Abstract
The responsiveness of patients with chronic kidney disease (CKD) to nephrologists’ care is unpredictable. We defined the longitudinal stages (LSs) 1–5 of estimated glomerular filtration rate (eGFR) by group-based trajectory modeling for repeated eGFR measurements of 7135 patients with CKD aged 20–90 years from a 13-year pre-end-stage renal disease (ESRD) care registry. Patients were considered nonresponsive to the pre-dialysis care if they had a more advanced eGFR LS compared with the baseline. Conversely, those with improved or stable eGFR LS were considered responsive. The proportion of patients with CKD stage progression increased with the increase in the baseline CKD stage (stages 1–2: 29.2%; stage 4: 45.8%). The adjusted times to ESRD and all-cause mortality in patients with eGFR LS-5 were 92% (95% confidence interval [CI] 86–96%) and 57% (95% CI 48–65%) shorter, respectively, than in patients with eGFR LS-3A. Among patients with baseline CKD stages 3 and 4, the adjusted times to ESRD and all-cause death in the nonresponsive patients were 39% (95% CI 33–44%) and 20% (95% CI 14–26%) shorter, respectively, than in the responsive patients. Our proposed Renal Care Responsiveness Prediction (RCRP) model performed significantly better than the conventional Kidney Failure Risk Equation in discrimination, calibration, and net benefit according to decision curve analysis. Non-responsiveness to nephrologists’ care is associated with rapid progression to ESRD and all-cause mortality. The RCRP model improves early identification of responsiveness based on variables collected during enrollment in a pre-ESRD program. Urgent attention should be given to characterize the underlying heterogeneous responsiveness to pre-dialysis care.
Similar content being viewed by others
Introduction
Delaying the progression of chronic kidney disease (CKD) to end-stage renal disease (ESRD), which requires renal replacement therapy, is a theoretically possible strategy for improving patient outcomes and radically reducing healthcare costs, particularly in countries with endemic ESRD. Identifying patients with CKD who respond and do not respond to nephrologists’ care is crucial but challenging, even in a managed care model, such as the multidisciplinary pre-dialysis care, first advocated in 1994 by the US National Institutes of Health1,2,3. Indeed, recent studies have shown inconsistent results regarding the potential of intense pre-dialysis care in slowing or arresting CKD progression4,5,6,7,8,9,10,11. For example, a recent study showed that among older veterans, the lower intensity of pre-dialysis management in the Veterans Affairs health care system than in Medicare was not associated with higher mortality11. Two studies that used a propensity score matched case–control design revealed a high dialysis rate among patients with pre-dialysis care during the progression of CKD to ESRD; however, neither study considered the potential competing risk of death5,6. By contrast, most studies based on the national pay-for-performance pre-dialysis care program showed overall improvements in mortality, dialysis rate, mature vascular access, and general cost saving8,9,10.
Prior studies have examined the longitudinal trajectories of estimated glomerular filtration rate (eGFR) in multiple CKD populations. Li et al. identified four main eGFR progression features based on an African American Study of Kidney Disease and Hypertension consisting of 846 patients. An important feature captured was the “fast-decline” trajectory, with a decline rate of at least 4 mL/min/1.73 m2/y. Similarly, the “fast-decline” characteristic of longitudinal eGFR trajectory is consistently recognized in diverse populations with CKD or diabetes12,13,14,15. However, whether this “fast-decline” trajectory can be mitigated through nephrologist-driven pre-dialysis care remains undetermined. In the present study, by using a national registry-based pre-ESRD cohort with a standardized care protocol, we defined the longitudinal CKD stage through modeling trajectories of all eGFR measurements following enrollment in the pre-dialysis program and defined the individual’s response to pre-dialysis care based on the discrepancy between baseline CKD stage at enrollment and the longitudinal CKD stage. We aimed to develope a prediction model to help early identification of non-responsiveness to pre-dialysis care.
