Abstract
Controversy exists regarding whether the rate of hepatocellular carcinoma (HCC) recurrence after orthotopic liver transplantation (OLT) differs when using livers from donation after controlled circulatory death (DCD) versus livers from donation after brain death (DBD). The aim of this cohort study was to analyze rates of HCC recurrence, patient survival, and graft survival after OLT for HCC, comparing recipients of DBD livers (n = 103) with recipients of uncontrolled DCD livers (uDCD; n = 41). No significant differences in tumor size, tumor number, serum alpha-fetoprotein, proportion of patients within Milan criteria, or pre-OLT bridging therapies were identified between groups, although the waitlist period was significantly shorter in the uDCD group (p = 0.040). HCC recurrence was similar between groups. Patient survival was similar between groups, but graft survival was lower in the uDCD group. Multivariate analysis identified recipient age (p = 0.031), pre-OLT bridging therapy (p = 0.024), and HCC recurrence (p = 0.048) as independent risk factors for patient survival and pre-OLT transarterial chemoembolization (p = 0.045) as the single risk factor for HCC recurrence. In conclusion, similar patient survival and lower graft survival were observed in the uDCD group. However, the use of uDCD livers appears to be justified due to a shorter waitlist time, and lower waitlist dropout and HCC recurrence rates.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) represents the sixth most common malignancy and the third leading cause of cancer-related deaths around the world1,2. It is frequently diagnosed incidentally or during screening programs, as people who develop HCC typically do not manifest symptoms until the tumor has reached a late stage.
Orthotopic liver transplantation (OLT) is considered the treatment of choice for patients with early-stage HCC (Milan criteria). In Spain, 1,227 patients underwent OLT during 2019, with 282 (23%) receiving transplants for HCC3. However, the number of available liver grafts remains insufficient to treat all patients who require an OLT for malignant or benign disease. To increase the liver graft pool and thereby decrease waitlist mortality, new strategies have been developed, such as the use of livers from marginal donors4, including livers donated after controlled circulatory death (cDCD)5,6, uncontrolled circulatory death (uDCD)7,8,9,10,11, and older donors12,13. Recently, liver grafts with major extended criteria (steatosis > 40%, age > 65 years, and prolonged cold ischemia time [CIT]) have been used in recipients with HCC without impairing patient survival or HCC recurrence14.
In Type 2 uDCD donation, the donor sustained a witnessed out-of-hospital cardiac arrest and underwent unsuccessful cardiopulmonary resuscitation, whereas in type 3 cDCD donation, organs are recovered after death confirmation from patients with irreversible brain injury or respiratory failure, in whom life-sustaining treatment has been withdrawn15. Liver grafts from both uDCD and cDCD donation are subjected to longer warm ischemia periods, compared to grafts donated after brain death (DBD), which results in a higher likelihood of ischemia/reperfusion injury (IRI). Use of uDCD livers has been associated with higher rates of primary nonfunction (PNF) and biliary complications (BCs) than OLT with cDCD and DBD donors. This has been attributed to the longer period of ischemia with uDCD, which is the sum of the donor circulatory arrest time, duration of cardiopulmonary resuscitation (CPR), duration of normothermic regional perfusion (NRP), and recipient warm ischemia time (WIT)7,11. However, use of uDCD livers may be justified given the length of time OLT candidates remain on the waitlist (mean, 124 days)3, and the associated dropout risk.
As patients with HCC often exhibit compensated, clinically stable disease with a relatively low Model for End-stage Liver Disease (MELD) score when placed on the waitlist, these individuals are usually ideal candidates for cDCD OLT, as they are able to tolerate potential complications related to the use of marginal livers. Nevertheless, several reports have demonstrated increased HCC recurrence after OLT using cDCD, as well as reduced patient and graft survival16,17,18. These results were explained in a mouse model, which showed that IRI is a strong stimulus for recurrent intrahepatic tumor growth in transplanted liver 16,18,19.
The aim of this study was to analyze use of liver grafts from uDCD donation in patients with HCC, comparing rates of patient and graft survival, HCC recurrence, and recurrence-free survival with a control group of patients with HCC who received livers from DBD donation.
