Abstract
Systemic juvenile idiopathic arthritis (sJIA) and cryopyrin-associated periodic syndrome (CAPS) share many common manifestations. We aim to identify an applicable method to assist disease discrimination. Inflammatory cytokines were measured in the plasma of patients with CAPS, sJIA with persistent disease course and healthy controls. Supernatants collected from non-stimulated peripheral blood mononuclear cells (PBMCs) and those undergone inflammasome stimulation tests utilizing lipopolysaccharide (LPS) with and without adenosine triphosphate (ATP) were investigated. Inflammatory cytokines in patient plasma fail to differentiate sJIA from CAPS. PBMCs from sJIA secrets higher amount of IL-1β and IL-18 while CAPS PBMCs produces more caspase-1 without stimulation. IL-1β, IL-18, and caspase-1 were significantly elevated among CAPS PBMCs (all p < 0.05) upon LPS stimulation, but not when additional ATPs were provided. Levels of cytokines and PBMC responses to the stimulation assays were similar among all sJIA patients regardless of their history of macrophage activation syndrome. Unstimulated PBMC activities and the LPS inflammasome stimulation assay without exogenic ATPs can assist the differentiation of CAPS from sJIA with persistent disease course.
Similar content being viewed by others
Introduction
Cryopyrin-associated periodic syndrome (CAPS) is a rare autoinflammatory syndrome characterized by overproduction of interleukin (IL)-1β, resulting from dysregulated NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome activity1,2. CAPS-related inflammation can cause fever, fatigue, skin irritation and musculoskeletal symptoms at the early stages3. Persisted uncontrolled inflammation, moreover, can lead to serious organ damages, including sensorineural hearing defect, renal amyloidosis, skeletal deformities and cognitive impairments1,4. To assist early diagnosis, provisional clinical classification criteria for autoinflammatory diseases and diagnostic criteria for CAPS have been introduced5. However, due to its board clinical phenotypes6, making a diagnosis of CAPS in clinic can be challenging7. In fact, the diagnosis of CAPS usually requires genetic evidence of NLRP3/cold-induced autoinflammatory syndrome 1 (CIAS1) germline gene mutations8, while mutations in NOD-like receptor family pyrin domain containing 12 (NLRP12) and somatic mosaicism of NLRP3 were also reported9,10.
Systemic juvenile idiopathic arthritis (sJIA), likewise, is a clinically diagnosed rheumatic disorder marked by over-activation of the innate immune system. Similar to CAPS, it is one of the most prevalent diseases within the scope of autoinflammation with heterogenic systemic manifestations2,11,12. While the course of sJIA may be monophasic or polycyclic, around half of the sJIA cases display a persistent disease course of relapsing high fever associating arthritis, skin rash, lymphadenopathy and serositis13. Additionally, around 6.7%–13% of the patients with sJIA experienced a potentially fatal condition, macrophage activation syndrome (MAS), portrayed by persistent fever, pan-cytopenia, liver abnormalities, lymphadenopathy, coagulopathy and neurological involvement14,15. Overlapping clinical manifestations, mutual hyper-active innate responses as well as common serum and cellular biomarkers of CAPS and sJIA, particularly those with polycyclic and persistent disease course, can sometimes cause confusion for clinicians3,16. While the advance of molecular genetic testing was believed to provide strong support to the diagnosis, in a large French study covering 821 cases suspicious with CAPS, only 16% of the cases were identified with NLRP3 mutations10. In addition, with more than 200 NLRP3 sequence variants registered in the Infevers database associating with CAPS, the pathogenic role of half of the variants are of uncertain significance17.
Many shared gene transcripts have been identified in patients with sJIA and CAPS18,19. Considerable body of works, additionally, have discussed about the gene expression profiling, cellular markers and serum biomarkers among the two diseases16. Limited data, however, were available directly comparing the markers between the two. Hence, it is not known whether or not these markers may be applicable in clinical settings to assist the disease discrimination. To investigate whether inflammatory markers and inflammasome stimulation tests can assist the differentiation of CAPS from persistent sJIA, we evaluated plasma inflammatory cytokine levels as well as the production of inflammatory cytokines produced by the peripheral blood mononuclear cells (PBMCs) upon lipopolysaccharide (LPS) with and without exogenic adenosine triphosphates (ATPs) stimulation in cases with CAPS, persistent sJIA and healthy controls. Moreover, we also evaluated the cytokines and inflammasome activities in sJIA patients with and without a history of MAS. Our data suggested that LPS inflammasome stimulation test without exogenic ATPs, and not plasma inflammatory cytokines perform better in differentiating between the two.