Methods
Study population
Taiwan’s National Health Insurance launched the Project of Integrated Care of CKD in 2002, initially targeting patients with an eGFR of < 60 mL/min/1.73 m2 or proteinuria (urine protein-to-creatinine ratio [uPCR] > 1 g/g creatinine). In 2007, the program used a multidisciplinary approach to focus on CKD stages 3b–54,16. China Medical University Hospital (CMUH), a tertiary medical center in Central Taiwan, joined the program in 2003 and prospectively enrolled consecutive patients with CKD who were willing to participate17. Details of the program were described in a previous study18. Biochemical markers of renal injury, including serum creatinine (S-Cre), blood urea nitrogen, and spot uPCR, were measured at least once every 3 months. We further integrated the CMUH pre-ESRD program with the CMUH electronic medical records (EMRs) containing laboratory tests, medications, special procedures, and admission records19. All enrolled patients were followed-up until initiation of long-term renal replacement therapy (hemodialysis, peritoneal dialysis, or transplantation), loss to follow-up, death, or December 31, 2016, whichever occurred first.
In the present study, we included participants of the pre-ESRD program from Jan 2003 to Dec 2015 who were aged 20–89 years, had no history of dialysis, and had at least 2 measurements of serum S-Cre during the follow-up period. Then, we excluded participants with a time between baseline and final S-Cre measurements of < 6 months. In total, 7135 participants with 156,295 records of S-Cre were included in the analysis (Figure S1). The study was approved by the Big Data Center of CMUH and the Research Ethical Committee/Institutional Review Board (REC/IRB) of China Medical University and informed consent was waived (CMUH105-REC3-068). All methods were performed in accordance with the relevant guidelines and regulations of REC/IRB.
Determination of kidney function
S-Cre levels were measured using the Jaffe rate method (kinetic alkaline picrate) at CMUH Central Laboratory by using a Beckman UniCel DxC 800 immunoassay system (Beckman Coulter Inc., Brea, CA, USA). The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation20. The S-Cre level at enrollment was used to define the baseline eGFR and the corresponding CKD stages according to the following cutoff values: > 90 [stage 1], 60–89.9 [stage 2], 45–59.9 [stage 3a], 30–44.9 [stage 3b], 15–29.9 [stage 4], and < 15 [stage 5] mL/min/1.73 m2. All S-Cre measurements of the enrolled participants were considered until the study endpoints. The quarterly average eGFR level was calculated if the patient had received more than one eGFR measurement in a 3-month period, and the individual’s eGFR trajectory was modeled based on quarterly average eGFR measures. For patient with only urine albumin-to-creatinine ratio (uACR) available, we converted uACR into uPCR based on the following equation derived from a Japanese study: \(\ln \left( {{\text{ACR}}} \right) = 1.32*\ln \left( {{\text{PCR}}} \right) - 2.64\)21. Proteinuria was defined as uPCR > 0.5 g/g creatinine. Details of other co-variables are summarized in the Supplementary Material.
Longitudinal stage of eGFR and Responsiveness to pre-dialysis care
Longitudinal stage of eGFR (eGFR LS) was determined based on the eGFR trajectories derived from group-based trajectory modeling (GBTM), which is described detail in the section of statistical analyses. Patients were considered nonresponsive to the pre-ESRD program if they reached a more advanced longitudinal stage of eGFR than the baseline CKD stage. Conversely, those with improved or stable CKD stages were defined as responsive patients.
Statistical analyses
Continuous variables were compared using the Wilcoxon rank sum test and expressed as median and interquartile range (IQR), whereas categorical variables were compared using the chi-square test and expressed as frequency (percentage). We used semiparametric GBTM to characterize the follow-up period trajectories of all eGFR measurements of the patients enrolled in the CMUH pre-ESRD program. Briefly, the PROC TRAJ macro developed using the SAS software was used to fit a semiparametric mixture model to the longitudinal data by using the maximum likelihood method22,23,24. This approach is useful when the number of subgroups and other information, such as the trajectory shapes of each subgroup, are unknown. We empirically compared 3-, 4-, 5-, and 6-group solutions and then optimized the number of subgroups by using Bayesian information criterion values (close to zero indicated a good fit); the trajectory shapes were determined according to the order of the polynomial (e.g., linear, quadratic, and cubic). The eGFR trajectories were determined before analysis of the risk of dialysis and mortality.