Patients and methods
Study population and study design
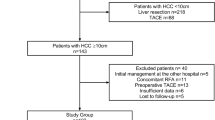
Between April 1986 and December 2016, 1,876 OLTs were performed in adults and children at our institution. From January 2006 to December 2016, 75 of these OLTs were performed using livers from uDCD donation. This retrospective cohort study compared 103 adults with HCC who underwent OLT using liver grafts from DBD donation (DBD Group) with 41 adults with HCC who underwent OLT with livers from uDCD donation (uDCD Group). There was a chronological correlation between cases and controls. This study was performed in accordance with the ethical guidelines of the declaration of Helsinki, and was approved by Institutional Review Board/Ethics Committee of “12 de Octubre” University Hospital. The organs were not procured from prisoners. The study was closed on October 31, 2018, after a minimum follow-up of 22 months after OLT.
Criteria for acceptance of uDCD and DBD liver grafts
All liver donors in this study were maintained with NRP for a maximum of 300 min before initiation of organ perfusion, in accordance with our previously described protocol for uDCD donors11. The criteria for acceptance of uDCD livers were as follows: donor age between 14 and 55 years; maximum transaminase levels < 4 times the upper limit of normal; and absence of alcoholic disease, drug addiction, history of cancer, hepatitis B and/or C infection, HIV infection, violent death, or abdominal trauma. Additional criteria, which were assessed at the time of organ procurement, included good appearance, consistency, and vascularization of the liver graft, and no evidence of ischemia of the gallbladder, common bile duct, or intestines.
Graft donor warm ischemia time (DWIT), also called pre-NRP WIT, was defined as the sum of circulatory arrest time and duration of pre-NRP CPR, whereas recipient WIT (RWIT) was defined as the interval from removal from cold preservation solution to completion of portal vein anastomosis. We routinely performed liver graft biopsy before cold perfusion and discarded grafts with fibrosis or > 30% macrosteatosis. We also discarded grafts when DWIT or RWIT exceeded the times established in the protocol. Once it is established that the liver graft from uDCD meets the criteria for OLT, the liver transplant team selects a recipient from their own waitlist that has previously signed the informed consent. This restriction (i.e. only offering the graft to the liver transplant team that has performed the procurement) was set in order to minimize ischemia time. In our hospital we have a single transplant waitlist that includes both patients that have only accepted livers from DBD and those who accept both donation types (DBD/uDCD).
Inclusion and exclusion criteria, candidate information, and transplant technique
The inclusion criteria for OLT recipients were age > 18 years, HCC as the main indication for transplant, and within the Milan20 or University of California, San Francisco (UCSF) criteria for transplantation21. We excluded patients who received partial or combined transplants, underwent retransplantation, were positive for HIV, underwent OLT for fulminant hepatitis, or received grafts from donors > 70 years of age (for DBD recipients). Use of uDCD livers was avoided in patients with previous abdominal surgery or a MELD score > 30. No exception points were added to the MELD score for patients with tumors > 2 cm in diameter. To prevent tumor progression, transarterial chemoembolization (TACE) was usually performed in patients with several tumors or a single tumor and ascites, whereas radiofrequency ablation was generally used in patients with a single tumor. Recipient hepatectomy was performed using the vena cava sparing technique (piggy-back). In most cases, biliary reconstruction was performed as a cholechocholedochostomy without T-tube.
Comparisons of donor and pre-OLT recipient variables
Donor variables common to both groups were compared between groups. These included age, sex, body mass index (BMI), cause of death, vasopressor use, transfusion, intensive care unit (ICU) stay, cardiac arrest, steatosis, cold ischemia time (CIT), and RWIT. The following pre-OLT recipient characteristics were also compared between groups: age, sex, BMI, OLT indication, MELD score, comorbidities, waitlist duration, and laboratory values. Likewise, a number of perioperative variables were compared between groups, including biliary reconstruction, transfusion of blood products, post-OLT liver function, immunosuppression, retransplantation, post-OLT complications, patient and graft survival, and recurrence-free survival. Pre- and post-OLT tumor characteristics and HCC recurrence were also compared between groups.
PNF was defined as severe clinical deterioration requiring retransplantation or progressing to death, which was the consequence of irreversible liver graft failure within 10 days after OLT, in the absence of vascular complications22. Among BCs, non-anastomotic biliary strictures (NABS) were defined as any stricture, dilation, or irregularity of the intrahepatic or extrahepatic bile ducts of the liver (hilum), whereas anastomotic biliary strictures (ABS) were defined as lesions localized to the anastomosis site23.
Immunosuppression
The immunosuppressive regimen consisted of tacrolimus and prednisone. Corticosteroids were usually discontinued between 3 and 6 months after OLT. Tacrolimus trough levels were maintained between 10–15 ng/mL during the 1st months after transplantation, between 8 and 12 ng/mL until the 6th month, and between 5 and 8 ng/mL thereafter. Mild acute rejection was treated with increasing the dose of tacrolimus, whereas moderate or severe episodes was treated with 1 g methylprednisolone intravenously for 3 days.