Material and methods
Study population
Eleven patients who fulfilled the 2017 diagnostic criteria for CAPS5 in a pediatric rheumatology clinic of a tertiary medical center in Taiwan were invited to participate the study at time of diagnosis or at time of treatment initiation with active diseases. Twelve cases diagnosed with sJIA according to the international league of associations for rheumatology classification11, with active disease for more than 24 months' duration and still remain in an active disease status were enrolled. Additionally, 11 healthy individuals were also invited as controls. Cases with CAPS have raised acute phase reactants and experienced at least 2 of the following clinical manifestations: recurrent fever, urticarial-like skin rashes, cold or stress triggered attacks, sensorineural hearing deficit, chronic aseptic meningitis, skeletomuscular symptoms or abnormalities, regardless of their family history. Patients diagnosed with sJIA suffered from arthritis and symptoms of systemic inflammation such as fever, skin rash and serositis before the age of 16. MAS was diagnosed according to the 2016 classification criteria for MAS complicating sJIA20. All subjects were excluded for infectious and oncological causes. Written informed consents were collected from all the participated subjects and/or their legal guardian. The research was in compliance with the Declaration of Helsinki and was approved by the Chang Gung Memorial Hospital Institutional Review Board (IRB No.: 201802287A3).
Genetic analysis
NLRP3/CIAS1 gene [NCBI RefSeqGene NC_000001.9] sequencing covering all 9 exomes were analyzed by sanger sequencing using the standard protocol with proper negative and positive controls in each polymerase chain reaction (PCR) test. Specifically, DNA fragments were amplified by ABI PCR machine with designated primers and FastStart Taq DNA polymerase (Roche, Basel, Switzerland). PCR was validated with gel electrophoresis, and the final products were purified with FavorPrep GEL/PCR Purification Kit (Favorgen, Taiwan) according to the manufacturer's protocol. Sequencing reaction was performed with Big Dye Terminator v. 3.1 Ready Reaction Cycle Sequencing kit (Applied Biosystems, Warrington, UK). The electrophoretic profiles of NLRP3 sequences were analyzed on the ABI 3500 Genetic Analyzer.
For those negative of NLRP3/CIAS1 missense mutations, whole exomes sequencing were performed to expand the coverage of other autoinflammatory disease related gene mutations. In detail, exome capture were performed using the Agilent SureSelect Human All Exon Kit V6 (Agilent Technologies) and massively parallel sequencing were carry out using the NovaSeq 6000 (Illumina, San Diego, CA). Raw image analyses and base calling were performed using Illumina’s Pipeline with default parameters. Sequence data were aligned to the reference human genome (hs37d5) using the Burrows-Wheeler Aligner21, and duplicate reads were removed using Picard tools. We use the Genome Analysis ToolKit (GATK 4.1.2) Haplotype Caller for variant calling of SNVs and short (< 50 bp) indels22. Annovar were utilized to catalogue the detected variations23. Then, we filtered variations with a homopolymer length > 6 (and synonymous substitutions) or that were common (> 1%) in dbSNP150 (http://www.ncbi.nlm.nih.gov/projects/SNP/), HapMap, the 1000 Genomes Project (http://www.1000genomes.org), the Exome Aggregation Consortium database and the Genome Aggregation Database (GnomAD, https://gnomad.broadinstitute.org). Integrated genome viewer was used to visualize the reads for manual checking.
Protein measurements
Levels of IL-1β, IL-6, IL-18, tumor necrosis factor alpha (TNF-α) and caspase-1 p20 within frozen plasma and culture soup were measured by sandwich enzyme-linked immunosorbent assay reagent kits (DY201-05, DY206-05, DY318-05, DY210-05 and DCA100, respectively) obtained from R&D system (Minneapolis, MN, USA). Serum amyloid A (SAA) was measured by sandwich enzyme-linked immunosorbent assay reagent kits (KA4865) obtained from Abnova (Taipei, Taiwan). The assays were performed according to the manufacturer’s instructions. Appropriate recombinant human protein were used to establish the standard curve for each assay, respectively.
Inflammasome stimulation test
After isolating human peripheral blood mononuclear cells via Ficoll-Paque gradient centrifugation, cells were counted and seeded onto 96-well plates at a concentration of 3 × 106 cells/ml in culture medium (RPMI medium with 10% heat-inactivated autoserum, 100 U/ml penicillin and 2 mM L-glutamine). The cells were either stimulated with LPS 1 µg/ml (Sigma) for 1 or 4 h, or primed with LPS 1 µg/ml for 1 h then stimulated with ATP 2 mM (Sigma) for another 1 or 4 h after cell seeding. Supernatants collected at 1 h after PBMC seeding without stimulation were used as non-stimulation controls. The culture supernatants were collected for the analysis of IL-1β, IL-18 and caspase-1 secretion with and without stimulations. All tests were performed in duplicates.
Statistical analysis
Continuous data were summarized as means ± SDs and compared by unpaired t-test. Nonparametric Mann–Whitney sum rank U test and Kruskal–Wallis (χ2) test were used for the between-group comparison. The level of significance is determined to be at 0.05. Statistical analyses and graphic presentation were carried out using Prism 6.01 software (GraphPad Software, Inc., San Diego, California).