To construct time-to-event analysis, person-years free of dialysis and mortality after the pre-ESRD enrollment were computed along with Kaplan–Meier survival functions. We evaluated the prospective associations of longitudinal eGFR trajectories and responsiveness to the pre-dialysis care with the risk of dialysis initiation and mortality by using multiple Cox proportional hazards models. The models were adjusted progressively (see footnotes of Table 3). To characterize the dialysis risk associated with exposures of interest, we performed a competing risk analysis according to the protocol provided by Fine and Gray, which minimized the potential bias introduced by a competing death risk25. Furthermore, we performed a parametric survival analysis under the Weibull distributions and applied the same adjustment strategy as in the Cox modeling. The relative survival time was estimated by exponentiating the coefficient of the main predictors (eGFR trajectories or responsiveness to pre-dialysis care). The relative time can be interpreted as the difference between the time required for individuals in the exposed population and nonexposed population to experience the events of interest. The model fitting was evaluated by using the Akaike information criterion. Due to missing data on some explanatory variables (e.g., urinary protein-to-creatinine ratio (uPCR) up to 28%; Table S1), we further performed multiple imputations with a fully conditional method in SAS, namely an iterative Markov chain Monte Carlo procedure, to replace the missing values for uPCR, comorbidities, and medications with imputed values. We specified the number of imputations as 20 and the number of iterations as 100. Exploratory subgroup analyses were performed to evaluate potential effect modification among patients with different longitudinal eGFR trajectories. To explore potential effect modifier in the association between responsiveness and main outcomes, we stratified patients on the basis of age older or younger than 65 years, sex, BMI category (< 24, 24–47, > 27 kg/m2), smoking status, CKD stage (3 vs. 4 or 5), uPCR higher or lower than 500 mg/g creatinine, serum uric acid (SUA) higher or lower than 7 mg/dL, diabetes, hypertension, and CVD at baseline. To evaluate the responsiveness prediction model, we compared the C-statistic between the reference Kidney Failure Risk Equation composed of age, sex, eGFR, uPCR, serum calcium, phosphorus, and albumin and our proposed model, Renal Care Responsiveness Prediction (RCRP), which additionally used DM, HTN, hemoglobin, and the history of NSAID exposure. We also plotted the observed versus predicted risk probability to reveal the differences in calibrations of all the responsiveness-prediction models. A decision curve analysis was then conducted to determine the clinical usefulness of the proposed prediction models quantifying the net benefit at different threshold probabilities26,27. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). The two-tailed statistical significance level of α was set at 0.05.
Ethical approval
The study was approved by the Research Ethical Committee/Institutional Review Board of China Medical University Hospital (CMUH105-REC3-068).
Results
Clinical characteristics across prospective eGFR longitudinal stage
The median (IQR) age at enrollment of all 7135 participants was 67.5 years (IQR 57.1–76.3); median follow-up duration was 2.40 and 4.25 years for ESRD events and death, respectively; and median (IQR) frequency of eGFR measurements was 16 times (IQR 9–30). We identified 6 eGFR trajectories by using GBTM that correspond well to the eGFR range of the current CKD stages of 1, 2, 3a, 3b, 4, and 5. (Fig. 1). We then considered eGFR LS-1 and LS-2 as the reference group. Compared with the reference group, those in the longitudinal eGFR LS-3B and LS-4 were considerable older and with a higher prevalence of CVD at baseline (Table 1). A decreasing trend of baseline BMI for increasing eGFR LSs was observed, which was opposite to the trend of the baseline prevalence of diabetes and hypertension. At baseline, nonsteroidal anti-inflammatory drug (NSAID) exposure was relatively prevalent, ranging from 21.9 for eGFR LS-5 to 28.6% for eGFR LS-3A. For exposure to radiocontrast, it ranged from 6.2 for eGFR LS-1-2 to 11.5% for eGFR LS-4 (Table 1).