Currently, we use a tacrolimus-based regimen with lower tacrolimus doses, which includes mycophenolate mofetil (MMF) or a mammalian target of rapamycin inhibitor (mTORi) in the presence of renal dysfunction, hypertension, diabetes, de novo tumor, or HCC as OLT indication. Conversion from tacrolimus to MMF or mTORi monotherapy is performed on long-term follow-up in recipients who undergo OLT for HCC or in patients with severe adverse tacrolimus effects but stable liver function.
Statistical analysis
Quantitative variables were expressed as mean and standard deviation or median and interquartile (25%–75%) range. Qualitative variables were expressed as percentages. Differences between qualitative variables were assessed by chi-square test or Fisher's exact test, as appropriate. Quantitative variables were compared using t-test or Mann–Whitney U test, depending on whether the data were normally distributed. Graft and patient survival rates were estimated using the Kaplan–Meier method, and survival curves were compared using the log-rank test. Donor and recipient variables with p values < 0.10 in univariate analysis were subsequently investigated in multivariate analysis using Cox's regression model to evaluate associations between baseline variables and patient or graft survival. Results were expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). P values < 0.05 were considered statistically significant. All analyses were performed with SPSS Statistics Version 24.
Ethical approval
This study was approved by our Institutional Review Board.
Informed consent
All patients included in this study firmed inform consent to treatment.
Results
Donor and recipient characteristics
uDCD donors were significantly younger than DBD donors (41.0 ± 10.0 y vs. 48.0 ± 13.0 y; p = 0.001). Mean BMI, as well as rates of cardiac arrest and vasopressor use, was significantly higher in the uDCD group. There were no statistically significant differences between groups for CIT, RWIT, and rates of donor microsteatosis or macrosteatosis.
During the period of this study, a total of 11 (7.6%) patients dropped out of the waitlist: 9 (6.2%) patients who had only signed the informed consent for DBD livers versus 2 (1.4%) patients who had signed the informed consent for both DBD and uDCD livers (p = 0.366). The dropout causes for patients in the DBD group were death in 6 patients, and tumor progression in 3; whereas in the DBD and uDCD group the causes were death in one, and tumor progression in the other patient.
Mean age was significantly higher in uDCD recipients than in DBD recipients (57.0 ± 8.1 y vs. 61.0 ± 6.6 y; p = 0.007). The prevalence of other OLT indications associated with HCC was similar in both groups, as was the prevalence of other morbidities. The median MELD score was significantly higher in recipients of uDCD donors (p = 0.008). Among laboratory variables, recipients of uDCD livers had significantly lower platelet counts and prothrombin rates (Table 1).
Perioperative characteristics and post-OLT complications
Types of biliary reconstruction techniques were similarly distributed in the two groups, whereas the volume of all intraoperative blood products was significantly higher in uDCD recipients. Liver function parameters were similar between groups on the 7th post-OLT day, but median gamma-glutamyl transpeptidase (GGT) and bilirubin were significantly lower in uDCD recipients on the 30th post-OLT day. Immunosuppressor use, hospital stay, or ICU stay did not differ significantly between groups.
Regarding post-OLT complications, the rate of PNF was significantly higher in uDCD recipients (4 cases; 9.7%) than in DBD recipients (1 case; 1.0%) (p = 0.023). Hepatic artery thrombosis occurred in 2 (1.9%) recipients in the DBD group and 3 (7.3%) patients in the uDCD group (p = 0.150). Fewer patients (9; 8.7%) developed BCs in the DBD group than in the uDCD group (10; 24.4%), but this finding did not reach statistical significance (p = 0.071). Rates of acute and chronic rejection were similar in both groups.
The rate of retransplantation was significantly higher in recipients of uDCD livers (5 patients; 12.2%) than in recipients of DBD livers (1 patient; 1.0%; p = 0.01). Reasons for retransplantation were PNF and NABS (4 patients and 1 patient, respectively) in the uDCD group. For the 1 patient who underwent retransplantation in the DBD group, PNF was the indication (Table 2).