Ethics approval and consent to participate
This study was approved of by the Institutional Review Board of the Chang Gung Memorial Hospital (IRB No.: 201802287A3).
Results
Clinical characteristics and inflammatory markers in cases with CAPS and sJIA
The average age of onset is 6.2 ± 5.7 years among cases with CAPS and 6.7 ± 4.8 for cases with persistent sJIA. Age at time of recruitment was 23.8 ± 18.0, 13.6 ± 7.8 and 18.3 ± 9.3 for CAPS, sJIA and healthy controls, respectively. The gender distribution, levels of acute phase reactants, laboratory profiles as well as the treatment regimens at time of sampling were summarized in Table 1. Three of the cases (25%) suffered from persistent sJIA experienced at least one MAS episodes after the diagnosis of sJIA.
Among the 11 patients with CAPS, three cases presented with a phenotype compatible with Muckle–Wells syndrome (MWS) while the other 8 experienced recurrent urticarial-like skin rashes especially upon cold or stress triggers. Five patients from 2 distinct family were found to harbor heterozygous c.1316 C > T (p.A439V) NLRP3 variants, which has been confirmed pathogenic according to the Infever database17. Additionally, two independent cases carrying heterozygous NLRP3 variants with undetermined clinical significance c.210 G > A (p.V70M) and c.1371 G > T (p.E457D) were also detached. The minor allele frequency for c.210 G > A and c.1371 G > T is 0.000741 and 0.00008 in the Trans-Omics for Precision Medicine program and 0.0077 and 0.0013 among the East Asians in the GnomAD database, respectively. Somatic mosaicism of NLRP3 were not identified using the sequencing technique applied. Cases diagnosed with persistent sJIA were negative for known genetic mutations associating autoinflammatory diseases. Clinical manifestations and relevant comorbidities of the patients diagnosed with CAPS were summarized in Supplementary Table 1.
Differences in the level of inflammatory cytokines between cases with CAPS and sJIA
CAPS and sJIA were both systemic inflammatory diseases with overactivated innate immune responses. To investigate whether there are differences in the plasma profile of inflammatory cytokines, levels of IL-1β, IL-6, IL-18, TNF-α and SAA were examined from patients’ plasma. As shown in Fig. 1, while an elevation in the level of IL-18 was observed in patients with CAPS when compared to the healthy controls (1366.1 ± 196.2 vs. 568.8 ± 171.9; p = 0.0008), the differences in levels of plasma IL-6 between CAPS and persistent sJIA were borderline significant (p = 0.051). Additionally, patients with sJIA have higher levels of inflammatory cytokines in all parameters tested when compared to healthy controls (38.6 ± 58.68 vs. 1.5 ± 0.7, 13.0 ± 11.7 vs. 0.8 ± 1.2, 1244.0 ± 612.8 vs. 568.8 ± 171.9, 10.8 ± 5.8 vs. 4.4 ± 1.6 and 11.6 ± 7.2 vs. 1.1 ± 0.6 for IL-1β, IL-6, IL-18, TNF-α and SAA; p = 0.04, 0.002, 0.002, 0.001 and 0.001 respectively).
Levels of inflammatory cytokines and serum amyloid A in patients’ plasma. Levels of inflammatory cytokines IL-1β, IL-6, IL-18, TNF-α and SAA were measured in cases with CAPS, sJIA and healthy controls. While the cytokines were globally elevated among the sJIA patients (p < 0.05), the level of IL-6 within the sJIA patient plasma is increased as compared to those with CAPS with borderline significance (p = 0.051). Data are presented with dot plot.* indicates p < 0.05. Abbreviations: CAPS- cryopyrin-associated periodic syndrome; sJIA-systemic juvenile idiopathic arthritis; HC- healthy controls; IL-1β- interleukin 1β; IL-6- interleukin 6; IL-18- interleukin 18; TNF⍺- tumor necrosis factor ⍺; SAA- serum amyloid A.
Differences in inflammasome stimulation test between cases with CAPS and sJIA
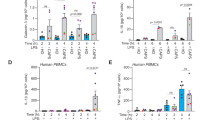
Considering the critical role of NLRP3 inflammasome in CAPS and its possible effect in sJIA8,24,25, we evaluated inflammatory cytokines production from patient PBMCs with and without NLRP3 stimuli using plain culture soup alone, LPS and LPS-primed ATP regimen. Shown in Fig. 2A, significant increase in the levels of IL-1β, IL-18 and caspase 1 were detected in the soup of PBMCs isolated from patients with CAPS at 1 h and 4 h upon LPS stimuli in comparison to those isolated from sJIA and healthy controls (all p < 0.05). While higher levels of IL-18 were produced by sJIA PBMCs consistently regardless of LPS stimuli when compared to those healthy controls (p = 0.01, 0.03, 0.05, without and 1 and 4 h of stimulation, respectively), the production of IL-1β and caspase-1 did not significantly increase in sJIA PBMCs as compared to healthy controls upon LPS stimulation (all p > 0.05). Interestingly, caspase-1 production is increased in the CAPS PBMCs when compared to those isolated from sJIA and healthy controls before additional stimulants were applied (all p < 0.05). Moreover, the levels of IL-1β were also slightly higher in the sJIA PBMCs as compared to the healthy controls before stimulation (p = 0.03).