Clinical characteristics by the responsiveness to pre-ESRD program
Only 3.8% of patients showed improvement in eGFR LS over the follow-up in patients with baseline CKD stage 5 (Fig. 2). Furthermore, the concordance matrix demonstrated a gradual increase in the proportion of CKD stage progression (LS more advanced than baseline CKD stage) with the increase in the baseline eGFR CKD stage (29.2% for stages 1–2; 36.2% for stage 3A; 37.3% for stage 3B; 45.8% for stage 4; Fig. 2). We then divided the study population based on the concordance between baseline CKD stage and eGFR LS into 3 groups: (1) Stage-Stable (4326, 60.8%); (2) Stage-Regression (617, 8.7%); and (3) Stage-Progression (2167, 30.5%). Patients in the Stage-Regression and Stage-Progression groups had comparable baseline eGFR (36.7 vs. 33.5 mL/min/1.73 m2, P = 0.76; Table 2). However, patients in the Stage-Progression group had significantly higher baseline levels of serum blood urea nitrogen, phosphorus, total cholesterol, and uPCR along with significantly lower baseline levels of serum albumin, hemoglobin, and urine creatinine compared with those in the other groups (Table 2). Also, responsive patients were more likely to have been exposed to NSAIDs and have better baseline levels of S-Cre, blood urea nitrogen, SUA, phosphorus, and uPCR than nonresponsive patients (Table S2). The eGFR slopes before enrollment to pre-dialysis care were comparable between responsive and nonresponsive patients (− 6.97 vs. − 7.89 mL/min/1.73 m2/y). The eGFR slope was then flattened to 0.21 mL/min/1.73 m2/y among responsive patients, whereas the annual eGFR slope kept declining at − 5.34 mL/min/1.73 m2/y in nonresponsive patients (Fig. 3). The dramatic slowness of declining eGFR slope was consistently observed for patients with baseline CKD stage 3b-5 after enrollment to pre-ESRD program (Figure S2).
The eGFR slope (red line), with the light red shaded area representing the 95% confidence interval, before and after enrollment of the entire study population into the pre-ESRD program. The eGFR slope was modeled using the growth piecewise linear mixed model through the incorporation of random effects. Blue and orange points represent eGFR measurements before and after enrollment into pre-ESRD program, respectively. The estimated value of eGFR slope (mL/min/1.73 m2/y) is shown.
Clinical prognosis of eGFR LS and the responsiveness to pre-ESRD program
Over the 21,662.36 and 33,472.81 person-years of follow-up, 1451 ESRD events and 2031 deaths occurred, respectively. ESRD and all-cause mortality incidence were 66.9 and 60.7 per 1000 person-years, respectively. Compared with patients with eGFR LS-3A, fully adjusted hazard ratios (HRs) for dialysis and all-cause mortality among patients with eGFR LS-5 were 70.0 (95% confidence interval [CI] 27.8–176.7) and 3.69 (95% CI 2.71–5.04), respectively. On the basis of parametric survival models, the adjusted times to the outcomes of ESRD and all-cause mortality in patients with eGFR LS-5 were 92% (95% CI 86–96%) and 57% (95% CI 48–65%) shorter, respectively, than those with eGFR LS-3A (Table 3). The adjusted HRs for ESRD and all-cause mortality of nonresponsive patients were 2.17 (95% CI 1.9–2.5) and 1.42 (95% CI 1.27–1.59), respectively, compared with those who responded to pre-dialysis care. From the parametric perspective, the adjusted time to ESRD and all-cause death in nonresponsive patients was 39% (95% CI 33–44%) and 20% (95% CI 14–26%) shorter, respectively, than that in responsive patients (Table 3). In the subgroup analysis, we found men and patients with an uPCR < 500 mg/g creatinine or a baseline serum phosphorus < 4 mg/dL who showed no response to pre-dialysis care were more vulnerable to develop ESRD (Table S3).