Pre-OLT and post-OLT tumor characteristics and post-OLT recurrence
Pre-OLT and post-OLT HCC characteristics (histological examination of the explant liver), as well as post-OLT HCC recurrence data, are depicted in Table 3. There were no statistically significant differences in mean tumor size or mean tumor number between recipients of DBD and uDCD livers. Alpha-fetoprotein (AFP) levels at the time of placement on the waitlist were not significantly different between the two groups. Similarly, we detected no significant differences in the percentage of patients in both groups within Milan criteria (86% in DBD vs. 95.1% in uDCD; p = 0.200) or UCSF criteria at the time of waitlist inclusion (16.6% in DBD vs. 4.9% in uDCD; p = 0.351). The time from HCC diagnosis to OLT was significantly longer in DBD recipients than in uDCD recipients (19.1 ± 7.1 mo vs. 12.3 ± 7.1 mo; p = 0.038). The time from OLT waitlist inclusion to OLT was also significantly longer in the DBD group than in the uDCD group (7.9 ± 5.4 mo vs. 5.3 ± 3.0 mo; p = 0.040). Similar proportions of patients in both groups (48.5% in DBD and 48.7% in uDCD; p = 0.413) received pre-OLT HCC bridging therapies; rates for pre-OLT TACE or radiofrequency ablation did not differ between groups. After histological examination of the explant liver, we observed no statistically significant differences between groups for median tumor size, number of tumors, and vascular or perineural invasion. The proportion of patients within Milan criteria decreased from pre-OLT to post-OLT (based on liver explant histological findings), although there were no differences between groups either pre- or post-OLT. There were corresponding increases in the percentage of patients within UCSF from pre-OLT to post-OLT, with no significant between-group differences at either time. The proportion of patients exceeding UCSF criteria after OLT were similar in the DBD group (19 patients; 18.4%) and uDCD group (7 patients; 17.1%; p = 0.528).
After a follow-up period of at least 22 months for all patients (mean, 52 ± 35 mo in the DBD group vs. 56 ± 44 mo in the uDCD group; p = 0.550), the rate of tumor recurrence was similar in both groups (7.8% in the DBD group vs. 7.3% in the uDCD group; p = 0.597). The median time from OLT to tumor recurrence diagnosis was longer in the DBD group than in the uDCD group, although the difference did not reach statistical significance (33 mo vs. 12 mo; p = 0.091). Locations of tumor recurrence were the liver, bones, or lungs, which were not significantly different between groups. Four (3.8%) patients died of tumor recurrence in the DBD group, whereas 1 (2.4%) patient died of recurrence in the uDCD group (p = 0.545).
Patient and graft survival and predictive factors
Patient and graft survival were lower in the uDCD group, but only graft survival reached a statistically significant difference. Overall, 1-, 3-, and 5-year patient survival rates were 85%, 78%, and 72%, respectively, in DBD recipients and 72%, 65%, and 61%, respectively, in uDCD recipients (p = 0.249). The 1-, 3-, and 5-year graft survival rates were 84%, 77%, and 71%, respectively, in DBD recipients and 65%, 58%, and 58%, respectively, in uDCD recipients (p = 0.021) (Figs. 1A,B). However, when we excluded PNF cases, graft survival did not differ between groups, with 1-, 3-, and 5-year graft survival rates of 82%, 79%, and 70%, respectively, in DBD recipients and 70%, 62%, and 61%, respectively, in uDCD recipients (p = 0.126).
(A) Patient survival: 1-, 3- and 5-year were 85%, 78% and 72%, respectively, in DBD recipients and 72%, 65% and 61%, respectively, in uDCD recipients (p = 0.249). (B) Graft survival: 1-, 3- and 5-year were 84%, 77%, and 71%, respectively, in DBD recipients and 65%, 58% and 58%, respectively, in uDCD recipients (p = 0.021). (C) Recurrence-free tumor survival: 1-, 3- and 5-year were 98%, 95% and 82%, respectively, in DBD recipients, and 91%, 79% and 79%, respectively, in uDCD recipients (p = 0.754).
Regarding recurrence-free survival, there were no statistically significant differences between groups. The 1-, 3-, and 5-year recurrence-free survival rates were 98%, 95%, and 82%, respectively, in DBD recipients and 91%, 79%, and 79%, respectively, in uDCD recipients (p = 0.754) (Fig. 1C).
On univariate analysis, recipient age, bridging therapies, and HCC recurrence were significantly associated with patient survival. On multivariate analysis, recipient age (HR, 1.06; 95% CI, 1.01–1.12; p = 0.031), use of bridging therapies (HR, 2.50; 95% CI, 1.12–5.55; p = 0.024), and HCC recurrence (HR, 2.58; 95% CI, 1.01–6.67; p = 0.048) remained significant predictors of patient survival. Other variables, including MELD score, hepatitis C virus (HCV) cirrhosis, alcoholic cirrhosis, AFP, downstaging therapy, and type of liver graft (uDCD/DBD), did not influence patient survival (Table 4).