Secretion of inflammatory cytokines upon in vitro stimulation test. Secretion of IL-1β, IL-18 and caspase-1 differentiates between patients with CAPS, sJIA and healthy controls. IL-1β, IL-18 and caspase-1 secretion were measured in the supernatant of PBMCs isolated from patients with CAPS, sJIA and control donors with medium only and 1 and 4 h after A) LPS stimulation, and B) LPS-primed ATP stimulation regimen. Data are shown as dot plat with means. * indicates p < 0.05. Abbreviations: CAPS- cryopyrin-associated periodic syndrome; sJIA-systemic juvenile idiopathic arthritis; HC- healthy controls; IL-1β- interleukin 1β; IL-18- interleukin 18; LPS- lipopolysaccharide; ATP- adenosine triphosphate; PBMC- peripheral blood mononuclear cells.
Extracellular ATP is a potent activator for NLRP3 inflammasome26. When PBMCs were provided with additional ATPs followed by LPS priming, the levels of caspase 1 and IL-1β increased by 2 to 4 folds universally, as shown in Fig. 2B. However, as the production of inflammatory cytokines were significantly increased, the differences in the levels of IL-1β, IL-18 and caspase 1 between those with or without diseases may be limited. Indeed, no significant differences in the inflammatory mediators were detached in the culture soup of PMBCs isolated from patient diagnosed with CAPS, sJIA and healthy controls (all p > 0.05).
Differences in plasma cytokine levels and inflammasome responses in sJIA patients with and without a propensity of MAS
To investigate whether the propensity of MAS correlated with inflammasome responses or levels of plasma cytokines in cases with sJIA, we compared the inflammatory mediators acquired from 3 sJIA who experienced at least one episodes of MAS during follow up to those without. As shown in Fig. 3A, no significant differences were observed in the levels of plasma IL-1β, IL-6, IL-18 or TNF-α between sJIA patients regardless of the history of MAS (all p > 0.05). Moreover, the production of IL-1β and IL-18 in PBMCs upon LPS stimuli were also similar (Fig. 3B).
Plasma cytokine levels and PBMC responses to in vitro stimulations test among sJIA patients with and without a history of MAS. Levels of plasma cytokines and responses to in vitro stimulations tests are comparable among sJIA patients regardless of the propensity of MAS. (A) Levels of IL-1β, IL-6, IL-18 and TNF-α were measured and compared between sJIA cases with and without a history of MAS. (B) IL-1β and IL-18 secretion were measured in the supernatant of PBMCs isolated from patients with sJIA with medium only and 1 and 4 h after LPS stimulation. Results between those with and without a history of MAS were compared. Data are presented with dot plot with means. Abbreviations: sJIA-systemic juvenile idiopathic arthritis; MAS- macrophage activation syndrome; IL-1β- interleukin 1β; IL-6- interleukin 6; IL-18- interleukin 18; TNF⍺- tumor necrosis factor ⍺; LPS- lipopolysaccharide; ATP- adenosine triphosphate.
Discussions
In the present study, we found that while inflammatory cytokines were generally elevated in persistent sJIA patients’ plasma as compared to the healthy controls, differences between CAPS and sJIA patients were not statistically significance. Moreover, the production of IL-1β, IL-18, and caspase-1 in the inflammasome activation assay were significantly increased among CAPS patients in comparison with sJIA patients or healthy individuals 1 or 4 h following LPS stimulation. This reaction, however, is masked by the universal elevation of inflammatory cytokines when exogenic ATPs were added. Finally, our data suggested that the levels of plasma cytokines and PBMC responses to the LPS stimulation assays were similar among all sJIA patients regardless of their propensity of MAS.