Prediction of responsiveness to the pre-ESRD program
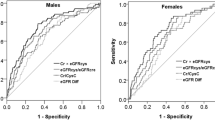
The significant positive associations of male sex, diabetes, hypertension, and uPCR level with nonresponsiveness to pre-dialysis care were identified in the multiple logistic regression. By contrast, baseline eGFR, age, NSAID use, hemoglobin, and serum albumin were negatively associated with nonresponsiveness to pre-dialysis care (Table 4). Compared with the conventional Kidney Failure Risk Equation (KFRE) 7-variable model, our proposed prediction model for responsiveness to pre-dialysis care using baseline demographics, comorbidities, medications, and biochemical profiles performed significantly better in terms of discrimination and calibration, with an area under the receiver operating characteristics of 0.74 (Fig. 4A, B). In decision curve analysis, our proposed model correctly identified an additional 3 nonresponsiveness patients for every 100 patients with CKD at the threshold probability of 20% compared with the KFRE 7-variable model (Fig. 4C).
Prediction performance of the proposed renal care responsiveness prediction (RCRP) model compared with the reference model using seven variables based on kidney failure risk estimation (KFRE). (A) Receiver operating characteristic curve, (B) calibration plots of predicted and observed progression probability, and (C) decision curve analysis.
Discussion
eGFR LSs were largely determined based on the baseline CKD stage when the patients were enrolled into the pre-ESRD program. The more advanced the baseline CKD stage, the less likely the patient was to remain in the baseline stage longitudinally even under the nephrologists’ care. The responsiveness to the nephrologists’ care based on the discrepancy between baseline CKD stage and eGFR-LS was well validated according to the improvement of eGFR declining slope before and after the pre-dialysis enrollment. Patients with poor responsiveness have significantly shorter dialysis-free survival time and higher mortality compared with those whose progression was halted by the pre-ESRD program. Our proposed complete predictive model outperformed the conventional KFRE in predicting CKD progression even under pre-dialysis care.
The fast-decline characteristic of eGFR trajectory was not observed among patients with advanced CKD under nephrologists’ care. Along with the slow declining eGFR slope after the pre-ESRD enrollment, the multidisciplinary pre-ESRD care program of Taiwan did halt the CKD progression; however, patients with CKD stage 5 at baseline still experienced rapid progression to ESRD with a much shorter median dialysis-free survival time of, for instance, approximately 5 months if the median dialysis-free survival time for patients with LS-3A eGFR is 5 years. The dialysis-free survival time were comparable with those of the Initiating Dialysis Early and Late randomized controlled trial28. Such rapid and irreversible progression to ESRD makes shared-decision making regarding the issues of dialysis modality and access preparation essential in pre-dialysis care for patients with stage 5 CKD at baseline. Despite the kidney function of patients with CKD under appropriate pre-dialysis care had been generally stabilized against rapid progression, the steep declining eGFR slopes before pre-dialysis care were observed particularly among patients with stages 4 and 5 CKD at baseline indicating rapid loss of kidney function before the enrollment of pre-ESRD program (Figure S2). This observation highlighted the importance of determining a method to detect and refer patients with early-stage CKD to the pre-ESRD program using innovative diagnostic approaches, such as a noninvasive CKD screening through portable ultrasound29.