On univariate analysis for predictors of HCC recurrence after transplantation, only use of TACE as therapy before OLT was significantly associated with recurrence (OR, 4.22; 95% CI, 1.15–20.06; p = 0.041). On multivariate Cox logistic regression analysis, TACE before OLT persisted as a significant risk factor for post-OLT tumor recurrence (OR, 3.93; 95% CI, 1.05–18.96; p = 0.045). Other variables did not influence HCC recurrence (Table 5).
Discussion
Regarding the indications of OLT for HCC our OLT team criteria coincides with the Consensus Statement and Recommendations of the Spanish Society for Liver Transplantation. Thus, we are in complete agreement with this Society that accepts OLT for patients beyond Milan but within “up-to-seven” UCSF criteria with alpha-fetoprotein < 400 ng/ml and radiological response to locoregional therapy24. Prior reports using DBD livers identified several predictive factors for post-OLT HCC recurrence, such as advanced donor age25,26, diabetes mellitus, severe donor steatosis18, WIT > 50 min17,27, CIT > 10 h27, increased tumor size and number20,21,25, vascular invasion27,28, poor tumor differentiation27,29, elevated pre-OLT AFP, exceeding Milan criteria27, grafts from a non-local share distribution21, and unfavorable tumor biology on pre-OLT imaging17.
The use of selected cDCD donors offers excellent long-term graft and patient survival that are comparable to those observed with DBD donors, although ischemic cholangiopathy and subsequent BCs constitute specific morbidities associated with cDCD livers30,31,32,33. A recent multicenter study suggests that use of postmortem NRP in cDCD donors may reduce rates of post-OLT BCs and graft loss34. Several other OLT teams have reported using livers from cDCD donation in patients with HCC16,32,35,36, with the current tendency to use livers from cDCD donation rather than DBD livers in recipients with HCC32,35 and low MELD scores. The general impression is that these marginal grafts are better tolerated by recipients with better physical condition and liver function37,38. Thus, patients with HCC and low MELD scores are probably ideal candidates for cDCD livers36,38.
Preliminary studies from the Scientific Registry of Transplant Recipients demonstrated that use of cDCD livers increased the risk of death in OLT recipients with HCC35 and was associated with inferior patient and graft survival16. More recently, other OLT series comparing outcomes between cDCD and DBD livers in patients with HCC showed no differences in HCC recurrence5,26,36, patient survival, or tumor-free survival5,36. Furthermore, Khorsandi et al.36 found no difference in survival between cDCD and DBD transplant recipients but noted that increased HCC size and number, microvascular invasion, and poor tumor differentiation were associated with increased cancer-specific mortality, thereby highlighting the importance of tumor biology on survival and recurrence post-OLT in patients with HCC.
As with cDCD livers, uDCD livers have often been used in patients with HCC7,8,9,10,11,39, reaching up to 85–90% of patients in two recent series9,10 (one of which involved 46% of patients exceeding the Milan criteria)9. These series also underline the importance of using uDCD livers in recipients with HCC who meet recommended indications and avoiding their use when post-OLT graft function is unpredictable9. In agreement with previous authors9, our results confirmed that use of uDCD livers confers the advantage of reducing waitlist time and the dropout rate, compared with DBD livers. Similar to donor data of previously reported studies7,8,9,10, the mean age of our uDCD donors was significantly lower than DBD donors, yet the mean age of our uDCD recipients was significantly higher.
As in other cDCD donation40,41 and uDCD donation experiences8,9,10,11,39,42 the main disadvantages of uDCD livers in our series, when compared with DBD livers, were the increased need for blood products and higher rates of PNF, ischemic BCs, and retransplantation. PNF is a severe complication of OLT with a high mortality rate, given that early retransplantation in the only treatment available. Its occurrence has been related to a series of risk factors, including the use of grafts from extended criteria donors, hemodynamically unstable recipients, or those that require transfusion of large volumes of blood products. Regarding the use of uDCD livers, an increase in PNF has been linked to the longer period of ischemia. The incidence of PNF is 1.1–7.2% for DBD OLT, 1.8–11.8% when using cDCD livers, and 5–25% for uDCD OLT22. However, on the 30th post-OLT day, liver function of our uDCD recipients was equivalent to that of DBD recipients, reflecting the good recovery of uDCD livers.
Overall, our results support the use of cDCD6,43 and uDCD livers9,10,11 to mitigate liver shortages, and mortality, especially in HCC patients. Because of better uDCD donor and recipient selection and management, our results have improved over the past 4 years, producing lower rates of PNF and BCs and better survival11,44.