Nirmala et al. recently reviewed the similarities and differences of the protein, cellular, mRNA and DNA markers among patients suffering from sJIA and CAPS16. While inflammatory markers including IL-6, IL-18, S100A8/A9 and S100A12 were noted to elevate under both conditions27,28,29,30,31, limited reports have yet directly compared CAPS and sJIA for their expression of biomarkers. In a case series reported by Ohnishi et al., one patient with active JIA presented with higher levels of serum IL-6 and IL-18 in comparison with those with CAPS and healthy controls3. S100A8 and S100A9 have also been found to increase significantly among cases with sJIA, particularly during active status, when compared to patients with neonatal onset multisystem inflammatory disease (NOMID) and other inflammatory disorders30. As the activity status of sJIA largely influenced the level of serum cytokines30,32, sampling sJIA patients during chronic active phases, our data on inflammatory cytokines show no differences between cases suffered from CAPS and sJIA (Fig. 1). Thus, while our data echoed previous reports on a global increase of proinflammatory cytokines in cases with sJIA and CAPS16, no single cytokine along is capable of assisting the differentiation of CAPS from cases with persistent sJIA.
Physiologically, NLRP3 cryopyrin, an intracellular sensor, forms inflammasome complex associating pro-caspase-1 and ASC proteins upon encountering its triggers26. The formation of inflammasome then catalyzes pro-IL-1β and pro-IL-18, produce upon the activation of nuclear factor kappa B (NF-κB) signaling, to their mature form and potently drives further inflammation26,33. According to our result, no spontaneous secretion of IL-1β and IL-18, but caspase 1 was noted in CAPS PBMCs before any stimulation was applied (Fig. 2A,B). This may be explained by the necessity of NF-κB signaling for the transcription of pro-IL-1β and pro-IL-18, before they can be catalyzed by the over-active NLRP3 inflammasomes34,35. On the contrary, caspase-1 is a direct interaction partner for the NLRP3 inflammasome and is critical for its proteolytic activity in processing the precursors of various inflammatory cytokines33. Even without additional triggers, altered NLRP3 proteins oligomerized with pro-caspase-1 and displayed a higher basal caspase-1 activity as compared to healthy controls36. Nonetheless, while several reports also demonstrated the requirement of NF-κB signaling for the production of IL-1β and IL-18 in in vitro stimulation assays3,37,38, others reported high unstimulated cytokine production, including IL-6, IL-18, TNF, interferon (IFN)-γ and IL-12p70 in PBMCs isolated from patients suffered from CAPS39,40. These differences may be influenced by the disease status and severity at time of sampling. Furthermore, PBMCs from sJIA secreted higher amount of IL-1β and IL-18 before any additional stimulants were provided (Fig. 2A,B). Although the exact mechanism is unknown, genes involved in innate immune reaction, such as IL-1, IL-18 and toll like receptor (TLR) signaling pathways have been found to upregulate in cases with sJIA41.
The most pronounced difference between CAPS and persistent sJIA in the present study is perhaps the reaction of PBMCs upon LPS stimulation (Fig. 2A). While different mutations and its capacity to auto-activate the inflammasome can lead to large variability in the responses to stimulation37, IL-1β, IL-18, and caspase-1 were significantly elevated among CAPS PBMCs (all p < 0.05) upon LPS stimulation, but not when additional ATPs were provided. Experiments carry out in MWS murine models revealed a lower threshold and enhanced production of IL-1β and IL-1842,43. Likewise, enhanced IL-1β, IL-18 and caspase-1 release have been shown in CAPS PMBCs in comparison to control PBMCs34,37. Mutations in NLRP3 is believed to drive inflammation in cases with CAPS. Although polymorphisms in NLRP3 have also been reported to associate with sJIA25, it did not lead to an altered inflammatory response upon TLR stimulation. Additionally, while Reiber et al. observed a significant increase of mature IL-1β, IL-18, and caspase-1 in culture supernatants 4 h following LPS stimulation37, our result suggested that the differences may be detected at an earlier time point. The rapid and colossal secretion of inflammatory cytokines upon LPS stimulation is a reflection of the efficient processing of pro-cytokines in CAPS with constitutive active NLRP3 inflammasomes1,2,4. Moreover, priming the PBMCs with LPS followed by addition ATP in the samples showed global elevation in the inflammasome related products regardless of the underlining diseases (Fig. 2B). This was distinct from Reiber’s report, perhaps because their PBMCs received dual stimulation with ATPs and LPS simultaneously at a dose 10 times higher37. ATP is a potent activator for NLRP3 inflammasome26. The exogenic ATPs provided in the present protocol somehow activated NLRP3 inflammasomes to a comparable degree as they were in CAPS and masked the differences when LPS stimulation was provided. Together, our data suggested that LPS stimulation test is better than LPS and ATP together in identifying CAPS from patients with persistent sJIA and healthy controls.
Marked increase of proinflammatory cytokines including IL-1, IL-6, IL-18, TNFα, IFNγ, ferritin and ST2 during MAS attacks portrayed a significant systemic inflammation in patients with various rheumatic diseases, particularly sJIA28,44,45,46,47. Recently, Canna et al. reported that gain of function mutations in NLRC4 can display a MAS like clinical presentation48. In addition, repetitive TLR stimulation via administration of CpG has been shown to induce a MAS-like features in mice49. Even though infections and inflammasome dysregulation have been considered as possible triggers for MAS44,45, the discrimination of immune responses between sJIA from CAPS with LPS stimulation tests in not jeopardized by the tendency for MAS in the present study with limited cases. Although further study may be required to confirm this observation, the similarity in the level of inflammatory cytokines and PBMC responses to LPS suggested that the induction of MAS in patients with sJIA may less likely result from NLRP3 inflammasome dysregulation.