Some studies have identified potential predictors of rapid CKD progression and CKD regression among diverse populations. In a study of 949 African American patients, only 3.3% of patients had a clearly improving eGFR slope over a 12-year follow-up. Lower proteinuria and blood pressure were associated with improved eGFR30. Another study conducted in France showed that 15.3% of 394 patients had improved eGFR measurements within a 2-year follow-up period even among patients with stage 4 or 5 CKD at baseline. Patients who showed improvement had lower urinary albumin-to-creatinine ratio (uACR) than those who did not31. Consistently, Borrelli et al. reported that 25% of patients with an eGFR range of 15–60 mL/min/1.73 m2 at baseline exhibited CKD regression under nephrologists’ care. Factors associated with CKD regression were low proteinuria, low blood pressure, high BMI, and absence of autosomal polycystic kidney disease32. A large study of 36,195 patients with stage 3 CKD in the United States showed that the key driving factors of accelerated CKD progression defined as a loss of eGFR > 4 mL/min/1.73 m2/y include proteinuria, high blood pressure, heart failure, anemia, and older age, regardless of diabetic status33. Another large study conducted in Hong Kong concluded that microalbuminuria and retinopathy are associated with an accelerated decline in eGFR defined as joint-latent class modeling among patients with diabetes with baseline eGFR > 60 mL/min/1.73 m215. However, the method of integrating these findings into daily practice is uncertain. In our study, we found that although the KFRE is useful and robust in predicting the risk of progression to ESRD, it is of little value for distinguishing nonresponders from responders to the nephrologists’ care34. Although eGFR variability based on serial eGFR measurements helps predict the responsiveness of patients to pre-ESRD programs, this predictor requires an additional observation period of, for instance, 1 year after the enrollment of pre-ESRD program to obtain multiple eGFR measurements35. To practically help clinicians predict nonresponders in a single clinical encounter, we proposed a predictive model, albeit complex, using variables that are required at the enrollment to pre-dialysis care in Taiwan and readily available in EMR to provide real-time prediction of care responsiveness. The moderate performance of both KFER and ours implied the difficulty in predicting clinical response to nephrologists’ care in the present population. We found men, patients without significant proteinuria, and those with relatively good baseline phosphorus control, who showed no response to pre-dialysis care pose a critical challenge to nephrologists as they are particularly vulnerable to rapid progression to ESRD. More targeted research efforts are needed to identify effective therapeutic strategies for in-time diagnosis and treatment of nonresponders.
The present study has several limitations. First, we did not validate the predictive performance of our proposed model in other populations, which would also require multiple eGFR measurements to define the CKD progression (status of responsiveness). Second, the study population was derived from the Han-Chinese population and was under a universal healthcare system. Therefore, the predictive model must be generalized to other populations with caution. Third, the possibility of residual confounding, such as medication and dietary nonadherence, and over-adjustment of variables that could be in the causal pathway cannot be completely excluded.
On the basis of discrepancy between baseline CKD stage and LS of eGFR, approximately 60% of the patients with CKD achieved disease stability or improvement in Taiwan’s pre-ESRD care program. Our proposed predictive model improves early identification of responsiveness to pre-dialysis care and facilitates decision sharing between clinicians and patients regarding therapeutic strategies. Large longitudinal databases with multiple eGFR measurements should be used to verify our definitions of disease progression and our model’s prediction validity.
References
White, C. A., Pilkey, R. M., Lam, M. & Holland, D. C. Pre-dialysis clinic attendance improves quality of life among hemodialysis patients. BMC Nephrol. 3, 3 (2002).
Stack, A. G. Impact of timing of nephrology referral and pre-ESRD care on mortality risk among new ESRD patients in the United States. Am. J. Kidney Dis. 41, 310–318. https://doi.org/10.1053/ajkd.2003.50038 (2003).
Tisher, C. C. et al. Morbidity and mortality of renal dialysis: An NIH consensus conference statement. Ann. Internal Med. 121(1), 62–70 (1994).
Lin, C. M., Yang, M. C., Hwang, S. J. & Sung, J. M. Progression of stages 3b–5 chronic kidney disease: preliminary results of Taiwan national pre-ESRD disease management program in Southern Taiwan. J. Formosan Med. Assoc. 112, 773–782. https://doi.org/10.1016/j.jfma.2013.10.021 (2013).