Regarding pre-OLT HCC characteristics, the only significant difference between our groups was the shorter interval between HCC diagnosis and OLT in uDCD recipients. Tumor size, tumor number, AFP level, and percentage of patients within Milan or UCSF criteria were not significantly different between types of OLT. Similarly, there was no significant difference in number of patients who received pre-OLT bridging therapies. Despite use of bridging therapies in patients on the waitlist, tumor progression continued during this period (proportion of patients exceeding Milan criteria increased from 59.2% to 83.4% in the DBD group vs. 65.8–95.1% in the uDCD group). However, the overall HCC recurrence rate after OLT was similar in both groups (7.8% in DBD vs. 7.3% in uDCD), with parallel distributions of metastasis locations. Other authors have reported similar HCC recurrence rates between DBD and cDCD liver recipients (12.1% vs. 12.3%)5.
Previous studies reported no differences in 1-year patient survival between recipients of uDCD and DBD livers but lower 1-year graft survival in uDCD liver recipients8,9,11,42. Our study confirmed similar overall patient survival, disease-free survival, and tumor recurrence rates at 5 years between recipients receiving DBD and uDCD livers but lower 5-year graft survival in the uDCD group, which was mainly attributed to a higher incidence of ischemic BCs and PNF. The increased risk of these complications with cDCD32,34 or uDCD livers7,9,10,39,44 is well known, and when we excluded patients with PNF (the number of which has decreased substantially during the past 4 years) from our analysis, graft survival was not significantly different between groups.
Our multivariate analysis revealed recipient age, use of bridging therapies, and HCC recurrence as independent risk factors for patient survival, whereas pre-OLT bridging therapy with TACE was the only risk factor for HCC recurrence. The authors of a recent study found no association between locoregional therapy and worse patient survival, and they considered tumor biology (size, number, differentiation, and microvascular invasion) to be more relevant for patient survival36. Nevertheless, the need for bridging therapies may reflect unfavorable HCC biology and, therefore, a higher risk of recurrence and lower survival. Thus, bridging locoregional therapies for patients within Milan criteria do not improve post-OLT survival or recurrences in the majority of patients who fail to achieve complete pathologic response45. It has been suggested that delaying OLT for 3 months after inclusion on the waitlist can reduce early HCC recurrence by excluding patients with poor tumor biology46.
There are some limitations to this study. Data were collected retrospectively and were therefore subject to the typical biases expected with this study design. Although this study represents the largest single-center experience using uDCD livers in recipients with HCC, the sample size was limited for analyzing risk factors by multivariate analysis.
Conclusion
In conclusion, when comparing uDCD and DBD livers in HCC recipients, similar patient survival but lower graft survival was observed in the uDCD group, which was attributed to the group’s higher incidence of PNF and BCs. Furthermore, HCC recurrence rates after OLT were similar with uDCD or DBD liver grafts, and TACE as a bridging therapy was identified as a risk factor for post-OLT recurrence. Other important advantages of using livers from uDCD in patients with HCC are the potential reduction in waiting list time, and consequently the decrease in the dropout rate of patients due to tumor progression or death.
Data availability
The dataset generated and analyzed for this study are available from the corresponding author on reasonable request.
Abbreviations
- ABS:
-
Anastomotic biliary stricture
- AFP:
-
Alpha-fetoprotein
- BC:
-
Biliary complication
- BMI:
-
Body mass index
- cDCD:
-
Controlled donor after circulatory death
- CI:
-
Confidence interval
- CIT:
-
Cold ischemia time
- CPR:
-
Cardio-pulmonary resuscitation
- DBD:
-
Donation after brain death
- DCD:
-
Donor after circulatory death
- DWIT:
-
Donor warm ischemia time
- FFP:
-
Fresh frozen plasma
- GGT:
-
Gamma-glutamyl transpeptidase
- GOT:
-
Glutamic oxaloacetic transaminase
- GPT:
-
Glutamic pyruvic transaminase
- HR:
-
Hazard ratio
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- ICU:
-
Intensive care unit
- INR:
-
International normalized ratio
- IRI:
-
Ischemia/reperfusion injury
- MELD:
-
Model for end-stage liver disease
- MMF:
-
Mycophenolate mofetil
- mTORi:
-
Mammalian target of rapamycin inhibitors
- NABS:
-
Non-anastomotic biliary stricture
- NRP:
-
Normothermic regional perfusion
- OLT:
-
Orthotopic liver transplantation
- OR:
-
Odds ratio
- PNF:
-
Primary nonfunction
- PRBC:
-
Packed red blood cells
- RWIT:
-
Recipient warm ischemia time
- TACE:
-
Transarterial chemoembolization
- uDCD:
-
Uncontrolled donor after circulatory death
- UCSF:
-
University California, San Francisco
- WIT:
-
Warm ischemia time
References
Ferlay, J. et al. Estimates of worldwide burden of cancer in 2008: Globocan 2008. Int. J. Cancer. 127, 2893–2917 (2010).