The present study is limited by its few case numbers and only CAPS patients with the clinical phenotype of familial cold autoinflammatory syndrome (FCAS) and MWS were studied. It would be worthwhile to also examine patients with NOMID phenotype since the disease activity and phenotype have also been implied with altered inflammasome responses39. Moreover, considering the association of aging and inflammasome activity50, the imperfectly age-matched healthy controls can also result in bias in the present study. Finally, the in vitro functional diagnostic tests were performed in CAPS and sJIA patients under medical treatment with a relatively long disease duration. Although in clinical settings, the consideration of rare diseases and genetic testing are usually reserved for those who suffered from persisted disease course or those who respond poorly to standard treatments, future validation of the results with treatment-naïve patients before being correctly diagnosed for CAPS or sJIA would definitely further strengthen the clinical application.
Conclusions
In summary, our data suggested that the rapid and excessive-production of IL-1β, IL-18 and caspase-1 in the LPS inflammasome stimulation assay on PBMC cultures can assist the differentiation of persistent sJIA and CAPS.
Data availability
All relevant data were displayed in the table and figures.
Abbreviations
- ATP:
-
Adenosine triphosphate
- CAPS:
-
Cryopyrin-associated periodic syndrome
- CIAS1:
-
Cold-induced autoinflammatory syndrome 1
- FCAS:
-
Familial cold autoinflammatory syndrome
- IFN:
-
Interferon
- IL:
-
Interleukin
- LPS:
-
Lipopolysaccharide
- MAS:
-
Macrophage activation syndrome
- MWS:
-
Muckle–Wells syndrome
- NF-κB:
-
Nuclear factor kappa B
- NLRP12:
-
NOD-like receptor family pyrin domain containing 12
- NLRP3:
-
NOD-like receptor family pyrin domain containing 3
- NOMID:
-
Neonatal onset multisystem inflammatory disease
- PBMCs:
-
Peripheral blood mononuclear cells
- PCR:
-
Polymerase chain reaction
- SAA:
-
Serum amyloid A
- sJIA:
-
Systemic juvenile idiopathic arthritis
- TLR:
-
Toll like receptor
- TNF-α:
-
Tumor necrosis factor alpha
References
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426. https://doi.org/10.1016/s1097-2765(02)00599-3 (2002).
Bodar, E. J., Drenth, J. P., van der Meer, J. W. & Simon, A. Dysregulation of innate immunity: hereditary periodic fever syndromes. Br. J. Haematol. 144, 279–302. https://doi.org/10.1111/j.1365-2141.2008.07036.x (2009).
Ohnishi, H. et al. Characterization of NLRP3 variants in Japanese cryopyrin-associated periodic syndrome patients. J. Clin. Immunol. 32, 221–229. https://doi.org/10.1007/s10875-011-9629-0 (2012).
Farasat, S., Aksentijevich, I. & Toro, J. R. Autoinflammatory diseases: clinical and genetic advances. Arch. Dermatol. 144, 392–402. https://doi.org/10.1001/archderm.144.3.392 (2008).
Kuemmerle-Deschner, J. B. et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann. Rheum. Dis. 76, 942–947. https://doi.org/10.1136/annrheumdis-2016-209686 (2017).
Kuemmerle-Deschner, J. B. & Haug, I. Canakinumab in patients with cryopyrin-associated periodic syndrome: an update for clinicians. Ther. Adv. Musculoskelet. Dis. 5, 315–329. https://doi.org/10.1177/1759720X13502629 (2013).
Toplak, N. et al. An international registry on autoinflammatory diseases: the Eurofever experience. Ann. Rheum. Dis. 71, 1177–1182. https://doi.org/10.1136/annrheumdis-2011-200549 (2012).
Hoffman, H. M., Mueller, J. L., Broide, D. H., Wanderer, A. A. & Kolodner, R. D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle–Wells syndrome. Nat. Genet. 29, 301–305. https://doi.org/10.1038/ng756 (2001).
Lasiglie, D. et al. Cryopyrin-associated periodic syndromes in Italian patients: evaluation of the rate of somatic NLRP3 mosaicism and phenotypic characterization. J. Rheumatol. 44, 1667–1673. https://doi.org/10.3899/jrheum.170041 (2017).
Cuisset, L. et al. Mutations in the autoinflammatory cryopyrin-associated periodic syndrome gene: epidemiological study and lessons from eight years of genetic analysis in France. Ann. Rheum. Dis. 70, 495–499. https://doi.org/10.1136/ard.2010.138420 (2011).
