic cell maturation. Science 299, 1400–1403 (2009).
Guérin-Dubiard, C. et al. Hen egg white fractionation by ion-exchange chromatography. J. Chromatogr. A. 1090, 58–67 (2005).
Hemmings, B., Zubenko, G., Hasilik, A. & Jones, E. Mutant defective in processing of an enzyme located in the lysosome-like vacuole of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 78, 435–439 (1981).
Holt, O., Gallo, F. & Griffiths, G. Regulating secretory lysosomes. J. Biochem. 140, 7–12 (2006).
Desai, S. & Bolton, S. A floating controlled-release drug delivery system: In vitro–in vivo evaluation. Pharm. Res. 10, 1321–1325 (1993).
Khafagy, E., Morishita, M., Onuki, Y. & Takayama, K. Current challenges in non-invasive insulin delivery systems: A comparative review. Adv. Drug Deliv. Rev. 59, 1521–1546 (2007).
Carino, G. & Mathiowitz, E. Oral insulin delivery. Adv. Drug Deliv. Rev. 35, 249–257 (1999).
Ghebre-Selassie, I. (ed.) Multiparticulate Oral Drug Delivery (CRC Press, 1994).
Lee, K. & Mooney, D. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 37, 106–126 (2012).
Pawar, S. & Edgar, K. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 33, 3279–3305 (2012).
Chan, L., Ching, A., Liew, C. & Heng, P. Mechanistic study on hydration and drug release behavior of sodium alginate compacts. Drug Dev. Ind. Pharm. 33, 667–676 (2007).
Liu, J. et al. Alginate oligosaccharides: Production, biological activities, and potential applications. Compr. Rev. Food Sci. Food Saf 18, 1859–1881 (2019).
Wan, L., Heng, P. & Chan, L. Drug encapsulation in alginate microspheres by emulsification. J. Microencapsul. 9, 309–316 (1992).
Khodagholi, F., Eftekharzadeh, B. & Yazdanparast, R. A new artificial chaperone for protein refolding: Sequential use of detergent and alginate. Protein J. 27, 123–129 (2008).
Rezaii, N. & Khodagholi, F. Evaluation of chaperone-like activity of alginate: Microcapsule and water-soluble forms. Protein J. 28, 124–130 (2009).
Mo, S., Son, E., Rhee, D. & Pyo, S. Modulation of TNF-α-induced icam-1 expression, NO and H2O2 production by alginate, allicin and ascorbic acid in human endothelial cells. Arch. Pharmacal Res. 26, 244 (2003).
An, Q. et al. Alginate-deriving oligosaccharide production by alginase from newly isolated Flavobacterium sp. LXA and its potential application in protection against pathogens. J. Appl. Microbiol. 106, 161–170 (2009).
Sattarahmady, N., Khodagholi, F., Moosavi-Movahedi, A., Heli, H. & Hakimelahi, G. Alginate as an antiglycating agent for human serum albumin. Int. J. Biol. Macromol. 41, 180–184 (2007).
Charruyer, A., Fong, S., Yue, L., Arron, S. & Ghadially, R. Phycosaccharide AI, a mixture of alginate polysaccharides, increases stem cell proliferation in aged keratinocytes. Exp. Dermatol. 25, 738–740 (2016).
Kawada, A. et al. Stimulation of human keratinocyte growth by alginate oligosaccharides, a possible co-factor for epidermal growth factor in cell culture. FEBS Lett. 408, 43–46 (1997).
Morvan, P., Pentecouteau, L., Grandpierre, M., Rougier, N. & Vallee, R. Adult human epidermal stem cells recruitment by an oligoalginate extract: 049. Int. J. Cosmet. Sci. 31, 316–317 (2009).
Kawada, A., Hiura, N., Tajima, S. & Takahara, H. Alginate oligosaccharides stimulate VEGF-mediated growth and migration of human endothelial cells. Arch. Dermatol. Res. 291, 542–547 (1999).
Tusi, S., Khalaj, L., Ashabi, G., Kiaei, M. & Khodagholi, F. Alginate oligosaccharide protects against endoplasmic reticulum-and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 32, 5438–5458 (2011).
Khan, S. et al. Overcoming drug resistance with alginate oligosaccharides able to potentiate the action of selected antibiotics. Antimicrob. Agents Chemother. 56, 5134–5141 (2012).
Tøndervik, A. et al. Alginate oligosaccharides inhibit fungal cell growth and potentiate the activity of antifungals against Candida and Aspergillus spp. PLoS ONE 9, e112518 (2014).
Abeyrathne, E., Lee, H. & Ahn, D. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—A review. Poult. Sci. 92, 3292–3299 (2013).
Nokhodchi, A. & Tailor, A. In situ cross-linking of sodium alginate with calcium and aluminum ions to sustain the release of theophylline from polymeric matrices. Il Farmaco. 59, 999–1004 (2004).
González-Rodrıguez, M., Holgado, M., Sanchez-Lafuente, C., Rabasco, A. & Fini, A. Alginate/chitosan particulate systems for sodium diclofenac release. Int. J. Pharm. 232, 225–234 (2002).
Pillay, V. & Fassihi, R. Unconventional dissolution methodologies. J. Pharm. Sci. 88, 843–851 (1999).
Mellman, I. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12, 575–625 (1996).
Gu, F. & Gruenberg, J. Biogenesis of transport intermediates in the endocytic pathway. FEBS Lett. 452, 61–66 (1999).
Yoon, J., Park, J., Kim, K., Kim, Y. & Min, J. Antimicrobial activity of the cell organelles, lysosomes, isolated from egg white. J. Microbiol. Biotechnol. 19, 1364–1368 (2009).
Xu, X., Iwamoto, Y., Kitamura, Y., Oda, T. & Muramatsu, T. Root growth-promoting activity of unsaturated oligomeric uronates from alginate on carrot and rice plants. Biosci. Biotechnol. Biochem. 67, 2022–2025 (2003).
Han, S. & Yang, Y. Antimicrobial activity of wool fabric treated with curcumin. Dyes Pigm. 64, 157–161 (2005).
Acknowledgements
This research was supported by Korea Basic Science Institute (National research Facilities and Equipment Center) grant funded by the Ministry of Education (Grant No. 2020R1A6C101A204) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2019R1A6A3A01096359).
Author information
Authors and Affiliations
Contributions
R.M.P., N.H.T.N., Y.-H.K., and J.M. designed the experiments and analyzed the results. R.M.P. and S.M.L. conducted experiments. R.M.P. analyzed the experimental data, N.H.T.N. conducted the data analysis. All authors contributed to writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, RM., Nguyen, NH.T., Lee, SM. et al. Alginate oligosaccharides can maintain activities of lysosomes under low pH condition. Sci Rep 11, 11504 (2021). https://doi.org/10.1038/s41598-021-91175-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-91175-6
This article is cited by
-
Recent advances in the production, properties and applications of alginate oligosaccharides - a mini review
World Journal of Microbiology and Biotechnology (2023)
-
Growth-promoting Effect of Alginate Oligosaccharides on Rhodobacter sphaeroides
Biotechnology and Bioprocess Engineering (2022)