Bruix, J. & Sheerman, M. Management of hepatocellular carcinoma: an update. Hepatology 53, 1020–1022 (2011).
Spanish National Transplant Organization (ONT). Dossier de Actividad en Trasplante Hepático (Dossier on Liver Transplantation Activity). 2019. Available at http://www.ont.es. Accessed (2020).
Busuttil, R. W. & Tanaka, K. The utility of marginal donors in liver transplantation. Liver Transpl. 9, 651–663 (2003).
Croome, K. P. et al. The use of donation after cardiac death allografts does not increase recurrence of hepatocellular carcinoma. Am. J. Transplant. 15, 2704–2711 (2015).
Goldberg, D. S. et al. Interpreting outcomes in DCDD liver transplantation: First report of the multicenter IDOL consortium. Transplantation 101, 1067–1073 (2017).
Otero, A. et al. Liver transplantation from Maastricht category 2 non-heart-beating donors. Transplantation 76, 1068–1073 (2003).
Fondevila, C. et al. Liver transplant using donors after unexpected cardiac death: novel preservation protocol and acceptance criteria. Am. J. Transplant. 7, 1849–1855 (2007).
Savier, E. et al. First experience of liver transplantation with type 2 donation after cardiac death in France. Liver Transpl. 21, 631–643 (2015).
De Carlis, R. et al. Liver grafts from donors after circulatory death on regional perfusion with extended warm ischemia compared with donors after brain death. Liver Transpl. 24, 1523–1535 (2020).
Jiménez-Romero, C. et al. Liver transplantation using uncontrolled donors after circulatory death: a 10-year single center experience. Transplantation 103, 2497–2505 (2019).
Jiménez-Romero, C. et al. Octogenarian liver grafts: Is their use for transplant currently justified?. World J. Gastroenterol. 23, 3099–3110 (2017).
Houben, P. et al. Differential influence of donor age depending on the indication for liver transplantation—a collaborative transplant study report. Transplantation 104, 779–787 (2020).
Lozanovski, V. J. et al. Liver grafts with major extended donor criteria may expand the organ pool for patients with hepatocellular carcinoma. J. Clin. Med. 8, 1692 (2019).
Kootstra, G. G., Daemen, J. H. & Oomen, A. P. Categories of non-heart-beating donors. Transplant. Proc. 27, 2893–2894 (1995).
Croome, H. P. et al. Inferior survival in liver transplant recipients with hepatocellular carcinoma receiving donation after cardiac death liver allografts. Liver. Transpl. 19, 1214–1223 (2013).
Kornberg, A. et al. Extended ischemia times promote risk of HCC recurrence in liver transplant patients. Dig. Dis. Sci. 60, 2832–2839 (2015).
Orci, L. A. et al. Donor characteristics and risk hepatocellular carcinoma recurrence after liver transplantation. Br. J. Surg. 102, 1250–1257 (2015).
Van der Bilt, J. D. et al. Ischemia/reperfusion accelerates the outgrowth of hepatic micrometastases in a highly standardized murine model. Hepatology 42, 165–175 (2005).
Mazzaferro, V. et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 334, 693–699 (1996).
Yao, F. Y. et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 33, 1394–1403 (2001).
Justo, I. et al. Incidence and risk factors of primary non-function after liver transplantation using grafts from uncontrolled donors after circulatory death. Clin. Transplant. 35, e14134 (2021).
Buis, C. I. et al. Nonanastomotic biliary strictures after liver transplantation, Part 1: radiological features and risk factors for early vs. late presentation. Liver. Transpl. 13, 708–718 (2007).
Rodríguez-Perálvarez, M. et al. Expanding indications of liver transplantation in Spain: consensus statement and recommendations by the spanish society of liver transplantation. Transplantation 105, 602–607 (2021).
Sharma, S., Gurakar, A. & Jabbour, N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver. Transpl. 14, 759–769 (2008).
Vagefi, P. A., Dodge, J. L., Yao, F. Y. & Roberts, J. Potential role of the donor in hepatocellular carcinoma recurrence after liver transplantation. Liver. Transpl. 21, 187–914 (2015).