Petty, R. E. et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001.: second revision, Edmonton, 2001. J. Rheumatol. 31, 390–392 (2004).
Holzinger, D., Kessel, C., Omenetti, A. & Gattorno, M. From bench to bedside and back again: translational research in autoinflammation. Nat. Rev. Rheumatol. 11, 573–585. https://doi.org/10.1038/nrrheum.2015.79 (2015).
Singh-Grewal, D., Schneider, R., Bayer, N. & Feldman, B. M. Predictors of disease course and remission in systemic juvenile idiopathic arthritis: significance of early clinical and laboratory features. Arthritis Rheum. 54, 1595–1601. https://doi.org/10.1002/art.21774 (2006).
Grom, A. A. & Mellins, E. D. Macrophage activation syndrome: advances towards understanding pathogenesis. Curr. Opin. Rheumatol. 22, 561–566. https://doi.org/10.1097/01.bor.0000381996.69261.71 (2010).
McClain, K. L. & Allen, C. E. Fire behind the fury: IL-18 and MAS. Blood 131, 1393–1394. https://doi.org/10.1182/blood-2018-02-828186 (2018).
Nirmala, N., Grom, A. & Gram, H. Biomarkers in systemic juvenile idiopathic arthritis: a comparison with biomarkers in cryopyrin-associated periodic syndromes. Curr. Opin. Rheumatol. 26, 543–552. https://doi.org/10.1097/BOR.0000000000000098 (2014).
Milhavet, F. et al. The infevers autoinflammatory mutation online registry: update with new genes and functions. Hum. Mutat. 29, 803–808. https://doi.org/10.1002/humu.20720 (2008).
Ogilvie, E. M., Khan, A., Hubank, M., Kellam, P. & Woo, P. Specific gene expression profiles in systemic juvenile idiopathic arthritis. Arthritis Rheum. 56, 1954–1965. https://doi.org/10.1002/art.22644 (2007).
Hinze, C. H. et al. Immature cell populations and an erythropoiesis gene-expression signature in systemic juvenile idiopathic arthritis: implications for pathogenesis. Arthritis Res. Ther. 12, R123. https://doi.org/10.1186/ar3061 (2010).
Ravelli, A. et al. 2016 Classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European league against rheumatism/American college of rheumatology/paediatric rheumatology international trials organisation collaborative initiative. Arthritis Rheumatol. 68, 566–576. https://doi.org/10.1002/art.39332 (2016).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760. https://doi.org/10.1093/bioinformatics/btp324 (2009).
DePristo, M. A. et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498. https://doi.org/10.1038/ng.806 (2011).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164. https://doi.org/10.1093/nar/gkq603 (2010).
Aksentijevich, I. et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 46, 3340–3348. https://doi.org/10.1002/art.10688 (2002).
Yang, C. A., Huang, S. T. & Chiang, B. L. Association of NLRP3 and CARD8 genetic polymorphisms with juvenile idiopathic arthritis in a Taiwanese population. Scand. J. Rheumatol. 43, 146–152. https://doi.org/10.3109/03009742.2013.834962 (2014).
Kelley, N., Jeltema, D., Duan, Y. & He, Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20133328 (2019).
de Benedetti, F. et al. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 34, 1158–1163. https://doi.org/10.1002/art.1780340912 (1991).
Maeno, N. et al. Highly elevated serum levels of interleukin-18 in systemic juvenile idiopathic arthritis but not in other juvenile idiopathic arthritis subtypes or in Kawasaki disease: comment on the article by Kawashima et al.. Arthritis Rheum. 46, 2539–2541. https://doi.org/10.1002/art.10389 (2002) (author reply 2541-2532).
Wittkowski, H. et al. S100A12 is a novel molecular marker differentiating systemic-onset juvenile idiopathic arthritis from other causes of fever of unknown origin. Arthritis Rheum. 58, 3924–3931. https://doi.org/10.1002/art.24137 (2008).
Frosch, M. et al. The myeloid-related proteins 8 and 14 complex, a novel ligand of toll-like receptor 4, and interleukin-1beta form a positive feedback mechanism in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 60, 883–891. https://doi.org/10.1002/art.24349 (2009).
Wittkowski, H. et al. MRP8 and MRP14, phagocyte-specific danger signals, are sensitive biomarkers of disease activity in cryopyrin-associated periodic syndromes. Ann. Rheum. Dis. 70, 2075–2081. https://doi.org/10.1136/ard.2011.152496 (2011).
Lotito, A. P., Campa, A., Silva, C. A., Kiss, M. H. & Mello, S. B. Interleukin 18 as a marker of disease activity and severity in patients with juvenile idiopathic arthritis. J. Rheumatol. 34, 823–830 (2007).