Chen, Y. R. et al. Effectiveness of multidisciplinary care for chronic kidney disease in Taiwan: A 3-year prospective cohort study. Nephrol. Dial Transpl. 28, 671–682. https://doi.org/10.1093/ndt/gfs469 (2013).
Cho, E. J. et al. Effect of multidisciplinary pre-dialysis education in advanced chronic kidney disease: Propensity score matched cohort analysis. Nephrology (Carlton) 17, 472–479. https://doi.org/10.1111/j.1440-1797.2012.01598.x (2012).
Winkelmayer, W. C., Liu, J., Chertow, G. M. & Tamura, M. K. Predialysis nephrology care of older patients approaching end-stage renal disease. Arch Intern. Med. 171, 1371–1378. https://doi.org/10.1001/archinternmed.2011.360 (2011).
Chen, P. M. et al. Multidisciplinary care program for advanced chronic kidney disease: Reduces renal replacement and medical costs. Am. J. Med. 128, 68–76. https://doi.org/10.1016/j.amjmed.2014.07.042 (2015).
Hsieh, H. M. et al. Economic evaluation of a pre-ESRD pay-for-performance programme in advanced chronic kidney disease patients. Nephrol. Dial. Transplant. 32, 1184–1194. https://doi.org/10.1093/ndt/gfw372 (2017).
Lin, M. Y. et al. Effect of national pre-ESRD care program on expenditures and mortality in incident dialysis patients: A population-based study. PLoS ONE 13, e0198387. https://doi.org/10.1371/journal.pone.0198387 (2018).
Kurella Tamura, M. et al. Dialysis initiation and mortality among older veterans with kidney failure treated in medicare vs the department of veterans affairs. JAMA Intern. Med. 178, 657–664. https://doi.org/10.1001/jamainternmed.2018.0411 (2018).
Xie, Y., Bowe, B., Xian, H., Balasubramanian, S. & Al-Aly, Z. Renal function trajectories in patients with prior improved eGFR slopes and risk of death. PLoS ONE 11, e0149283. https://doi.org/10.1371/journal.pone.0149283 (2016).
Krolewski, A. S., Skupien, J., Rossing, P. & Warram, J. H. Fast renal decline to end-stage renal disease: An unrecognized feature of nephropathy in diabetes. Kidney Int. 91, 1300–1311. https://doi.org/10.1016/j.kint.2016.10.046 (2017).
Burckhardt, P., Nagin, D. S. & Padman, R. Multi-trajectory models of chronic kidney disease progression. AMIA Annu. Symp. Proc. 2016, 1737–1746 (2016).
Jiang, G. et al. Progression of diabetic kidney disease and trajectory of kidney function decline in Chinese patients with Type 2 diabetes. Kidney Int. 95, 178–187. https://doi.org/10.1016/j.kint.2018.08.026 (2019).
Tsai, C. W. et al. Uric acid predicts adverse outcomes in chronic kidney disease: A novel insight from trajectory analyses. Nephrol. Dial. Transpl. 33(2), 231–41 (2018).
Tsai, C. W. et al. Uric acid predicts adverse outcomes in chronic kidney disease: a novel insight from trajectory analyses. Nephrol. Dial. Transpl. https://doi.org/10.1093/ndt/gfx297 (2017).
Ting, I. W. et al. Joint longitudinal low calcium high phosphorus trajectory associates with accelerated progression, acute coronary syndrome and mortality in chronic kidney disease. Sci. Rep. 10, 9682. https://doi.org/10.1038/s41598-020-66577-7 (2020).
Liang, H. Y., Lo, Y. C., Chiang, H. Y., Chen, M. F. & Kuo, C. C. Validation and comparison of the 2003 and 2016 diastolic functional assessments for cardiovascular mortality in a large single-center cohort. J. Am. Soc. Echocardiogr. 33, 469–480. https://doi.org/10.1016/j.echo.2019.11.013 (2020).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Yamamoto, K. et al. The total urine protein-to-creatinine ratio can predict the presence of microalbuminuria. PLoS ONE 9, e91067. https://doi.org/10.1371/journal.pone.0091067 (2014).