Nagai, S. et al. Ischemia time impacts recurrence of hepatocellular carcinoma after liver transplantation. Hepatology 61, 895–904 (2015).
Lim, K. C. et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann. Surg. 254, 108–113 (2011).
DuBay, D. et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann. Surg. 253, 166–172 (2011).
De Oliveira, M. L. et al. Biliary complications after liver transplantation using grafts from donors after cardiac death: results from matched control study in a single large volume center. Ann. Surg. 254, 716–722 (2011).
Dubbeld, J. et al. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br. J. Surg. 97, 744–753 (2010).
Grewal, H. P. et al. Liver transplantation using controlled donation after cardiac death donors: an analysis of a large single-center experience. Liver. Transpl. 15, 1028–1035 (2009).
Doyle, M. B. M. et al. Outcomes using grafts from donors after cardiac death. J. Am. Coll. Surg. 221, 142–152 (2015).
Hessheimer, A. et al. Normothermic regional perfusion versus super rapid recovery in controlled donation after circulatory death liver transplantation. J. Hepatol. 70, 658–665 (2019).
Jay, C. et al. A comprehensive risk assessment of mortality following donation after cardiac death liver transplant—an analysis of the national registry. J. Hepatol. 55, 808–813 (2011).
Khorsandi, S. E. et al. Does donation after cardiac death utilization adversely affect hepatocellular cancer survival?. Transplantation 100, 1916–1924 (2016).
Schaubel, D. E., Sima, C. S., Goodrich, N. P., Feng, S. & Merion, R. M. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am. J. Transplant. 8, 419–425 (2008).
Croome, K. P., Lee, D. D., Keaveny, A. P. & Taner, C. B. Improving national results in liver transplantation using grafts from donation after cardiac death donors. Transplantation 100, 2640–2647 (2016).
Fondevila, C. et al. Applicability and results of Maastricht type 2 donation after cardiac death liver transplantation. Am. J. Transplant. 12, 162–170 (2012).
Foley, D. P. et al. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann. Surg. 253, 817–825 (2011).
Axelrod, D. A. et al. National assessment of early biliary complications following liver transplantation: incidence and outcomes. Liver. Transpl. 20, 446–456 (2014).
Suarez, F. et al. Biliary complications after liver transplantation from Maastricht category-2 non-heart-beating donors. Transplantation 85, 9–14 (2008).
Morrisey, P. E. & Monaco, A. P. Donation after circulatory death: Current practices, ongoing challenges, and potential improvement. Transplantation 97, 258–264 (2014).
Jimenez-Romero, C. et al. Biliary complications after liver transplantation from uncontrolled donors after circulatory death: incidence, management, and outcome. Liver. Transpl. 26, 80–91 (2020).
Agopian, V. G. et al. Impact of pretransplant bridging locoregional therapy for patients with hepatocellular carcinoma within Milan criteria undergoing liver transplantation: analysis of 3601 patients from the US multicenter HCC transplant consortium. Ann. Surg. 266, 525–535 (2017).
Schlansky, B., Chen, Y., Scott, D. L., Austin, D. & Naugler, W. E. Waiting times predicts survival after liver transplantation for hepatocellular carcinoma: a cohort study using the United Network for Organ Sharing registry. Liver. Transpl. 20, 1045–1056 (2014).
Author information
Authors and Affiliations
Contributions
A.N. Study design, data collection, analysis and interpretation of the data, manuscript writing, and revision of the manuscript. I.J. Study design, analysis and interpretation of the data, manuscript writing, and revision of the manuscript. A.M. Data collection, interpretation of the data, critically review of the manuscript, and final approval of the manuscript. Ó.C. Analysis and interpretation of data, critically review of the manuscript, and final approval of the manuscript. A.M. Analysis and interpretation of data, critically review of the manuscript, and final approval of the manuscript. J.C. Data collection, interpretation of the data, critically review of the manuscript, and final approval of the manuscript. ÁG.-S. Data collection, interpretation of data, critically review of the manuscript, and final approval of the manuscript. M.S.G. Analysis and interpretation of the data, critically review of the manuscript, and final approval of the manuscript. M.G.-C. Data collection and final approval of the manuscript. C.J.-R. Study design, data collection, analysis and interpretation of data, manuscript writing, and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nutu, A., Justo, I., Marcacuzco, A. et al. Liver transplantation for hepatocellular carcinoma using grafts from uncontrolled circulatory death donation. Sci Rep 11, 13520 (2021). https://doi.org/10.1038/s41598-021-92976-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-92976-5