Keller, M., Ruegg, A., Werner, S. & Beer, H. D. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831. https://doi.org/10.1016/j.cell.2007.12.040 (2008).
Bauernfeind, F. G. et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791. https://doi.org/10.4049/jimmunol.0901363 (2009).
Man, S. M. & Kanneganti, T. D. Regulation of inflammasome activation. Immunol. Rev. 265, 6–21. https://doi.org/10.1111/imr.12296 (2015).
Verma, D. et al. Two adult siblings with atypical cryopyrin-associated periodic syndrome due to a novel M299V mutation in NLRP3. Arthritis Rheum. 62, 2138–2143. https://doi.org/10.1002/art.27489 (2010).
Rieber, N. et al. A functional inflammasome activation assay differentiates patients with pathogenic NLRP3 mutations and symptomatic patients with low penetrance variants. Clin. Immunol. 157, 56–64. https://doi.org/10.1016/j.clim.2015.01.003 (2015).
Stack, J. H. et al. IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocytes from familial cold autoinflammatory syndrome patients. J. Immunol. 175, 2630–2634. https://doi.org/10.4049/jimmunol.175.4.2630 (2005).
Haverkamp, M. H., van de Vosse, E., Goldbach-Mansky, R. & Holland, S. M. Impaired cytokine responses in patients with cryopyrin-associated periodic syndrome (CAPS). Clin. Exp. Immunol. 177, 720–731. https://doi.org/10.1111/cei.12361 (2014).
Janssen, R., Verhard, E., Lankester, A., Ten Cate, R. & van Dissel, J. T. Enhanced interleukin-1beta and interleukin-18 release in a patient with chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 50, 3329–3333. https://doi.org/10.1002/art.20494 (2004).
Quartier, P. et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial). Ann. Rheum. Dis. 70, 747–754. https://doi.org/10.1136/ard.2010.134254 (2011).
Meng, G., Zhang, F., Fuss, I., Kitani, A. & Strober, W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity 30, 860–874. https://doi.org/10.1016/j.immuni.2009.04.012 (2009).
Jiang, H. et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 214, 3219–3238. https://doi.org/10.1084/jem.20171419 (2017).
Schulert, G. S. & Grom, A. A. Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu. Rev. Med. 66, 145–159. https://doi.org/10.1146/annurev-med-061813-012806 (2015).
Grom, A. A., Horne, A. & De Benedetti, F. Macrophage activation syndrome in the era of biologic therapy. Nat. Rev. Rheumatol. 12, 259–268. https://doi.org/10.1038/nrrheum.2015.179 (2016).
Fall, N. et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 56, 3793–3804. https://doi.org/10.1002/art.22981 (2007).
Ishikawa, S., Shimizu, M., Ueno, K., Sugimoto, N. & Yachie, A. Soluble ST2 as a marker of disease activity in systemic juvenile idiopathic arthritis. Cytokine 62, 272–277. https://doi.org/10.1016/j.cyto.2013.03.007 (2013).
Canna, S. W. et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat. Genet. 46, 1140–1146. https://doi.org/10.1038/ng.3089 (2014).
Behrens, E. M. et al. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J. Clin. Investig. 121, 2264–2277. https://doi.org/10.1172/JCI43157 (2011).
Sebastian-Valverde, M. & Pasinetti, G. M. The NLRP3 inflammasome as a critical actor in the inflammaging process. Cells https://doi.org/10.3390/cells9061552 (2020).
Acknowledgements
We thank GAA corp. Taipei, Taiwan for their support in NLRP3 gene sequencing and the Genomic Medicine Core Laboratory at Chang Gung Memorial Hospital for analyzing the whole exome sequencing data. We also thank Pi-Shuang Chu and Pei-Chun Liao for their contribution in sample collection and assistance on the in vitro stimulation tests.
Funding
This work was supported by the Chang-Gung memorial Hospital research grant CMRPG3G1191 and CMRPG3J1901-2.
Author information
Authors and Affiliations
Contributions
C.-Y.W. have designed and drafted the work; C.-Y.W., W.-L.F. and H.-Y.Y. have interpreted the data; J.-L.H., W.-I.L., Y.-M.C. have assist case recruitment and made substantial contributions to the conception and substantively revised it; and C.-Y.W., J.-L.H. and W.-I.L. have finalized the manuscript for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, CY., Fan, WL., Chiu, YM. et al. Lipopolysaccharide stimulation test on cultured PBMCs assists the discrimination of cryopyrin-associated periodic syndrome from systemic juvenile idiopathic arthritis. Sci Rep 11, 11903 (2021). https://doi.org/10.1038/s41598-021-91354-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-91354-5
This article is cited by
-
Glycocalyx Disruption Triggers Human Monocyte Activation in Acute Heart Failure Syndromes
Cardiovascular Drugs and Therapy (2024)