Nagin, D. S., Lynam, D., Raudenbush, S. & Roeder, K. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychol. Methods 4, 139–157 (1999).
Nagin, D. S. & Odgers, C. L. Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol. 6, 109–138. https://doi.org/10.1146/annurev.clinpsy.121208.131413 (2010).
Jones, B. L., Nagin, D. S. & Roeder, K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol. Methods Res. 29, 374–393 (2001).
Fine, J. P. & Gray, R. J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 94, 496–509 (1999).
Balachandran, V. P., Gonen, M., Smith, J. J. & DeMatteo, R. P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 16, e173-180. https://doi.org/10.1016/S1470-2045(14)71116-7 (2015).
Vickers, A. J. & Elkin, E. B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 26, 565–574. https://doi.org/10.1177/0272989X06295361 (2006).
Cooper, B. A. et al. The initiating dialysis early and late (IDEAL) study: Study rationale and design. Perit. Dial. Int. 24, 176–181 (2004).
Kuo, C. C. et al. Automation of the kidney function prediction and classification through ultrasound-based kidney imaging using deep learning. NPJ Digit. Med. 2, 29. https://doi.org/10.1038/s41746-019-0104-2 (2019).
Hu, B. et al. Kidney function can improve in patients with hypertensive CKD. J. Am. Soc. Nephrol. 23, 706–713. https://doi.org/10.1681/ASN.2011050456 (2012).
Weis, L. et al. Renal function can improve at any stage of chronic kidney disease. PLoS ONE 8, e81835. https://doi.org/10.1371/journal.pone.0081835 (2013).
Borrelli, S. et al. Epidemiology of CKD regression in patients under nephrology care. PLoS ONE 10, e0140138. https://doi.org/10.1371/journal.pone.0140138 (2015).
Go, A. S. et al. Contemporary rates and predictors of fast progression of chronic kidney disease in adults with and without diabetes mellitus. BMC Nephrol. 19, 146. https://doi.org/10.1186/s12882-018-0942-1 (2018).
Tangri, N. et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305, 1553–1559. https://doi.org/10.1001/jama.2011.451 (2011).
Tsai, C. W. et al. First-year estimated glomerular filtration rate variability after pre-end-stage renal disease program enrollment and adverse outcomes of chronic kidney disease. Nephrol. Dial. Transplant. 34, 2066–2078. https://doi.org/10.1093/ndt/gfy200 (2019).
Acknowledgements
We appreciate the data exploration, statistical analysis, manuscript preparation, and the support of the iHi Clinical Research Platform from the Big Data Center of CMUH. We would like to thank the Health and Welfare Data Science Center (HWDC), Ministry of Health Welfare, and Health Data Science Center, China Medical University Hospital for providing administrative, technical, and funding support.
Funding
This study was supported by the Ministry of Science and Technology of Taiwan (Grant No. 108-2314-B-039-038-MY3 and 109-2321-B-468-001).
Author information
Authors and Affiliations
Contributions
E.K.K., M.H.H., H.Y.C., and C.C.K. designed the study, conducted statistical analysis and drafted the manuscript. D.R.C., C.T.L., I.W.T., and H.C.Y. collected data and critically revised the manuscript. C.C.W., P.S.C., and C.C.K. participated in the literature search, data interpretation, and manuscript editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
King, E.K., Hsieh, MH., Chang, D.R. et al. Prediction of non-responsiveness to pre-dialysis care program in patients with chronic kidney disease: a retrospective cohort analysis. Sci Rep 11, 13938 (2021). https://doi.org/10.1038/s41598-021-93254-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93254-0
This article is cited by
-
Discovery and prioritization of genetic determinants of kidney function in 297,355 individuals from Taiwan and Japan
Nature Communications (2024)